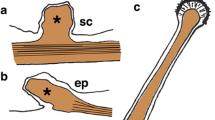
Abstract
Typically, presynaptic terminals form a synapse directly on the surface of postsynaptic processes such as dendrite shafts and spines. However, some presynaptic terminals invaginate—entirely or partially—into postsynaptic processes. We survey these invaginating presynaptic terminals in all animals and describe several examples from the central nervous system, including giant fiber systems in invertebrates, and cup-shaped spines, electroreceptor synapses, and some specialized auditory and vestibular nerve terminals in vertebrates. We then examine mechanoreceptors and photoreceptors, concentrating on the complex of pre- and postsynaptic processes found in basal invaginations of the cell. We discuss in detail the role of vertebrate invaginating horizontal cell processes in both chemical and electrical feedback mechanisms. We also discuss the common presence of indenting or invaginating terminals in neuromuscular junctions on muscles of most kinds of animals, and especially discuss those of Drosophila and vertebrates. Finally, we consider broad questions about the advantages of possessing invaginating presynaptic terminals and describe some effects of aging and disease, especially on neuromuscular junctions. We suggest that the invagination is a mechanism that can enhance both chemical and electrical interactions at the synapse.












Similar content being viewed by others
References
Achim, K., & Arendt, D. (2014). Structural evolution of cell types by step-wise assembly of cellular modules. Current Opinion in Genetics & Development, 27, 102–108.
Acsády, L., Katona, I., Martínez-Guijarro, F. J., Buzsáki, G., & Freund, T. F. (2000). Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus. Journal of Neuroscience, 20(18), 6907–6919.
Adriaensen, D., Brouns, I., Van Genechten, J., & Timmermans, J. P. (2003). Functional morphology of pulmonary neuroepithelial bodies: Extremely complex airway receptors. The Anatomical Record Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 270(1), 25–40.
Ammermüller, J., & Kolb, H. (1996). Functional architecture of the turtle retina. Progress in Retinal and Eye Research, 15(2), 393–433.
Andres, K. H., von During, M., & Petrasch, E. (1988). The fine structure of ampullary and tuberous electroreceptors in the South American blind catfish Pseudocetopsis spec. Anatomy and Embryology (Berlin), 177(6), 523–535.
Arama, J., Abitbol, K., Goffin, D., Fuchs, C., Sihra, T. S., Thomson, A. M., et al. (2015). GABAA receptor activity shapes the formation of inhibitory synapses between developing medium spiny neurons. Frontiers in Cellular Neuroscience, 9, #290. (291–218).
Arbuthnott, E. R., Ballard, K. J., Boyd, I. A., Gladden, M. H., & Sutherland, F. I. (1982). The ultrastructure of cat fusimotor endings and their relationship to foci of sarcomere convergence in intrafusal fibres. Journal of Physiology, 331, 285–309.
Arden, G. B., Gorin, M. B., Polkinghorne, P. J., Jay, M., & Bird, A. C. (1988). Detection of the carrier state of X-linked retinoschisis. American Journal of Ophthalmology, 105(6), 590–595.
Arden, G. B., & Hogg, C. R. (1985). Rod-cone interactions and analysis of retinal disease. British Journal of Ophthalmology, 69(6), 404–415.
Atsumi, S. (1977). Development of neuromuscular-junctions of fast and slow muscles in chick-embryo—Light and electron-microscopic Study. Journal of Neurocytology, 6(6), 691–709.
Attwell, D., Barbour, B., & Szatkowski, M. (1993). Nonvesicular release of neurotransmitter. Neuron, 11(3), 401–407.
Atwood, H. L., & Johnston, H. S. (1968). Neuromuscular synapses of a crab motor axon. Journal of Experimental Zoology, 167, 457–470.
Atwood, H. L. (1976). Organization and synaptic physiology of crustacean neuromuscular systems. Progress in Neurobiology, 7(Pt 4), 291–391.
Atwood, H. L., & Morin, W. A. (1970). Neuromuscular and axoaxonal synapses of the crayish opener muscle. Journal of Ultrastructure Research, 32(3), 351–369.
Babai, N., & Thoreson, W. B. (2009). Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. Journal of Physiology, 587(Pt 10), 2353–2364.
