Abstract
Patients with end-stage kidney disease, whether or not on renal replacement therapy, have an impaired immune system. This is clinically manifested by a large percentage of patients unresponsive to the standard vaccination procedure for hepatitis B virus (HBV). In this study, the immune response to HBV vaccination is related to the in vitro function of monocyte-derived dendritic cells (moDC). We demonstrate that mature moDC from nonresponders to HBV vaccination have a less mature phenotype, compared to responders and healthy volunteers, although this did not affect their allostimulatory capacity. However, proliferation of autologous T cells in the presence of tetanus toxoid and candida antigen was decreased in non-responders. Also, HLA-matched CD4+ hsp65-specific human T-cell clones showed markedly decreased proliferation in the group of non-responders. Our results indicate that impairment of moDC to stimulate antigen-specific T cells provides an explanation for the clinical immunodeficiency of patients with end-stage kidney disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with chronic kidney disease (CKD) have an impaired immune system, which is most evident in patients with CKD class IV and V (glomerular filtration rate <30 ml/min) [1]. Clinically, this manifests itself by an increased susceptibility to infections [2], decreased delayed-type hypersensitivity responses [3] and an increased risk of virus-related cancer [4]. In addition, the immune response to antigens such as tetanus toxoid [5], diphtheria [6] and hepatitis B surface antigen (HBsAg) [7] is decreased in patients with CKD.
The immune response to hepatitis B virus (HBV) vaccination in patients with CKD shows an inverse relation with renal function [8]. Despite an intensified vaccination, up to 40% of the patients undergoing chronic intermittent haemodialysis (CIHD) do not achieve protective levels of antibody and are considered to be vaccine non-responders [9]. In addition, the peak antibody titres are lower [9], and the antibody titres tend to decrease more rapidly in most of the patients on CIHD, compared to healthy controls [10].
The mechanism underlying the phenomenon of CKD-associated hepatitis B immune non-responsiveness is largely unknown, but the role for dendritic cells (DC) may be pivotal. DC have a central role in the induction and regulation of immunity against various antigens [11]. The function of DC is regulated by a process of differentiation and their life cycle includes several stages characterised by distinct functions [12]. Immature DC take up antigen and process it into peptides that are presented on the cell surface as HLA class II peptide complexes. Immature DC undergo a process of terminal differentiation which can be initiated by various stimuli e.g., pro-inflammatory cytokines, products of microbial pathogens or ligation of CD40 [12]. Mature DC activate naive and antigen-specific T helper cells [13] that are able to support the differentiation of B cells into antibody-secreting plasma cells and memory B cells. Also, there is evidence that B cells may differentiate into plasma cells without the interference of T helper cells but in the presence of DC only [14, 15].
A model of monocyte-derived dendritic cells (moDC) can mimic the terminal differentiation process from immature into mature DC, and is used to study DC in vitro [16]. Recently, we have shown that moDC derived from patients with CKD class IV–V, as well as patients on CIHD, have a defective terminal differentiation [17]. In the present study we have related the phenotype and various functions of moDC from patients on CIHD to their antibody response after hepatitis B vaccination. Our results reveal that dysfunction of DC may contribute to the diminished immune response to T-cell-dependent antigens among patients with CKD receiving renal replacement therapy.
Patients, materials, and methods
Subjects
All patients had undergone a hepatitis B vaccination procedure, which consists of 4 intra-muscular injections with 40 µg of hepatitis B vaccine (HB-VAX, APMSD) at 0, 1, 6 and 12 months. If the antibody response was below 10 IU/ml, the patients received two additional boosters. Patients were considered to be non-responders if the antibody response was below 10 IU/ml after six vaccinations. Responders are defined as patients with an antibody response above 10 IU/ml after 4 vaccinations (median response 200 IU/ml). All healthy volunteers were vaccinated by standard regime of 3 vaccinations whereafter their antibody titre was at least 1000 IU/ml. No patient was suffering from a malignancy or autoimmune disease, or was taking immunosuppressive medication. We included ten non-responders, ten responders and eight healthy volunteers. Patients were recruited from a single centre haemodialysis unit at our university hospital. The non-responders were recruited prospectively within a period of two years, with subsequent selection of age- and sex-matched responders. The clinical characteristics of the patients included in the study are shown in Table 1. Besides CKD, diabetes mellitus and age also influence the immune response to HBV vaccination [8]. As shown in Table 1, responders and non-responders did not differ in age and the number of patients with diabetes mellitus was similar in both groups of patients. Patients were dialysed three times weekly for 4 h and water for dialysis was prepared by the use of reverse osmosis according to European guidelines. The bacteriological quality of the dialysate was measured at regular intervals according to European guidelines (endotoxin levels <0.25 IU/ml and <100 colony forming units/ml). All patients were dialysed with haemophane (MA-12H) (Kawasumi Laboratory Inc., Tokyo, Japan) dialyser membranes, and received recombinant erythropoeitin in a dosage to maintain haemoglobin levels ≥7.0 mmol/l, and were all treated with 1α,25-dihydroxy vitamin D3. All patients included gave informed consent and the local medical ethical committee approved the study.
