Abstract
Despite the availability of an effective vaccine and antiviral treatments, hepatitis B is still a global public health problem. Hepatitis B vaccination can prevent the disease. Vaccination induces long-lasting protective immune memory, and the identification of memory cell subsets can indicate the effectiveness of vaccines. Here, we compared the frequency of CD4+ memory T cell subsets between responders and nonresponders to HB vaccination. Besides, the frequency of IFN-γ+ memory T cells was compared between studied groups. Study participants were grouped according to their anti-HBsAb titer. For restimulation of CD4+ memory T cells, peripheral blood mononuclear cells (PBMCs) were cultured in the presence of HBsAg and PHA for 48 h. Besides, PMA, ionomycin, and brefeldin were added during the last 5 h of incubation to induce IFN-γ production. Flow cytometry was used for analysis. There was a statistically significant difference in the frequency of CD4+CD95+, CD4+CD95Hi, and CD4+CD95low/med T stem cell memory (TSCM) cells between responder and nonresponder groups. However, the comparison of the frequency of memory T cells producing IFN-γ showed no differences. Our results identified a possible defect of immunological CD4+ memory T cell formation in nonresponders due to their lower frequency of CD4+ TSCM cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis is a term used for a variety of inflammatory liver diseases which may eventually lead to liver failure, cirrhosis, and hepatocellular carcinoma. This inflammation is divided into two main categories: noninfectious and infectious, which is induced by hepatitis A, B, C, D, and E viruses as well as cytomegalovirus, and Epstein–Barr virus [1]. Hepatitis B and C viruses are the major causes of cirrhosis and liver cancer [2, 3]. Hepatitis B virus (HBV), a member of the Hepadnaviridae family [4], has a small, partially double-stranded, relaxed-circular DNA genome that encodes seven proteins: precore/E antigen (HBeAg), large (L-), medium (M-), and small (S-) surface antigen (HBsAg), core protein, polymerase, and X protein (HBx) [5, 6]. Nowadays, it is well known that hepatocyte infection with the virus is non-cytopathic and can be transient or chronic depending on the ability of the host immune system to clear the infection [7].
Despite the availability of effective vaccines and antiviral treatments, infection with HBV is considered a global health problem [8, 9]. In the past 2 decades, many drugs have been developed to treat this disease. Their main problems are the inability to eradicate HBV, side effects, the necessity for regular injections, and the high cost of treatment. In addition to the suggested treatments, hepatitis B (HB) vaccination seems to be the most effective strategy to prevent and control the infection [10,11,12,13,14]. The first generation of the HBV vaccine was the serum of people who produced large amounts of antibodies against HBsAg (passive). Subsequently, advances in DNA recombination technology led to the development of the second generation of HBV vaccines (DNA recombinant vaccines) (active) [15, 16].
After vaccination, measurement of the humoral immune response against HBsAg is an immune marker indicating the presence or absence of protective antibodies against HBV infection. According to this factor, seroprotection is accepted when anti-HBsAb titer reaches more than 10 mIU/ml, therefore, people who did not develop corresponding anti-HBsAb titers, even after administration of two complete series of the HBV vaccine, are considered nonresponders [15, 17, 18].
After HBV exposure, protection induced by the vaccine occurs through two mechanisms; the first is the neutralization of the virus by anti-HB antibodies, and the second involves the activation of CD4+ T memory cells, which subsequently activate memory B cells to secrete anti-HBs antibody [15]. Several studies have shown that vaccine-induced antibody levels are gradually declining, while memory cell formation in healthy recipients will remain for more than 15 years [19,20,21,22].
Although, many successful vaccines primarily act by generating antibodies, producing vaccines that can provoke a population of highly-specific T cells is completely on demand. These types of vaccines should have the ability to generate large, effective, and long-lived populations of memory T cells [23, 24]. Advances in the multi-parameter flow cytometry technique have provided the ability to define the heterogeneity of T cells [25]. Based on the differential expression of CD28, CCR7, CD45RO, and CD95, there are six major groups of quiescent T cells, including naïve T cell (TN), stem cell memory T cell (TSCM), central memory T cell (TCM), transitional memory T cell (TTM), effector memory T cell (TEM), and terminal effector T cell (TTE). These cells are supposed to be generated from TN during a linear model called linear differentiation [23, 26,27,28,29]. Despite many advances in this field, there are still questions about the formation and maintenance of immunological memory after vaccination [23]. Accordingly, the identification of memory cell subsets can indicate the effectiveness of vaccines like the HB vaccine. In the present study, we, therefore, aimed to determine the frequency of CD4+ memory T cell subsets and compare these cell quantities between responders and nonresponders to the HB vaccine.
Materials and methods
Subjects
All study participants were selected from the health care staff of hospitals affiliated with Shiraz University of Medical Sciences (SUMS), Shiraz, Iran. This study was approved by the Ethics Committee of the university (ethics code: IR.SUMS.REC.1397.779).
