Abstract
This review has been compiled to assess publications related to the clinical application of direct cerebral tissue oxygenation (PtiO2) monitoring published in international, peer-reviewed scientific journals. Its goal was to extract relevant, i.e. positive and negative information on indications, clinical application, safety issues and impact on clinical situations as well as treatment strategies in neurosurgery, neurosurgical anaesthesiology, neurosurgical intensive care, neurology and related specialties. For completeness’ sake it also presents some related basic science research. PtiO2 monitoring technology is a safe and valuable cerebral monitoring device in neurocritical care. Although a randomized outcome study is not available its clinical utility has repeatedly been clearly confirmed because it adds a monitoring parameter, independent from established cerebral monitoring devices. It offers new insights into cerebral physiology and pathophysiology. Pathologic values have been established in peer-reviewed research, which are not only relevant to outcome but are treatable. The benefits clearly outweigh the risks, which remains unchallenged in all publications retrieved. It is particularly attractive because it offers continuous, real-time data and is available at the bedside.

Similar content being viewed by others
References
Albano C, Comandante L, Nolan S (2005) Innovations in the management of cerebral injury. Crit Care Nurs Q 28:135–149
Alessandri B, Hoelper BM, Behr R, Kempski O (2004) Accuracy and stability of temperature probes for intracranial application. J Neurosci Methods 139:161–165
Baumgartl H, Lubbers DW (1983) Microaxial needle sensors for polarographic measurements of local O2 in the cellular range of living tissue. In: Gnaiger E, Foerstner H (eds) Polarographic oxygen sensors. Springer, Berlin Heidelberg New York, pp 37–65
Becker A, Kuhnt T, Liedtke H, Krivokuca A, Bloching M, Dunst J (2002) Oxygenation measurements in head and neck cancers during hyperbaric oxygenation. Strahlenther Onkol 178:105–108
Belda FJ, Aguilar G, Soro M, Maruenda A (2004) Ventilatory management of the severely brain-injured patient (in Spanish). Rev Esp Anestesiol Reanim 51:143–150
Brawanski A, Faltermeier R, Rothoerl RD, Woertgen C (2002) Comparison of near-infrared spectroscopy and tissue p(O2) time series in patients after severe head injury and aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 22:605–611
Burger R, Bendszus M, Vince GH, Roosen K, Marmarou A (2002) A new reproducible model of an epidural mass lesion in rodents. Part I: characterization by neurophysiological monitoring, magnetic resonance imaging, and histopathological analysis. J Neurosurg 97:1410–1418
Carre E, Cantais E, Darbin O, Terrier JP, Lonjon M, Palmier B, Risso JJ (2004) Technical aspects of an impact acceleration traumatic brain injury rat model with potential suitability for both microdialysis and PtiO2 monitoring. J Neurosci Methods 140:23–28
Carvi y Nievas M, Toktamis S, Hollerhage HG, Haas E (2005) Hyperacute measurement of brain-tissue oxygen, carbon dioxide, pH, and intracranial pressure before, during, and after cerebral angiography in patients with aneurysmatic subarachnoid hemorrhage in poor condition. Surg Neurol 64:362–367; discussion 367
Cavus E, Dorges V, Wagner-Berger H, Stadlbauer KH, Steinfath M, Wenzel V, Bein B, Scholz J (2005) Changes of local brain tissue oxygen pressure after vasopressin during spontaneous circulation. Acta Neurochir (Wien) 147:283–290; discussion 290
Clausen T, Khaldi A, Zauner A, Reinert M, Doppenberg E, Menzel M, Soukup J, Alves OL, Bullock MR (2005) Cerebral acid-base homeostasis after severe traumatic brain injury. J Neurosurg 103:597–607
Clausen T, Scharf A, Menzel M, Soukup J, Holz C, Rieger A, Hanisch F, Brath E, Nemeth N, Miko I, Vajkoczy P, Radke J, Henze D (2004) Influence of moderate and profound hyperventilation on cerebral blood flow, oxygenation and metabolism. Brain Res 1019:113–123
Dings J, Jager A, Meixensberger J, Roosen K (1998) Brain tissue pO2 and outcome after severe head injury. Neurol Res 20(Suppl 1):S71–S75