Isolation and culture of cells
Peripheral blood mononuclear cells (PBMC) were isolated from 50 ml venous blood by Ficoll-Paque plus (Amersham Biosciences AB, Uppsala, Sweden) density gradient centrifugation. Monocytes were isolated by positive selection using an automatic magnetic cell sorter (Miltenyi Biotec, Bergisch Gladbach, Germany) in combination with CD14 coupled magnetic beads (10 µl beads for maximal 60×106 PBMC) (Miltenyi Biotec). CD14 positive fraction (purity always >95%) was plated in 6-well plates (Costar, Cambridge, MA) in a density of 1.5×106 cells per well and cultured for 11 days at 37°C in humidified 5% CO2. As culture medium we used RPMI 1640 (Cambrex, Verviers, Belgium) supplemented with 100 IU/ml penicillin (Cambrex), 100 µg/ml streptomycin (Cambrex), L-glutamin (Cambrex) at 2 mM final concentration and 10% heat-inactivated AB+ pooled human serum. For the cultivation procedure, 50 ng/ml recombinant human GM-CSF (Leucomax, Sandoz, Germany) and 40 ng/ml recombinant human IL-4 (Tebu-bio, Heerhugowaard, The Netherlands) were used. Fresh medium and cytokines were added at days 3, 6 and 8. During the last 3 days of culture a cytokine cocktail containing IL-1β (10 ng/ml), IL-6 (10 ng/ml), TNFα (20 ng/ml) (Tebu-bio) and PGE2 (1 µg/ml) (Sigma-Aldrich, Zwijndrecht, The Netherlands) was added as a maturation stimulus. Viability of all cells was determined by Trypan blue exclusion.
Immunofluorescence staining and flow cytometric analysis
Antibodies used for cell surface staining included CD86, anti-HLA-ABC (Serotec, Oxford, UK), CD83 (Pharmingen, San Diego, USA), anti-HLA-DR (Becton Dickinson, San Jose, USA) and anti-CCR7 (R&D, Minneapolis, USA). All antibodies were mouse monoclonal antibodies conjugated to either fluorescein isothiocyanate (FITC) or phycoerythrin (PE). Appropriate isotype controls were used at the same protein concentration as the test antibody. Data acquisition and analysis was performed on a FACSCalibur (Becton-Dickinson) using CellQuest Pro software (Becton-Dickinson). Results are expressed as mean fluorescence intensity (MFI).
FITC-labelled albumin internalisation
FITC-labelled bovine serum albumin (50 µg/ml) (Sigma-Aldrich) was added to 1×105 moDC at 37°C. Uptake of the same concentration of FITC-labelled albumin at 0°C served as negative control. Both groups were incubated for 60 min in the dark. After incubation, cells were washed twice in ice-cold Facsflow (Becton Dickinson) and analysed by FACSCalibur (Becton Dickinson). Data represent the differences in geometric mean of moDC after FITC-albumin uptake at 37°C minus 0°C.