The participants were divided into two groups of responders (n = 13) and nonresponders (n = 15) according to their anti-HBsAb titers registered at the hospital infection control centers. Responders had antibody titers > 100 mIU/ml, and nonresponder subjects had antibody titers < 10 mIU/ml after administration of at least two complete series of the HB vaccine (repeating the whole schedule of vaccine after the first schedule). After obtaining written informed consent, the blood samples were taken under sterile conditions and transferred to the laboratory enclosed in an ice pack. The patients with hepatic infections and HIV, cancers, autoimmune diseases, and alcohol users were excluded from the study.
Isolation of mononuclear cells from peripheral blood
Five milliliters of peripheral blood were overlaid on Ficoll-Paque (inno-train DIAGNOSTIK GMBH, Germany) in sterile conditions to isolate mononuclear cells by density gradient centrifugation. The plasma layer was removed, aliquoted, and sorted at − 20 °C for identification of anti-HBs Ab level. The peripheral blood mononuclear cells (PBMCs) were then carefully aspirated from the Ficoll-plasma interface, washed two times with 1 × phosphate-buffered saline (PBS), counted with Trypan blue dye (Shellmax, China), and prepared in appropriate concentration for further studies.
Determination of HBsAb with enzyme linked immunoassay (ELISA) technique
Anti-HBsAb plasma level was checked using an HBsAb ELISA kit (DIA.PRO Diagnostic Bioprobes Srl, Italy) according to the manufacturer’s instructions. In brief, the sample was applied on microwells coated with highly purified HBsAg, which specifically captured anti-HBs antibodies and formed antibody‐antigen complexes. The amount of conjugate bound and, hence, the color in the wells was directly related to the concentration of antibodies in the sample.
Activation for restimulation of CD4+ memory T cells
Two million of PBMCs were cultured in a final volume of 1000 µl complete culture media (CM10) [RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin–streptomycin (Pen-Strep), and 1% glutamine (all from Shellmax)] per well. The optimal concentrations of HBsAg (Razi Institute, Iran) and phytohemagglutinin (PHA; Invitrogen, USA) were determined to be 4 and 1 µg/ml for lymphocyte activation. The cells were then exposed to HBsAg and PHA for 48 h at 37 °C in a humidified atmosphere supplemented with 5% CO2.
Activation for IFN-γ production by CD4+ T cells
Two million of PBMCs were cultured in a final volume of 1000 µl CM10 per well. The cells were stimulated with HBsAg (4 µg/ml) (Razi Institute) and PHA (1 µg/ml) (Invitrogen) during a 48 h incubation at 37 °C in a humidified atmosphere supplemented with 5% CO2. In the last 5 h, 25 ng/ml of phorbol myristate acetate, 500 ng/ml of ionomycin (both from Sigma-Aldrich, USA), and 0.7 µl of brefeldin A as a Golgi stopper (BD Biosciences, USA) were added to this cocktail.
Cell staining for assessment of CD4+ memory T cell subsets
As previously described [30], to investigate the frequency of CD4+ memory T cell subsets, after stimulation time, PBMCs were collected, washed with 1 × PBS, and stained with appropriate fluorescent-labeled antibodies (FITC-conjugated anti-CCR7 clone: G043H7, PE-conjugated anti-CD95 clone: Dx2, PerCP/Cy5.5-conjugated anti-CD4 clone: RPA-T4, and APC-conjugated anti-CD45RO clone: UCHL1; all from Biolegend, USA) and incubated in the dark for 30 min at room temperature. After that, the cells were washed twice using 2 ml of 1 × PBS to remove unbound antibodies and fixed in 300 µl of paraformaldehyde (PFA; 10 mg/ml; Merk, Germany) for 15 min at 4 °C. Following washing with 3 ml of 1 × PBS, in the last step, the cells were suspended in 500 µl of 1 × PBS and acquired on a 4-color BD FACSCalibur™ flow cytometer (BD Biosciences) (~ 200 × 103 events). FlowJo software (version X.0.7; Tree Star, Inc., Ashland, OR, USA) was used for data analysis.
The mean fluorescent intensity (MFI) of CD95 was also evaluated on CD95+ and CD95Hi TSCM. To normalize the MFI of CD95 in different subsets, the MFI of CD95 in positive cells (CD95+ TSCM or CD95Hi TSCM) was divided by the MFI of CD95 in the negative population (TN).