Dings J, Meixensberger J, Amschler J, Hamelbeck B, Roosen K (1996) Brain tissue pO2 in relation to cerebral perfusion pressure, TCD findings and TCD-CO2-reactivity after severe head injury. Acta Neurochir (Wien) 138:425–434
Dings J, Meixensberger J, Amschler J, Roosen K (1996) Continuous monitoring of brain tissue PO2: a new tool to minimize the risk of ischemia caused by hyperventilation therapy. Zentralbl Neurochir 57:177–183
Dings J, Meixensberger J, Jager A, Roosen K (1998) Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 43:1082–1095
Dings J, Meixensberger J, Roosen K (1997) Brain tissue pO2-monitoring: catheter stability and complications. Neurol Res 19:241–245
Gelabert-Gonzalez M, Fernandez-Villa JM, Ginesta-Galan V (2002) Intra-operative monitoring of brain tissue O2 (PtiO2) during aneurysm surgery. Acta Neurochir (Wien) 144:863–866; discussion 866–867
Gonzalez H, Hunter CJ, Bennet L, Power GG, Gunn AJ (2005) Cerebral oxygenation during postasphyxial seizures in near-term fetal sheep. J Cereb Blood Flow Metab 25:911–918
Gracias VH, Guillamondegui OD, Stiefel MF, Wilensky EM, Bloom S, Gupta R, Pryor JP, Reilly PM, Leroux PD, Schwab CW (2004) Cerebral cortical oxygenation: a pilot study. J Trauma 56:469–472; discussion 464–472
Hare GM, Hum KM, Kim SY, Barr A, Baker AJ, Mazer CD (2004) Increased cerebral tissue oxygen tension after extensive hemodilution with a hemoglobin-based oxygen carrier. Anesth Analg 99:528–535, table of contents
Hartl R, Bardt TF, Kiening KL, Sarrafzadeh AS, Schneider GH, Unterberg AW (1997) Mannitol decreases ICP but does not improve brain-tissue pO2 in severely head-injured patients with intracranial hypertension. Acta Neurochir Suppl 70:40–42
Hemphill JC 3rd, Morabito D, Farrant M, Manley GT (2005) Brain tissue oxygen monitoring in intracerebral hemorrhage. Neurocrit Care 3:260–270
Hlatky R, Valadka AB, Robertson CS (2005) Intracranial pressure response to induced hypertension: role of dynamic pressure autoregulation. Neurosurgery 57:917–923; discussion 917–923
Hoelper BM, Alessandri B, Heimann A, Behr R, Kempski O (2005) Brain oxygen monitoring: in-vitro accuracy, long-term drift and response-time of Licox- and Neurotrend sensors. Acta Neurochir (Wien) 147:767–774
Ibanez J, Vilalta A, Mena MP, Vilalta J, Topczewski T, Noguer M, Sahuquillo J, Rubio E (2003) Intraoperative detection of ischemic brain hypoxia using oxygen tissue pressure microprobes (in Spanish). Neurocirugia (Astur) 14:483–489; discussion 490
Imberti R, Bellinzona G, Riccardi F, Pagani M, Langer M (2003) Cerebral perfusion pressure and cerebral tissue oxygen tension in a patient during cardiopulmonary resuscitation. Intensive Care Med 29:1016–1019
Jaeger M, Schuhmann MU, Soehle M, Meixensberger J (2006) Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med 34:1783–1788
Jaeger M, Soehle M, Meixensberger J (2003) Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J Neurol Neurosurg Psychiatry 74:513–515
Jaeger M, Soehle M, Meixensberger J (2005) Brain tissue oxygen (PtiO2): a clinical comparison of two monitoring devices. Acta Neurochir Suppl 95:79–81
Jaeger M, Soehle M, Schuhmann MU, Winkler D, Meixensberger J (2005) Correlation of continuously monitored regional cerebral blood flow and brain tissue oxygen. Acta Neurochir (Wien) 147:51–56; discussion 56
Jia J, Lin YQ, Liu WF, Zhong TA, Zhang J, Ye Y, Xu YQ (2005) Study of the effects of mild hypothermia on cerebral PO2, PCO2 and pH and body temperature in patients with acute severe head injury. Chin J Traumatol 8:138–141
Jodicke A, Hubner F, Boker DK (2003) Monitoring of brain tissue oxygenation during aneurysm surgery: prediction of procedure-related ischemic events. J Neurosurg 98:515–523
Johnston AJ, Steiner LA, Chatfield DA, Coleman MR, Coles JP, Al-Rawi PG, Menon DK, Gupta AK (2003) Effects of propofol on cerebral oxygenation and metabolism after head injury. Br J Anaesth 91:781–786
Johnston AJ, Steiner LA, Chatfield DA, Coles JP, Hutchinson PJ, Al-Rawi PG, Menon DK, Gupta AK (2004) Effect of cerebral perfusion pressure augmentation with dopamine and norepinephrine on global and focal brain oxygenation after traumatic brain injury. Intensive Care Med 30:791–797