Banerjee, S., Venkatesan, A., & Bhat, M. A. (2017). Neurexin, Neuroligin and Wishful Thinking coordinate synaptic cytoarchitecture and growth at neuromuscular junctions. Molecular and Cellular Neuroscience, 78, 9–24.
Barber, V. C., & Graziadei, P. (1967). The fine structure of cephalopod blood vessels. 3. Vessel innervation. Zeitschrift für Zellforschung und mikroskopische Anatomie, 77(2), 162–174.
Bargmann, W., Lindner, E., & Andres, K. H. (1967). Uber Synapsen an Endokrinen Epithelzellen Und Die Definition Sekretorischer Neuron - Untersuchungen Am Zwischenlappen Der Katzenhypophyse. Zeitschrift für Zellforschung und mikroskopische Anatomie, 77(2), 282–298.
Bargmann, W., von Hehn, G., & Lindner, E. (1968). On the cells of the brown fatty tissue and their innervation. Zeitschrift für Zellforschung und mikroskopische Anatomie, 85(4), 601–613.
Barlow, R. B., Hitt, J. M., & Dodge, F. A. (2001). Limulus vision in the marine environment. Biological Bulletin, 200(2), 169–176.
Baumgarten, H. G., Björklund, A., Holstein, A. F., & Nobin, A. (1972). Organization and ultrastructural identification of catecholamine nerve terminals in neural lobe and pars intermedia of rat pituitary. Zeitschrift für Zellforschung und mikroskopische Anatomie, 126(4), 483–517.
Beccari, N. (1911). La costituzione, i nuclei terminali e le vie di connessione del nervo acustico nella Lacerta muralis. Arch ital Anat Embryol, 10, 646–698.
Benedeczky, I., Csoknya, M., Fekete, E., & Halasy, K. (1990). Ultrastructure of the nerve-muscle junction in the pharynx of the earthworm, Lumbricus terrestris. Zoomorphology, 109(6), 337–341.
Benedeczky, I., Mala, J., & Sehnal, F. (1980). Ultrastructural-study on the innervation of prothoracic glands in Galleria mellonella. General and Comparative Endocrinology, 41(3), 400–407.
Beramendi, A., Peron, S., Casanova, G., Reggiani, C., & Cantera, R. (2007). Neuromuscular junction in abdominal muscles of Drosophila melanogaster during adulthood and aging. Journal of Comparative Neurology, 501(4), 498–508.
Bergman, R. A. (1967). Motor nerve endings of twitch muscle fibers in Hippocampus hudsonius. Journal of Cell Biology, 32(3), 751–757.
Berthold, C. H., Kellerth, J. O., & Conradi, S. (1979). Electron microscopic studies of serially sectioned cat spinal alpha-motoneurons. I. Effects of microelectrode impalement and intracellular staining with the fluorescent dye “Procion Yellow”. Journal of Comparative Neurology, 184(4), 709–740.
Best, A. C. G., & Bone, Q. (1973). Terminal neuromuscular junctions of lower chordates. Zeitschrift für Zellforschung und mikroskopische Anatomie, 143(4), 495–504.
Biazik, J., Ylä-Anttila, P., Vihinen, H., Jokitalo, E., & Eskelinen, E. L. (2015). Ultrastructural relationship of the phagophore with surrounding organelles. Autophagy, 11(3), 439–451.
Birks, R., Huxley, H. E., & Katz, B. (1960). The fine structure of the neuromuscular junction of the frog. Journal of Physiology-London, 150(1), 134–144.
Blagburn, J. M., Alexopoulos, H., Davies, J. A., & Bacon, J. P. (1999). Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: A structural study. Journal of Comparative Neurology, 404(4), 449–458.
Boaro, S. N., Soares, J. C., & König, B., Jr. (1998). Comparative structural analysis of neuromuscular junctions in mice at different ages. Annals of Anatomy, 180(2), 173–179.
Bone, Q. (1972). The dogfish neuromuscular junction: Dual innervation of vertebrate striated muscle fibres? Journal of Cell Science, 10(3), 657–665.