Cell staining for assessment of CD4+ IFN-γ+ memory T cells
At the end of stimulation time, PBMCs were collected, washed with 1 × PBS, and stained with fluorescent-labeled antibodies for both surface and intracellular markers. At first, an APC-conjugated anti-CD45RO antibody (clone: UCHL1; Biolegend) was added, and the cells were incubated in the dark for 30 min at room temperature. Then, they were washed twice with 2 ml of 1 × PBS and fixed with 300 µl of PFA (10 mg/ml; Merk) for 15 min at 4 °C. After washing with 3 ml of 1 × PBS, in the next step, the cells were permeabilized using 1 ml of 1 × Perm/Wash buffer (Biolegend) and were incubated in the dark for 15 min at room temperature. The fluorescent-labeled antibodies (FITC-conjugated anti-IFN-γ clone: B27 and PerCP/Cy5.5-conjugated anti-CD4 clone: RPA-T4; Biolegend) were then added, and incubation was done in the dark for 30 min at room temperature. The cells were washed twice with 1 × Perm/Wash buffer and then fixed with 300 µl of PFA for 15 min at 4 °C. Finally, the cells were washed with 3 ml of 1 × PBS, suspended in 500 µl of 1 × PBS, and were acquired on a 4-color BD FACSCalibur™ flow cytometer (BD Biosciences) (~ 200 × 103 events). FlowJo software (version X.0.7; Tree Star, Inc.) was used for data analysis.
As the mean expression of IFN-γ, the MFI of this cytokine was also determined. To report the MFI of IFN-γ, the MFI of each IFN-γ expressing T cell was normalized by the corresponding IFN-γ negative population.
Statistical analysis
SPSS software (version 22.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis. Before comparing, the normal distribution of variables was first evaluated using the Kolmogorov–Smirnov test. As the data could not pass the normality test, the nonparametric Mann–Whitney U test was used. Moreover, a nonparametric Spearman’s rank correlation test was done to assess the relationship between two quantitative variables. All data were presented as mean ± SEM, and p-values < 0.05 were considered significant.
Results
The characteristics of the study subjects
In this study, 15 people with anti-HBs antibody titer less than 10 mIU/ml were recruited as the nonresponder group. These individuals had no increase in their anti-HBsAb titer after administration of at least 2 complete series of the HBV vaccine. Besides, 13 subjects with an antibody titer of more than 100 mIU/ml were included in the responder group (Table 1). The individuals with inflammatory diseases (i.e., autoimmune disorders and cancers), alcohol users, and those infected by HIV, HBV, and HCV were excluded from the investigation.
The frequency of different CD4+ memory T cell subsets in the peripheral blood of HB vaccine responders and nonresponders after stimulation with HBsAg
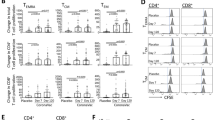
Our data analysis in flow cytometry relied on the following gating strategy: lymphocytes were gated based on their relative size (forward scatter) and granularity (side scatter) (Fig. 1A). Then, lymphocytes with high expression of CD4 marker (CD4+) were separated (Fig. 1B), and the frequency of different memory T cell subsets was determined based on the expression of CCR7, CD45RO, and CD95 markers. All frequencies were reported in the CD4+ T cell population. Among CCR7+CD45RO− population (Fig. 1C), CD95− cells were considered TN cells, and those with CD95 expression were introduced as TSCM. TN and TSCM cells were determined in red and black squares, respectively, in Fig. 1H. CCR7+CD45RO+ cells (Fig. 1E) expressing CD95 were considered TCM cells (Fig. 1J). Besides, according to the expression level of CD45RO (Fig. 1F and G), two groups of TCM were further introduced: CD45ROHi TCM (Fig. 1K) and CD45ROlow/med TCM (Fig. 1L). The CCR7−CD45RO+ cells (Fig. 1D) expressing CD95 were considered TEM (Fig. 1I). Regarding the high expression of CD95 on memory T cell subsets after activation, CD95Hi memory T cells were also evaluated (Fig. 1M-Q). The frequency of CD95low/med memory T cells was also calculated by subtracting the frequency of CD95Hi memory T cell from the frequency of CD95+ memory T cell.
Gating strategy to identify the frequency of CD4+ memory T cell subsets in peripheral blood of HB vaccine responders and nonresponders after stimulation. Lymphocytes were gated based on their relative size (forward scatter) and granularity (side scatter) (A). Then, lymphocytes with high expression of CD4 (CD4+) were determined (B). The frequencies of different memory T cell subsets were then defined based on the expression of CCR7, CD45RO, and CD95 markers in the CD4+ population (C–Q). Regarding different levels of CD95 expression on memory T cell subsets, two groups of cells, CD95+ and CD95Hi cells, were evaluated (H–Q). TN: T naïve; TSCM: T stem cell memory; TCM: T central memory; TEM: T effector memory
Frequency of CD4+ memory T cell subsets in responders and nonresponders to HB vaccine
The purpose of this part of the experiment was to determine the differences in the frequency of the CD4+ memory T cell subsets between the responder and nonresponder groups. Median (IQ25–75) and mean ± SEM of the frequency of cell subsets in each group and p-values of their differences were detailed in Table 2. As shown, there were no statistically significant differences in the frequencies of various memory T cell subsets except for CD4+CD95+ (P-value = 0.023), CD4+CD95Hi (P-value = 0.001), and CD4+CD95low/med (P-value = 0.032) TSCM cells between responder and nonresponder groups. The CD95 expression (based on MFI) on CD4+ TSCM was also compared, however, there were no statistical significant differences between the two groups (Table 2). The results obtained from responder and nonresponder groups were shown in Supplementary Tables S1 and S2, respectively.