Kett-White R, Hutchinson PJ, Al-Rawi PG, Gupta AK, Pickard JD, Kirkpatrick PJ (2002) Adverse cerebral events detected after subarachnoid hemorrhage using brain oxygen and microdialysis probes. Neurosurgery 50:1213–1221; discussion 1221–1212
Kiening KL, Unterberg AW, Bardt TF, Schneider GH, Lanksch WR (1996) Monitoring of cerebral oxygenation in patients with severe head injuries: brain tissue PO2 versus jugular vein oxygen saturation. J Neurosurg 85:751–757
Lang EW, Czosnyka M, Mehdorn HM (2003) Tissue oxygen reactivity and cerebral autoregulation after severe traumatic brain injury. Crit Care Med 31:267–271
Lang EW, Lagopoulos J, Griffith J, Yip K, Yam A, Mudaliar Y, Mehdorn HM, Dorsch NW (2003) Cerebral vasomotor reactivity testing in head injury: the link between pressure and flow. J Neurol Neurosurg Psychiatry 74:1053–1059
Lubbers DW, Baumgartl H (1997) Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kidney Int 51:372–380
Maas AI, Fleckenstein W, de Jong DA, van Santbrink H (1993) Monitoring cerebral oxygenation: experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir Suppl (Wien) 59:50–57
McLeod AD, Igielman F, Elwell C, Cope M, Smith M (2003) Measuring cerebral oxygenation during normobaric hyperoxia: a comparison of tissue microprobes, near-infrared spectroscopy, and jugular venous oximetry in head injury. Anesth Analg 97:851–856
Meixensberger J, Baunach S, Amschler J, Dings J, Roosen K (1997) Influence of body position on tissue-pO2, cerebral perfusion pressure and intracranial pressure in patients with acute brain injury. Neurol Res 19:249–253
Meixensberger J, Jager A, Dings J, Baunach S, Roosen K (1998) Multimodal hemodynamic neuromonitoring-quality and consequences for therapy of severely head injured patients. Acta Neurochir Suppl 71:260–262
Meixensberger J, Renner C, Simanowski R, Schmidtke A, Dings J, Roosen K (2004) Influence of cerebral oxygenation following severe head injury on neuropsychological testing. Neurol Res 26:414–417
Mulvey JM, Dorsch NW, Mudaliar Y, Lang EW (2004) Multimodality monitoring in severe traumatic brain injury: the role of brain tissue oxygenation monitoring. Neurocrit Care 1:391–402
Ng I, Lim J, Wong HB (2004) Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery 54:593–597; discussion 598
Okonkwo DO, Wagner J, Melon DE, Alden T, Stone JR, Helm GA, Jane JA Sr (2003) Trans-sodium crocetinate increases oxygen delivery to brain parenchyma in rats on oxygen supplementation. Neurosci Lett 352:97–100
Raabe A, Gottschalk A, Hommel M, Dubben HH, Strandl T (2005) No effect of the hemoglobin solution HBOC-201 on the response of the rat R1H tumor to fractionated irradiation. Strahlenther Onkol 181:730–737
Reinert M, Barth A, Rothen HU, Schaller B, Takala J, Seiler RW (2003) Effects of cerebral perfusion pressure and increased fraction of inspired oxygen on brain tissue oxygen, lactate and glucose in patients with severe head injury. Acta Neurochir (Wien) 145:341–349; discussion 349–350
Reithmeier T, Lohr M, Pakos P, Ketter G, Ernestus RI (2005) Relevance of ICP and ptiO(2) for indication and timing of decompressive craniectomy in patients with malignant brain edema. Acta Neurochir (Wien) 147:947–952
Rossi S, Balestreri M, Spagnoli D, Bellinzona G, Valeriani V, Bruzzone P, Maestri M, Stocchetti N (2000) Oxygen delivery and oxygen tension in cerebral tissue during global cerebral ischaemia: a swine model. Acta Neurochir Suppl 76:199–202
Rossi S, Stocchetti N, Longhi L, Balestreri M, Spagnoli D, Zanier ER, Bellinzona G (2001) Brain oxygen tension, oxygen supply, and oxygen consumption during arterial hyperoxia in a model of progressive cerebral ischemia. J Neurotrauma 18:163–174
Sarrafzadeh AS, Kiening KL, Bardt TF, Hartl R, Schneider GH, Unterberg AW (1997) Monitoring cerebral oxygenation: a methodological comparison (in German). Anasthesiol Intensivmed Notfallmed Schmerzther 32:S224–S230
Sarrafzadeh AS, Kiening KL, Bardt TF, Schneider GH, Unterberg AW, Lanksch WR (1998) Cerebral oxygenation in contusioned vs. nonlesioned brain tissue: monitoring of PtiO2 with Licox and Paratrend. Acta Neurochir Suppl 71:186–189
Sarrafzadeh AS, Kiening KL, Callsen TA, Unterberg AW (2003) Metabolic changes during impending and manifest cerebral hypoxia in traumatic brain injury. Br J Neurosurg 17:340–346