Allogeneic mixed leukocyte reaction
The stimulatory capacity of moDC was assessed in an allogeneic mixed leukocyte reaction (alloMLR). For all assays, CD14 negative fractions from the same healthy donor were used as responder cells. After isolating monocytes by positive selection using autoMACS, the negative fraction was resuspended in RPMI 1640 containing 10% heat-inactivated human serum and 10% dimethyl sulphoxide (Sigma-Aldrich). Aliquots were stored at −80°C for 14 days. Responder cells were thawed immediately before use, washed and resuspended in RPMI 1640 containing 10% human serum and plated 5×104 cells per well. Decreasing numbers of moDC were mixed with the responder cells. As control for background proliferation, responder cells were cultured in the absence of moDC. As a positive control for T-cell proliferation, responder cells were stimulated with phytohaemagglutinin (PHA; 2 µg/ml of purified PHA-HA16; Murex Biotech Ltd. Kent, England) or phorbol myristate acetate (PMA; 100 ng/ml; Calbiochem, Darmstadt, Germany) and ionomycine (10 µg/ml; Calbiochem). Cells in triplicate wells of a 96-well round bottom plate (Nunc, Roskilde, Denmark) were cultured for 6 days and proliferation was measured by incorporation of 3H-thymidine (0.5 µCi/well, Amerham Pharmacia, Biotech, Little Chalfont, UK) for the last 8 h of culture. The 3H-thymidine incorporation into DNA was assessed by a liquid scintillation spectrophotometer (Betaplate 1205; LKB Wallac) and expressed in counts per minute (cpm).
Recall antigen assay
Tetanus toxoid (SVM, Bilthoven, the Netherlands) or Candida albicans antigen (ARTU, Biological N.V., Lelystad, the Netherlands) was used to test for recall antigen-specific T-cell responses. Antigen was added in a final concentration of 35 limit of flocculation units (Lf)/ml for tetanus toxoid, and 5 µg/ml for Candida albicans. T cells were prepared using the autologous CD14 negative fraction and were plated at 1×105 cells per well with 2×103 moDC. As control for background proliferation, responder cells were cultured in the absence of moDC and in the presence of antigen. Cells in triplicate wells of a 96-well round bottom plate (Nunc) were cultured for 5 days and subsequently pulsed with 1 µCi 3H-thymidine for the last 8 h of culture. 3H-thymidine incorporation was measured using a liquid scintillation counter. Data were expressed in cpm.
CD4+ hsp65-specific T-cell assay
The Mycobacterium tuberculosis and M. leprae hsp65-specific, HLA-DR3 and HLA-DR1 restricted CD4+ T-cell clones used in this study recognise an hsp65 determinant, corresponding to peptide residues 3–13 (p3–13) or 411–425 (p411–425), respectively [18, 19] (both kindly provided by Dr. T.H. Ottenhof and Dr. K.E. van Meijgaarden, Leiden, the Netherlands). HLA-DR-matched immature moDC were mixed with 10 µg/ml of appropriate peptides or with 10 µg/ml protein (hsp65). Hsp65-specific T cells (104) were cultured with different numbers of immature moDC in 96-well round bottom plates (Costar) in triplicates for 3 days. Likewise, monocytes were tested for their capability to stimulate hsp65-specific T cells. 3H-thymidine incorporation was measured on day 4 after a 16-h pulse. For background proliferation, moDC were cultured in the presence of T cells but in the absence of antigen. As a positive control for T-cell proliferation, responder cells were stimulated with PHA (2 µg/ml of purified PHA-HA16; Murex Biotech Ltd.).
Statistical analysis
For moDC, the MFI (phenotype and FITC-labelled albumin uptake) was logarithmic transformed, and differences between groups were compared by Student’s two-tailed unpaired t-test, assuming equal variances. Data obtained from T-cell proliferation assays were analysed by two-tailed unpaired Mann-Whitney U-test. These data are expressed as mean±standard error of the mean, and for FITC-labelled albumin uptake assay data were expressed as geometric mean±standard deviation. For all assays, P-value <0.05 was considered significant. Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Changes in cell surface markers in response to cytokines
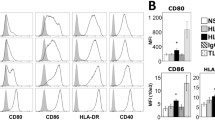
Monocytes from controls, responders and non-responders were differentiated to similar immature moDC with respect to immunophenotype and light microscopic appearances (data not shown). We then provided a cytokine cocktail that drives all immature moDC into CD83-positive mature moDC and analysed the phenotype of mature moDC from the 3 groups. The expression of DC-related cell surface molecules was significantly lower on mature moDC from both responders and non-responders compared to healthy controls (Fig. 1). This is in line with our previous results [16]. However, there was a graded decrease in cell surface expression of MHC molecules class I and II, and CD83 from healthy controls to non-responders. The cell surface expression of MHC class II and CD83 was significantly lower in non-responders compared to responders (P<0.05).