Correlation of anti-HBsAb level and age of participants with the frequencies of different cell subsets
Our results showed no correlation between the anti-HBsAb titer and the frequencies of various CD4+ memory T cell subsets in both responder and nonresponder groups. Moreover, there was no correlation between the age of nonresponders and the percentages of different cell subsets, while a negative correlation was observed between the age of responders and the frequency of CD4+ TN (P-value = 0.029, rs = − 0.6). On the other hand, the positive correlations were found between the responders’ age and the frequencies of CD4+ TCM subsets (CD45RO+ TCM: P-value = 0.043, rs: 0.57; and CD45ROlow/med TCM: P-value = 0.014, rs = 0.66) (Fig. 2, Table S3).
Production of IFN-γ by CD4+and CD4− memory T cells of responders and nonresponders to HB vaccine after stimulation
To determine the frequency of IFN-γ+ memory lymphocytes (CD4+CD45RO+IFN-γ+ and CD4−CD45RO+IFN-γ+) after gating the lymphocyte population-based on their relative size (forward scatter) and granularity (side scatter) (Fig. 3A), CD4+ and CD4− lymphocytes were defined (Fig. 3B and C). Then, the frequency of IFN-γ producing cells was determined in each population (Fig. 3D-G).
Frequency of IFN-γ+ memory lymphocytes in responders and nonresponders to HB vaccine
In the second section of the study, the PBMCs were stimulated and the frequencies of different IFN-γ+ cells were compared between responders and nonresponders to the HB vaccine. The median (IQ25–75) and mean ± SEM of the frequencies in each group and the p-values of their differences were summarized in Table 3. As shown, there were no statistical differences in the frequencies of various IFN-γ producing subsets between responder and nonresponder participants.
Discussion
HBV is one of the main reasons for cirrhosis and liver cancer worldwide [2, 8]. Different medications are in clinical use to treat this disease. Because of the challenges during the treatment of hepatitis, vaccination is more efficient as a prophylactic approach [10,11,12,13]. The vaccine provides protection via neutralization of the virus by anti-HBsAb and activation of CD4+ T memory cells [15]. Although vaccine-induced antibody levels are gradually declining, it has been shown that memory cells are maintained in healthy vaccine recipients for more than 15 years [19,20,21].
The goal of vaccination is to induce long-lasting protective immune memory [24]. The identification of memory cell subsets may indicate the effectiveness of vaccines. Therefore, we designed the present study to analyze the frequency of CD4+ memory T cell subsets (TSCM, TCM, and TEM cells) and compare these cell quantities between responders and nonresponders to the HB vaccine. These cells were determined based on the differential expression of CCR7, CD45RO, and CD95 markers [23, 26].
Pieces of evidence showed that specific TSCM cells were produced during normal immune responses against pathogens [31,32,33]. Furthermore, a negative correlation between the severity of disease and the frequency of circulating TSCM cells in chronic viral and parasitic infections was observed [34]. Accordingly, the protective role of TSCM cells makes them an attractive candidate in vaccination and adoptive T cell therapy [34,35,36,37,38,39]. Our results showed that the nonresponder participants had a lower frequency of CD4+CD95+, CD4+CD95Hi, and CD4+CD95low/med TSCM than responders to the HB vaccine. However, other subsets, including naïve, central memory, and effector memory CD4+ T cells, were not statistically different between the studied groups.
There are some possible mechanisms underlying impaired antibody response to HB vaccination. For instance, several studies revealed a negative correlation between the frequency of regulatory T (Treg) cells and the seroconversion rate after HB vaccination [40, 41]. In addition, decreased frequency and function of monocyte-derived dendritic cells (moDCs) and subsequently attenuated memory T cell induction had been considered a probable reason for being nonresponder to HB vaccine [42]. However, the long-term memory T cell response has not been fully elucidated in this regard [43]. To the best of our knowledge, no similar studies could be found regarding the frequency of various memory T cell subsets, particularly TSCM cells after vaccination, and their comparison between responders and nonresponders of healthy recipients. In the case of vaccination, few studies existed about the induction of TSCM cells following vaccination against yellow fever (YF) and the application of CpG-B based cancer vaccines [44, 45]. In a study by Scriba et al., it was suggested that vaccine-induced TSCM contributed to long-term memory formation and proliferative capacity of the vaccine-induced T cell response to mycobacteria [46]. Moreover, Schlom et al. made a vaccine directed against a transcription factor named Twist that has a role in the metastatic process. They showed an increase in CD4+ TSCM cells in vaccinated mice in comparison with PBS-treated mice. This revealed that the TSCM population could generate an antitumor activity [47, 48]. Based on the findings of these studies and our results which showed a lower frequency of TSCM in nonresponders, it can be concluded that the TSCM cells play a crucial role in long-lasting immunological memory response preservation [34]. Also, we assumed that the memory response in nonresponders to the HB vaccine probably had an immunological defect in the memory CD4+ T cell formation. Consequently, a lower frequency of TSCM cells might play a principal role in the absence of protection even after several HB vaccine injections in the nonresponder group.