Sarrafzadeh AS, Kiening KL, Unterberg AW (2003) Neuromonitoring: brain oxygenation and microdialysis. Curr Neurol Neurosci Rep 3:517–523
Sarrafzadeh AS, Peltonen EE, Kaisers U, Kuchler I, Lanksch WR, Unterberg AW (2001) Secondary insults in severe head injury-do multiply injured patients do worse? Crit Care Med 29:1116–1123
Sarrafzadeh AS, Sakowitz OW, Callsen TA, Lanksch WR, Unterberg AW (2002) Detection of secondary insults by brain tissue pO2 and bedside microdialysis in severe head injury. Acta Neurochir Suppl 81:319–321
Sarrafzadeh AS, Unterberg AW, Lanksch WR (1998) Bedside-microdialysis for early detection of vasospasm after subarachnoid hemorrhage. Case report and review of the literature. Zentralbl Neurochir 59:269–273
Scheufler KM, Lehnert A, Rohrborn HJ, Nadstawek J, Thees C (2004) Individual value of brain tissue oxygen pressure, microvascular oxygen saturation, cytochrome redox level, and energy metabolites in detecting critically reduced cerebral energy state during acute changes in global cerebral perfusion. J Neurosurg Anesthesiol 16:210–219
Schneider GH, Sarrafzadeh AS, Kiening KL, Bardt TF, Unterberg AW, Lanksch WR (1998) Influence of hyperventilation on brain tissue-PO2, PCO2, and pH in patients with intracranial hypertension. Acta Neurochir Suppl 71:62–65
Smith MJ, Stiefel MF, Magge S, Frangos S, Bloom S, Gracias V, Le Roux PD (2005) Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med 33:1104–1108
Soehle M, Jaeger M, Meixensberger J (2003) Online assessment of brain tissue oxygen autoregulation in traumatic brain injury and subarachnoid hemorrhage. Neurol Res 25:411–417
Stiefel MF, Heuer GG, Abrahams JM, Bloom S, Smith MJ, Maloney-Wilensky E, Grady MS, LeRoux PD (2004) The effect of nimodipine on cerebral oxygenation in patients with poor-grade subarachnoid hemorrhage. J Neurosurg 101:594–599
Stiefel MF, Heuer GG, Smith MJ, Bloom S, Maloney-Wilensky E, Gracias VH, Grady MS, LeRoux PD (2004) Cerebral oxygenation following decompressive hemicraniectomy for the treatment of refractory intracranial hypertension. J Neurosurg 101:241–247
Stiefel MF, Spiotta A, Gracias VH, Garuffe AM, Guillamondegui O, Maloney-Wilensky E, Bloom S, Grady MS, LeRoux PD (2005) Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg 103:805–811
Strege RJ, Lang EW, Stark AM, Scheffner H, Fritsch MJ, Barth H, Mehdorn HM (2003) Cerebral edema leading to decompressive craniectomy: an assessment of the preceding clinical and neuromonitoring trends. Neurol Res 25:510–515
Tolias CM, Reinert M, Seiler R, Gilman C, Scharf A, Bullock MR (2004) Normobaric hyperoxia-induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J Neurosurg 101:435–444
Unterberg AW, Kiening KL, Hartl R, Bardt T, Sarrafzadeh AS, Lanksch WR (1997) Multimodal monitoring in patients with head injury: evaluation of the effects of treatment on cerebral oxygenation. J Trauma 42:S32–S37
Unterberg AW, Sarrafzadeh AS, Lanksch WR (1999) Skull-brain injury in polytrauma-results of neurosurgery (in German). Anasthesiol Intensivmed Notfallmed Schmerzther 34(Suppl 1):S13–S19
Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS (1998) Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med 26:1576–1581
van Santbrink H, Maas AI, Avezaat CJ (1996) Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery 38:21–31
van Santbrink H, Schouten JW, Steyerberg EW, Avezaat CJ, Maas AI (2002) Serial transcranial Doppler measurements in traumatic brain injury with special focus on the early posttraumatic period. Acta Neurochir (Wien) 144:1141–1149
Wilensky EM, Bloom S, Leichter D, Verdiramo AM, Ledwith M, Stiefel M, LeRoux P, Grady MS (2005) Brain tissue oxygen practice guidelines using the LICOX CMP monitoring system. J Neurosci Nurs 37:278–288
Author information
Authors and Affiliations
Additional information
Comments
Ignacio J. Previgliano, Buenos Aires, Argentina
Brain tissue oxygenation, as demonstrated in this complete systematic review by Lang et al., is a promising technology. I agree that it offers continuous, real-time data and is available at the bedside safely. The rapid correlation between the indicated treatment and the regional response allowed by the devices is stimulating.