Low expression of CD83, CD86, HLA class I and II, and chemokine receptor CCR7 on mature moDC from non-responders and responders to hepatitis B vaccination. Responders (n=10) and non-responders (n=10) compared to healthy controls (n=8). Expression of cell surface antigens is represented as MFI on the Yaxes. P<0.05 is considered significant and depicted as ↔
FITC-labelled albumin uptake
The uptake of albumin occurs via macropinocytosis [20]. If immature moDC differentiate into mature moDC, they lose their ability to take up antigen. Therefore determination of albumin uptake is used to monitor terminal differentiation [20, 21]. Immature moDC from all three groups were equally capable of taking up albumin. Mature moDC from controls showed the lowest uptake of FITC-labelled albumin compared to moDC from patients. Internalisation of FITC-albumin was similar between the two groups of patients (Fig. 2).
No difference between moDC from responder and non-responders in uptake of FITC-labelled albumin. Uptake of FITC-labeled albumin by immature and cytokine-induced mature moDC derived from controls (n=8), non-responders (n=9) and responders (n=9). Endocytosis was measured by flowcytometry, and is denoted as geometric mean of the fluorescents intensity±SD. * P<0.05 is considered significant by unpaired Student’s t-test
Stimulatory properties of MoDC in T-cell proliferation tests
The mature moDC were highly stimulatory for allogeneic T cells, even at moDC to T cell ratios of 1:300. However, no differences were observed in the allostimulatory capacity of moDC between non-uraemic controls, responders and non-responders (data not shown).
The stimulatory capacity of moDC for antigen-specific T cells was studied using the recall antigens tetanus toxoid and candida albicans antigen. Both responders and nonresponders showed less antigen-specific T-cell proliferation compared to healthy controls. The proliferation of autologous T cells in the presence of tetanus toxoid and candida antigen was most impaired by moDC from nonresponders (Fig. 3). PHA- and PMA-induced proliferation (the positive control for T-cell proliferation) was similar between the different groups (data not shown).
Immature moDC from non-responders have an impaired ability to induce autologous T-cell proliferation in the presence of recall antigens. Proliferative response of autologous T cells to moDC and the recall antigens tetanus toxoid and Candida albicans. Data are analysed by Mann-Whitney U-test and are expressed in counts per minute (cpm)±SEM. P<0.05 is considered significant
CD4+ HSP65-specific T-cell assay
A difference in T-cell composition/function between both groups of patients may exist, thereby influencing the results of the recall antigen assay. Therefore, moDC from patients were used as antigen-presenting cells for HLA-DR-matched CD4+ antigen-specific human T-cell clones. In agreement with the recall assays, the proliferation of CD4+ antigen-specific T cells was markedly decreased in patients who did not respond to hepatitis B vaccination (Fig. 4, P=0.05). There was no difference in the proliferation of CD4+ antigen-specific T cells in response to the peptide or whole protein (Fig. 4). Compared to monocytes, immature moDC had a 100-fold higher stimulatory capacity for CD4+ antigen-specific T cells (data not shown).
Immature moDC from non-responders have an impaired ability to induce T-cell proliferation in the presence of recall antigens. To exclude autologous T-cell anomalies as a cause for diminished T-cell responses upon recall antigens, autologous T cells were replaced by an HLA-matched hsp65-specific T-cell clone. Patients that were included have been immunophenotyped DR1 or DR3 (non-responders n=4; responders n=3). P=0.05 and is depicted as *
Discussion
Patients with renal failure are generally poor responders to vaccination with various antigens including HbSag. Even in patients responding to vaccination, the antibody titre is lowered compared to age-matched healthy individuals. Dysfunctional DC may underlie this impaired immune response [17]. In this study we tested the hypothesis that functional impairment of moDC is also related to the responsiveness to hepatitis B vaccination within the group of patients with renal failure. Our results indicate that moDC, from patients on CIHD who showed the most inadequate immune response (non-responders to hepatitis B vaccination), are phenotypically also the most immature and have a poor stimulatory capacity for memory T cells.