In this study, we also compared the frequency of TCM and TEM between studied groups and observed no statistically significant differences. Previous studies had shown that the frequency of these subsets was correlated with the efficacy of several vaccines like ZOSTAVAX, Influenza, Malaria, and human papillomavirus type 16 (HPV-16) vaccines [49,50,51,52,53]. No study reported the comparison of the frequency of these subsets between responders and nonresponders in the normal population against the HB vaccine. However, some studies investigated these cells after HB vaccination in subjects with a different conditions. For example, Litjens et al. conducted a study on end-stage renal disease (ESRD) patients. They showed that the production of specific CD4+ TEM cells was impaired in the patients after administration of the HB vaccine in comparison with healthy controls [54]. Another investigation that was done in a normal population showed a positive association between the frequency and absolute numbers of HBsAg-specific IL-2 producing CD4+ TEM cells and HBsAb titer [55]. Moreover, Marchant et al. performed a study on the subjects who received the Engerix-B vaccine. They observed that the HBsAg-specific memory CD4+ T cells included both TCM and TEM cells [56]. Two separate studies about HIV-infected individuals who were vaccinated against HBV had shown that the seroconversion stimulated by the vaccine positively correlated with the development of T cell immunological memory [57, 58].
No significant differences observed in our study could be related to stimulation conditions as it was not completely specific because we used HBsAg for stimulation and did not check the specific memory T cells using tetramer staining. In addition, due to the restriction in inclusion criteria of our samples, particularly the nonresponder subjects, the sample size was relatively small. Also, we did not have precise information on the time point of the last vaccination of the subjects of our study, which might have effects on the frequency of memory T cell populations [23].
In this study, we also investigated the correlation between the responders’ age and memory T cell subsets. There was a negative correlation between the age of responders and the frequency of TN. Besides, a positive correlation was found between the age of responders and the frequency of CD45RO+CD95+ TCM and CD45ROlow/medCD95+ TCM. There were similar studies that examined the correlation between the age of healthy individuals and the frequency of different memory T cell subsets [59,60,61,62]. Based on our findings and other studies, it can be implied that the turnover and long lifespan of TN cells are decreasing during aging, probably due to a decline in thymic output and/or depletion of naïve repertoire by activation. Accordingly, the thymus becomes unable to substitute the lost TN cells in the periphery. In contrast to TN, cumulative exposure to foreign pathogens and environmental antigens induces the accumulation of memory T cells with aging, which is in line with our study [63].
Several studies focus on IFN-γ production as an indicator of cellular immunity, and some of them have identified a clear dominance of the T helper (Th)1 phenotype after HB vaccination. Moreover, a strong correlation has been shown between the HBsAg-specific IFN-γ+ T cell response and HBsAb level. In the last part of our study, we evaluated the frequency of memory T cells secreting IFN-γ in studied groups. In agreement with Makhlouf et al. study, memory CD4+ T cells secreting IFN-γ were detectable after in vitro activation by HBsAg in both responder and nonresponder individuals. Although, Makhlouf’s study showed that the percentage of CD4+CD45RO+IFN-γ+ memory T cells was significantly higher in the responder participants than in nonresponders, we did not find any statistical differences neither in the frequency nor in the mean expression of IFN-γ in the CD4+CD45RO+IFN-γ+ memory T cells.
The limitations mentioned earlier, including nonspecific detection of memory T cells, small sample size, and the uncertain time interval between sampling and vaccination, are likely to affect our results regarding memory T cells secreting IFN-γ. Most likely, using more specific methods for detection of memory T cells against HBsAg producing IFN-γ should be taken into consideration in future studies. Also, the interval of the last vaccine administration and the time of investigations must be kept in mind.
In conclusion, based on our observations, it is likely that nonresponders to the HB vaccine have a defect in their immunological memory CD4+ T cell formation due to their lower frequency of TSCM cells compared to the responders. It may play an important role in lower anti-HBsAb production after HB vaccination in nonresponders. For further research, using recombinant HB vaccine containing safe adjuvants, administration of a vaccine with different intervals and doses of injections in nonresponders may increase the frequency of memory T cell subsets, especially TSCM cells.
References
Clemente MG, Schwarz K. Hepatitis: general principles. Pediatr Rev. 2011;32:333–40. https://doi.org/10.1542/pir.32-8-333.
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. https://doi.org/10.1016/j.jhep.2006.05.013.
Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int J Mol Sci. 2019;20:1358. https://doi.org/10.3390/ijms20061358.
Schaefer S. Hepatitis B virus taxonomy and hepatitis B virus genotypes. World J Gastroenterol. 2007;13:14–21. https://doi.org/10.3748/wjg.v13.i1.14.
Lamontagne RJ, Bagga S, Bouchard MJ. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163–86. https://doi.org/10.20517/2394-5079.2016.05.