Beyond the applications updated in the review there is a potential use in early brain death diagnosis that should be borne in mind as a matter of research. Estimation of the best cerebral perfusion pressure and hyperventilation therapy control are, in my opinion, among the most important applications.
Nevertheless, there are some important issues to highlight regarding this technology. The Neurotrend device has been dropped off the market by Codman, Johnson and Johnson, so Licox is the only device available. There are no papers assessing sensitivity, specificity, positive and negative value as well as positive or negative likelihood ratio and pre- or post-test odds for the method as a prognostic or diagnostic tool, according to the principles of evidence-based medicine. There are also controversies on which region should be monitored: non-injured tissue, penumbra area or injured tissue, as well as which are the normal values or the pathologic threshold. Another controversy is the one that confronts regional vs global monitoring systems.
Even though well conducted and serious, most of the research has been done in Germany where the Gesellschaft für Medizinische Sondentechnik (GMS) developed the polarographic cell device, as is noted in the review references.
In view of these critical considerations that should be taken into account, brain tissue oxygenation should be considered a new star in the neurocritical monitoring constellation in which none is more important than the other and all of them are still looking for their right place.
Comment
Alexander Brawanski, Regensburg, Germany
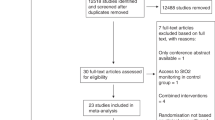
In their paper the authors review the development and clinical application of the registration of cerebral oxygenation (PtiO2). They reviewed the pertinent literature from 2004 to 2006 with the aim of collecting the relevant information on clinical applicability, safety issues and clinical usefulness. All in all 1,400 publications were scanned. Mostly PtiO2 monitoring was used in traumatic brain injury (TBI) followed by subarachnoid hemorrhage. Less commonly it was used intraoperatively during cerebrovascular surgery. Other rarer applications, like during cardiopulmonary resuscitation or cerebral angiography, are included in the review. The authors report that PtiO2 monitoring is mostly used clinically in conjunction with additional cerebral monitoring devices like intracranial pressure (ICP) monitoring, jugular venous saturation monitoring, cerebral microdialysis and near infrared spectroscopy, just to name a few. Two different devices are on the market based on different technology and giving slightly different data. The main results of this review are that this methodology is safe and has an impact on clinical treatment of the above-mentioned patient groups. A safety range of tissue oxygen levels could be established, below that the outcome of the studied patients was significantly worse. Thus PtiO2 monitoring has a prognostic value. Negative effects of hyperventilation were detected and it was applied more safely as hypoxic cerebral oxygen levels were avoided. The indication for decompressive craniectomy in severe brain swelling could be guided by cerebral oxygen monitoring more safely. PtiO2 has also been proven useful in monitoring the use of vasoactive drugs given in neurocritical care as well as in elucidating effects of nimodipine and mannitol. There were no severe side effects using PtiO2 in patients. Both available probes measure oxygen tension sufficiently. However, the Licox technology seems to be more accurate than the Neurotrend monitor. The often quoted disadvantage of the “locality” of the measuring devices—they measure an area of a few cubic millimeters—could be alleviated by several publications. These could show that there is a certain random positioning error. On the other hand these local measurements correlated well with more global methods like cerebral blood flow. The authors conclude that PtiO2 monitoring is safe, clinically useful, and it improves the treatment of ICU patients. They favor, however, a multiparameter approach, meaning that other “brain” parameters should be monitored in addition. All in all this is a very informative study giving relevant and up-to-date information about this important monitoring technique.
Rights and permissions
About this article
Cite this article
Lang, E.W., Mulvey, J.M., Mudaliar, Y. et al. Direct cerebral oxygenation monitoring—a systematic review of recent publications. Neurosurg Rev 30, 99–107 (2007). https://doi.org/10.1007/s10143-006-0062-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-006-0062-4