Recently, we showed that moDC from patients with CKD stage IV and V, with or without haemodialysis, have a similar differentiation to immature moDC as healthy controls [17]. However, upon stimulation with diverse maturation stimuli, like a cocktail of pro-inflammatory cytokines and CD40 ligand, moDC from patients showed an impaired terminal differentiation and functionality.
In this study these results were confirmed, as mature moDC from CIHD patients, compared to healthy controls, showed less terminal differentiation as judged by the expression of cell surface molecules studied. However, the moDC from non-responders showed the most profound decrease in the expression of these cell surface molecules, noticeably the HLA molecules and CD83. This was not reflected by a lowered stimulatory capacity for allogeneic T cells, but evidently present when the moDC from nonresponders were tested as antigen-presenting cells for memory T cells. This held true, not only for autologous tetanus toxoid and candida albicans antigen-specific T cells, but also for an HLA-matched hsp65 antigen-specific T-cell line.
Besides the expression of MHC and co-stimulatory molecules, the T-cell stimulatory capacity of moDC is influenced by their cytokine production and their ability to take up and process antigen effectively. We already documented that mature moDC from patients on CIHD do not differ from healthy controls with respect to their Th1 and Th2 polarisation capacity, or cytokine production like IL-10, IL-12p70 and IL-15 [17]. The results obtained in this study do not indicate a difference in antigen uptake and processing. For instance, the uptake of FITC-labelled albumin was similar between responders and non-responders. In addition, a difference in antigen processing seems unlikely because the moDC loaded with total protein induced a similar proliferative response of the hsp-65-specific T cells as compared to moDC loaded with peptide only. Therefore, the decreased stimulatory capacity of moDC from patients on CIHD, and more specifically nonresponders, seems to be related to the decreased expression of molecules necessary for T-cell stimulation.
There is a paucity of data on the function of antigen presentation in patients with CKD, but our results are remarkably consistent with the conclusions of previous studies. Meuer et al. found a correlation between a decreased T-cell proliferation response in vitro and a low or no antibody response in vivo [22]. This response could be restored to normal using healthy donor-derived monocytes as antigen-presenting cells. Similar results were found for tetanus toxoid-specific T-cell responses in patients on CIHD [23]. It should be emphasised that these studies used monocyte populations contaminated with DC and that the precise contribution of either cell type is therefore not defined. Even minor contamination of DC may influence results substantially because the DC are many times more potent as antigen-presenting cell than monocytes. In our hands, highly purified monocytes have little or no capacity for antigen presentation to allogeneic T cells and autologous memory T cells (this study and [24]). Therefore, the results obtained in previous studies may in fact be related to a difference in function of DC. In addition, we observed a strong negative correlation between the number of peripheral blood DC and the stage of CKD [25]. Therefore, loss of renal function not only negatively affects the in vitro maturation of monocytes to DC, as is shown in this study, but also the presence of circulating DC in vivo.
At present it is not clear which factors underlie the impaired terminal differentiation of moDC. One must assume that the difference is already present at the level of monocytes as these are the starting population of cells, which are cultured under identical circumstances for both patients and healthy controls. A number of studies have pointed out an activated status of monocytes derived from patients on CIHD compared to healthy controls. Furthermore, a high production of TNF-α and IL-6 by LPS-stimulated monocytes was shown, which probably represents significant pre-activation of these cells, correlated with the unresponsiveness to HBV vaccination among dialysis patients [26]. Therefore, one may hypothesise that pre-activation of monocytes impairs the full maturation into DC, but data to support this concept are as yet not available.
In conclusion, DC of patients on CIHD show an impaired terminal differentiation and relative dysfunction as antigen-presenting cells for memory T cells. This is most evident in patients that are not able to mount a sufficient antibody response after HBV vaccination. Dysfunction of DC may therefore lie at the heart of the disturbed immune system of patients with CKD.