Locarnini S, Zoulim F. Molecular genetics of HBV infection. Antivir Ther. 2010;15:3–14. https://doi.org/10.3851/IMP1619.
Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479:672–86. https://doi.org/10.1016/j.virol.2015.02.031.
Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of hepatitis B virus infection and impact of vaccination on disease. Clin Liver Dis. 2016;20:607–28. https://doi.org/10.1016/j.cld.2016.06.006.
Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6:589–99. https://doi.org/10.12998/wjcc.v6.i13.589.
Locarnini S, Hatzakis A, Chen D-S, Lok A. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J Hepatol. 2015;62:S76–86. https://doi.org/10.1016/j.jhep.2015.01.018.
Kao J-H, Chen D-S. Global control of hepatitis B virus infection. Lancet infect Dis. 2002;2:395–403. https://doi.org/10.1016/s1473-3099(02)00315-8.
Chen D-S. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50:805–16. https://doi.org/10.1016/j.jhep.2009.01.002.
Saco TV, Strauss AT, Ledford DK. Hepatitis B vaccine non-responders: possible mechanisms and solutions. Ann Allergy Asthma Immunol. 2018;121:320–7. https://doi.org/10.1016/j.anai.2018.03.017.
Zhao H, Zhou X, Zhou Y-H. Hepatitis B vaccine development and implementation. Hum Vaccin Immunother. 2020;16:1533–44. https://doi.org/10.1080/21645515.2020.1732166.
Michel M-L, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol. 2010;58:288–95. https://doi.org/10.1016/j.patbio.2010.01.006.
Das S, Ramakrishnan K, Behera SK, Ganesapandian M, Xavier AS, Selvarajan S. Hepatitis B vaccine and immunoglobulin: key concepts. J Clin Transl Hepatol. 2019;7:165–71. https://doi.org/10.14218/JCTH.2018.00037.
Gelinas L, Abu-Raya B, Ruck C, Cai B, Kollmann TR. Hepatitis B virus vaccine induced cell-mediated immunity correlates with humoral immune response following primary vaccination during infancy. Immuno Horizons. 2017;1:42–52. https://doi.org/10.4049/immunohorizons.1700015.
Balamurali V, Sujith R, Leela K, Jayaprakash T. Overcoming strategies for non-responders in HBV vaccination. J Adv Microbiol. 2020;20:54–62. https://doi.org/10.9734/jamb/2020/v20i830276.
Davis JP. Experience with hepatitis A and B vaccines. Am J Med. 2005;118:7–15. https://doi.org/10.1016/j.amjmed.2005.07.011.
Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24:572–7. https://doi.org/10.1016/j.vaccine.2005.08.058.
Wang RX, Boland GJ, van Hattum J, de Gast GC. Long term persistence of T cell memory to HBsAg after hepatitis B vaccination. World J Gastroenterol. 2004;10:260–3. https://doi.org/10.3748/wjg.v10.i2.260.
Simons BC, Spradling PR, Bruden DJ, et al. A longitudinal hepatitis B vaccine cohort demonstrates long-lasting hepatitis B virus (HBV) cellular immunity despite loss of antibody against HBV surface antigen. J Infec Dis. 2016;214:273–80. https://doi.org/10.1093/infdis/jiw142.
Macallan DC, Borghans JA, Asquith B. Human T cell memory: a dynamic view. Vaccines. 2017;5:5. https://doi.org/10.3390/vaccines5010005.
Castellino F, Galli G, Del Giudice G, Rappuoli R. Generating memory with vaccination. Eur J Immunol. 2009;39:2100–5. https://doi.org/10.1002/eji.200939550.
Chattopadhyay PK, Gierahn TM, Roederer M, Love JC. Single-cell technologies for monitoring immune systems. Nat Immunol. 2014;15:128–35. https://doi.org/10.1038/ni.2796.
Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43:2797–809. https://doi.org/10.1002/eji.201343751.
Hojyo S, Tumes D, Murata A, Tokoyoda K. Multiple developmental pathways lead to the generation of CD4 T-cell memory. Int Immunol. 2020;32:589–95. https://doi.org/10.1093/intimm/dxaa051.
Gasper DJ, Tejea MM, Suresh M. CD4 T-cell memory generation and maintenance. Crit Rev Immunol. 2014;34:121–46. https://doi.org/10.1615/critrevimmunol.2014010373.
Khanal S, Schank M, El Gazzar M, Moorman JP, Yao ZQ. HIV-1 Latency and viral reservoirs: existing reversal approaches and potential technologies, targets, and pathways involved in HIV latency studies. Cells. 2021;10:475. https://doi.org/10.3390/cells10020475.
Vahidi Y, Faghih Z, Talei A-R, Doroudchi M, Ghaderi A. Memory CD4+ T cell subsets in tumor draining lymph nodes of breast cancer patients: a focus on T stem cell memory cells. Cell Oncol. 2018;41:1–11. https://doi.org/10.1007/s13402-017-0352-6.