References
Litjens NH, van Druningen CJ, Betjes MG (2006) Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol 118:83–1
Allon M, Depner TA, Radeva M et al (2003) Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol 14:1863–870
Selroos O, Pasternack A, Virolainen M (1973) Skin test sensitivity and antigen-induced lymphocyte transformation in uraemia. Clin Exp Immunol 14:365–70
Maisonneuve P, Agodoa L, Gellert R et al (1999) Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 354:93–9
Kruger S, Seyfarth M, Sack K et al (1999) Defective immune response to tetanus toxoid in hemodialysis patients and its association with diphtheria vaccination. Vaccine 17:1145–150
Kreft B, Klouche M, Kreft R et al (1997) Low efficiency of active immunization against diphtheria in chronic hemodialysis patients. Kidney Int 52:212–16
Crosnier J, Jungers P, Courouce AM et al (1981) Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in French haemodialysis units: I, Medical staff. Lancet 1:455–59
DaRoza G, Loewen A, Djurdjev O et al (2003) Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 42:1184–192
Stevens CE, Alter HJ, Taylor PE et al (1984) Hepatitis B vaccine in patients receiving hemodialysis. Immunogenicity and efficacy. N Engl J Med 311:496–01
de Graeff PA, Dankert J, de Zeeuw D et al (1985) Immune response to two different hepatitis B vaccines in haemodialysis patients: a 2-year follow-up. Nephron 40:155–60
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–52
Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machine. Cell 106:255–58
Grouard G, Durand I, Filgueira L et al (1996) Dendritic cells capable of stimulating T cells in germinal centres. Nature 384:364–67
Dubois B, Vanbervliet B, Fayette J et al (1997) Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med 185:941–51
Fayette J, Durand I, Bridon JM et al (1998) Dendritic cells enhance the differentiation of naive B cells into plasma cells in vitro. Scand J Immunol 48:563–70
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:1109–118
Verkade MA, van Druningen CJ, Vaessen LM et al (2007) Functional impairment of monocyte-derived dendritic cells in patients with severe chronic kidney disease. Nephrol Dial Transplant 22:128–38
Van Schooten WC, Elferink DG, Van Embden J et al (1989) DR3-restricted T cells from different HLA-DR3-positive individuals recognize the same peptide (amino acids 2-12) of the mycobacterial 65-kDa heat-shock protein. Eur J Immunol 19:2075–079
Anderson DC, van Schooten WC, Barry ME et al (1988) A mycobacterium leprae-specific human T cell epitope cross-reactive with an HLA-DR2 peptide. Science 242:259–61
Sallusto F, Cella M, Danieli C et al (1995) Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182:389–00
Kitajima T, Caceres-Dittmar G, Tapia FJ et al (1996) T cellmediated terminal maturation of dendritic cells: loss of adhesive and phagocytotic capacities. J Immunol 175:2340–347
Meuer SC, Hauer M, Kurz P et al (1987) Selective blockade of the antigen-receptor-mediated pathway of T cell activation in patients with impaired primary immune responses. J Clin Invest 80:743–49
Gibbons RA, Martinez OM, Garovoy MR (1990) Altered monocyte function in uremia. Clin Immunol Immunopathol 56:66–0
Verkade MA, Van De Wetering J, Klepper M et al (2004) Peripheral blood dendritic cells and GM-CSF as an adjuvantfor hepatitis B vaccination in hemodialysis patients. Kidney Int 66:614–21
Hesselink DA, Betjes MG, Verkade MA et al (2005) The effects of chronic kidney disease and renal replacement therapy on circulating dendritic cells. Nephrol Dial Transplant 20:1868–873
Girndt M, Kaul H, Leitnaker CK et al (2001) Selective sequestration of cytokine-producing monocytes during hemodialysis treatment. Am J Kidney Dis 37:954–63
Acknowledgement
The authors are grateful to Dr. T.H. Ottenhof and Dr. K.E. van Meijgaarden for kindly providing the Mycobacterium tuberculosis and M. leprae hsp65-specific, HLADR3 and HLA-DR1 restricted CD4+ T cell clones.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Verkade, M.A., van Druningen, C.J., op de Hoek, C.T. et al. Decreased antigen-specific T-cell proliferation by moDC among hepatitis B vaccine non-responders on haemodialysis. Clin. Exper.Med. 7, 65–71 (2007). https://doi.org/10.1007/s10238-007-0127-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-007-0127-x