Axelsson-Robertson R, Ju JH, Kim H-Y, Zumla A, Maeurer M. Mycobacterium tuberculosis-specific and MHC class I-restricted CD8+ T-cells exhibit a stem cell precursor-like phenotype in patients with active pulmonary tuberculosis. Int J Infect Dis. 2015;32:13–22. https://doi.org/10.1016/j.ijid.2014.12.017.
Vigano S, Negron J, Ouyang Z, et al. Prolonged antiretroviral therapy preserves HIV-1-specific CD8 T cells with stem cell-like properties. J Virol. 2015;89:7829–40. https://doi.org/10.1128/JVI.00789-15.
Lugli E, Gattinoni L, Roberto A, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8:33–42. https://doi.org/10.1038/nprot.2012.143.
Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med. 2017;23:18–27. https://doi.org/10.1038/nm.4241.
Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. https://doi.org/10.1111/j.1600-065X.2010.00979.x.
Flynn JK, Gorry PR. Stem memory T cells (TSCM) their role in cancer and HIV immunotherapies. Clin Transl Immunology. 2014;3: e20. https://doi.org/10.1038/cti.2014.16.
Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell–like properties. Nat Med. 2011;17:1290–7. https://doi.org/10.1038/nm.2446.
Schmueck-Henneresse M, Sharaf R, Vogt K, et al. Peripheral blood–derived virus-specific memory stem T cells mature to functional effector memory subsets with self-renewal potency. J Immunol. 2015;194:5559–67. https://doi.org/10.4049/jimmunol.1402090.
Lugli E, Dominguez MH, Gattinoni L, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–9. https://doi.org/10.1172/JCI66327.
Li J, Tan D, Liu H, Li K. CD4 (+) CD25 (+) FoxP3 (+) T regulatory cells in subjects responsive or unresponsive to hepatitis B vaccination. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:1046–51. https://doi.org/10.3969/j.issn.1672-7347.2011.11.003.
del PozoBaladoMdel M, Leal M, Méndez Lagares G, et al. Increased regulatory T cell counts in HIV-infected nonresponders to hepatitis B virus vaccine. J Infect Dis. 2010;202:362–9. https://doi.org/10.1086/653707.
Verkade MA, Van Druningen C, Op de Hoek C, Weimar W, Betjes MG. Decreased antigen-specific T-cell proliferation by moDC among hepatitis B vaccine non-responders on haemodialysis. Clin Exp Med. 2007;7:65–71. https://doi.org/10.1007/s10238-007-0127-x.
Cassaniti I, Calarota SA, Adzasehoun KM, et al. Memory T cells specific for HBV enumerated by a peptide-based cultured enzyme-linked immunospot assay in healthy HBV-vaccinated subjects. Hum Vaccin Immunother. 2016;12:2927–33. https://doi.org/10.1080/21645515.2016.1204500.
Marraco SAF, Soneson C, Cagnon L, et al. Long-lasting stem cell–like memory CD8+ T cells with a naïve-like profile upon yellow fever vaccination. Sci Transl Med. 2015;7:28ra248. https://doi.org/10.1126/scitranslmed.aaa3700.
Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. https://doi.org/10.1586/erv.10.174.
Mpande CA, Dintwe OB, Musvosvi M, et al. Functional, antigen-specific stem cell memory (TSCM) CD4+ T cells are induced by human mycobacterium tuberculosis infection. Front Immunol. 2018;9:324. https://doi.org/10.3389/fimmu.2018.00324.
Ardiani A, Gameiro SR, Palena C, et al. Vaccine-mediated immunotherapy directed against a transcription factor driving the metastatic process. Cancer Res. 2014;74:1945–57. https://doi.org/10.1158/0008-5472.CAN-13-2045.
Khan MA, Chen HC, Zhang D, Fu J. Twist: a molecular target in cancer therapeutics. Tumor Biol. 2013;34:2497–506. https://doi.org/10.1007/s13277-013-1002-x.
Nienen M, Stervbo U, Mölder F, et al. The role of pre-existing cross-reactive central memory CD4 T-cells in vaccination with previously unseen influenza strains. Front Immunol. 2019;10:593. https://doi.org/10.3389/fimmu.2019.00593.
Trieu MC, Zhou F, Lartey S, et al. Long-term maintenance of the influenza-specific cross-reactive memory CD4+ T-cell responses following repeated annual influenza vaccination. J Infect Dis. 2017;215:740–9. https://doi.org/10.1093/infdis/jiw619.
Sei JJ, Cox KS, Dubey SA, et al. Effector and central memory poly-functional CD4+ and CD8+ T cells are boosted upon ZOSTAVAX® vaccination. Front Immunol. 2015;6:553. https://doi.org/10.3389/fimmu.2015.00553.
Moncunill G, De Rosa SC, Ayestaran A, et al. RTS, S/AS01E malaria vaccine induces memory and polyfunctional T cell responses in a pediatric African phase III trial. Front Immunol. 2017;8:1008. https://doi.org/10.3389/fimmu.2017.01008.
Diniz MO, Sales NS, Silva JR, Ferreira LCS. Protection against HPV-16–associated tumors requires the activation of CD8+ effector memory T cells and the control of myeloid-derived suppressor cells. Mol Cancer Ther. 2016;15:1920–30. https://doi.org/10.1158/1535-7163.MCT-15-0742.
Litjens NHR, Huisman M, van den Dorpel M, Betjes MGH. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J Am Soc Nephrol. 2008;19:1483–90. https://doi.org/10.1681/ASN.2007090971.
Litjens NH, Huisman M, Hijdra D, Lambrecht BM, Stittelaar KJ, Betjes MG. IL-2 producing memory CD4+ T lymphocytes are closely associated with the generation of IgG-secreting plasma cells. J Immunol. 2008;181:3665–73. https://doi.org/10.4049/jimmunol.181.5.3665.
Stubbe M, Vanderheyde N, Goldman M, Marchant A. Antigen-specific central memory CD4+ T lymphocytes produce multiple cytokines and proliferate in vivo in humans. J Immunol. 2006;177:8185–90. https://doi.org/10.4049/jimmunol.177.11.8185.
Giacomet V, Masetti M, Nannini P, et al. Humoral and cell-mediated immune responses after a booster dose of HBV vaccine in HIV-infected children, adolescents and young adults. PLoS ONE. 2018;13:e0192638. https://doi.org/10.1371/journal.pone.0192638.
Pessoa SD, Miyamoto M, Ono E, Gouvêa AF, de Moraes-Pinto MI, Succi RC. Persistence of vaccine immunity against hepatitis B virus and response to revaccination in vertically HIV-infected adolescents on HAART. Vaccine. 2010;28:1606–12. https://doi.org/10.1016/j.vaccine.2009.11.045.
Li M, Yao D, Zeng X, et al. Age related human T cell subset evolution and senescence. Immun Ageing. 2019;16:1–7. https://doi.org/10.1186/s12979-019-0165-8.
Hong MS, Dan JM, Choi JY, Kang I. Age-associated changes in the frequency of naive, memory and effector CD8+ T cells. Mech Ageing Dev. 2004;125:615–8. https://doi.org/10.1016/j.mad.2004.07.001.
Kang I, Hong MS, Nolasco H, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–81. https://doi.org/10.4049/jimmunol.173.1.673.
Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P. Age related changes in T cell mediated immune response and effector memory to respiratory syncytial virus (RSV) in healthy subjects. Immun Ageing. 2010;7:1–8. https://doi.org/10.1186/1742-4933-7-14.
Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4+ versus effector memory and terminally differentiated memory cells in CD8+ compartment. Mech Ageing Dev. 2006;127:274–81. https://doi.org/10.1016/j.mad.2005.11.001.
Zhu CL, Liu P, Chen T, et al. Presence of immune memory and immunity to hepatitis B virus in adults after neonatal hepatitis B vaccination. Vaccine. 2011;29:7835–41. https://doi.org/10.1016/j.vaccine.2011.07.098.
Krawczyk A, Ludwig C, Jochum C, et al. Induction of a robust T-and B-cell immune response in non-and low-responders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine. 2014;32:5077–82. https://doi.org/10.1016/j.vaccine.2014.06.076.
Van Damme P, Dionne M, Leroux-Roels G, et al. Persistence of HBsAg-specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J Viral Hepat. 2019;26:1066–75. https://doi.org/10.1111/jvh.13125.
Lu CY, Ni YH, Chiang BL, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis. 2008;197:1419–26. https://doi.org/10.1086/587695.
Werner JM, Abdalla A, Gara N, Ghany MG, Rehermann B. The hepatitis B vaccine protects re-exposed health care workers, but does not provide sterilizing immunity. Gastroenterology. 2013;145:1026–34. https://doi.org/10.1053/j.gastro.2013.07.044.
Makhlouf NA, Farghaly AM, Zaky S, et al. The efficacy of hepatitis B vaccination program in Upper Egypt: flow cytometry and the evaluation of long term immunogenicity. J Med Virol. 2016;88:1567–75. https://doi.org/10.1002/jmv.24506.
Acknowledgements
This study was supported by Shiraz University of Medical Sciences (grant no. 15186). In addition, we will be ever grateful to Prof. Dr. Reinhold E. Schmidt for guiding us in the selection of the samples, and we are sorry that he has not lived to see our graduate.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by Shiraz University of Medical Sciences (SUMS), Shiraz, Iran, grant number 15186.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The protocol of this study was approved by the Ethics Committee of the university (ethics code: IR.SUMS.REC.1397.779).
Consent to participate
All enrolled subjects provided written informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vakili, M.E., Faghih, Z., Sarvari, J. et al. Lower frequency of T stem cell memory (TSCM) cells in hepatitis B vaccine nonresponders. Immunol Res 70, 469–480 (2022). https://doi.org/10.1007/s12026-022-09278-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09278-9