Bone, Q., & Ryan, K. P. (1974). On the structure and innervation of the muscle bands of Doliolum (Tunicata: Cyclomyaria). Proceedings of the Royal Society of London, Series B: Biological Sciences, 187(1088), 315–327.
Bosch, E. (1990). Ultrastructure of the electrotonic and chemical components of the lateral-to-motor and medial-to-motor synapses in crayfish nerve cord. Journal of Comparative Neurology, 299(4), 446–461.
Bradacs, H., Cooper, R., Msghina, M., & Atwood, H. (1997). Differential physiology and morphology of phasic and tonic motor axons in a crayfish limb extensor muscle. Journal of Experimental Biology, 200(Pt 4), 677–691.
Brenowitz, S., & Trussell, L. O. (2001). Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. Journal of Neuroscience, 21(23), 9487–9498.
Budnik, V., Zhong, Y., & Wu, C. F. (1990). Morphological plasticity of motor axons in Drosophila mutants with altered excitability. Journal of Neuroscience, 10(11), 3754–3768.
Burighel, P., Lane, N. J., Fabio, G., Stefano, T., Zaniolo, G., Carnevali, M. D., et al. (2003). Novel, secondary sensory cell organ in ascidians: In search of the ancestor of the vertebrate lateral line. Journal of Comparative Neurology, 461(2), 236–249.
Burton, P. M. (2008). Insights from diploblasts; the evolution of mesoderm and muscle. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 310(1), 5–14.
Carr, C. E., & Boudreau, R. E. (1996). Development of the time coding pathways in the auditory brainstem of the barn owl. Journal of Comparative Neurology, 373(4), 467–483.
Case, N. M., Gray, E. G., & Young, J. Z. (1972). Ultrastructure and synaptic relations in the optic lobe of the brain of Eledone and Octopus. Journal of Ultrastructure Research, 39(1), 115–123.
Castejón, O. J., & Villegas, G. M. (1964). Fine structure of the synaptic contacts in the stellate ganglion of the squid. Journal of Ultrastructure Research, 10, 585–598.
Cavey, M. J., & Cloney, R. A. (1976). Ultrastructure and differentiation of ascidian muscle. I. Caudal musculature of the larva of Diplosoma macdonaldi. Cell and Tissue Research, 174(3), 289–313.
Chang, F. L., & Greenough, W. T. (1984). Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Research, 309(1), 35–46.
Charvet, B., Malbouyres, M., Pagnon-Minot, A., Ruggiero, F., & Le Guellec, D. (2011). Development of the zebrafish myoseptum with emphasis on the myotendinous junction. Cell and Tissue Research, 346(3), 439–449.
Chien, P., & Koopowitz, H. (1972). The ultrastructure of neuromuscular systems in Notoplana acticola, a free-living polyclad flatworm. Zeitschrift für Zellforschung und mikroskopische Anatomie, 133(2), 277–288.
Clément, P. (1977). Ultrastructural research on rotifers. Archiv f Hydrobiologie Beih, 8, 270–297.
Cobb, J. L. (1978). An ultrastructural study of the dermal papulae of the starfish, Asterias rubens, with special reference to innervation of the muscles. Cell and Tissue Research, 187(3), 515–523.
Cobb, J. L. S., & Laverack, M. S. (1966). Lantern of Echinus esculentus (L). 3. Fine structure of lantern retractor muscle and its innervation. Proceedings of the Royal Society Series B-Biological Sciences, 164(997), 651–658.
Cohen, A. I. (1973). An ultrastructural analysis of the photoreceptors of the squid and their synaptic connections. 3. Photoreceptor terminations in the optic lobes. Journal of Comparative Neurology, 147(3), 399–426.
Conradi, S., & Skoglund, S. (1969). Observations on the ultrastruture and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the cat during postnatal development. Acta Physiologica Scandinavica. Supplementum, 333, 5–52.
Coupland, R. E. (1965). Electron microscopic observations on structure of rat adrenal medulla.2. Normal innervation. Journal of Anatomy, 99, 255–272.
Cousin, C. E., & Dorsey, C. H. (1991). Nervous system of Schistosoma mansoni cercaria: Organization and fine structure. Parasitology Research, 77(2), 132–141.
Cutz, E., Pan, J., Yeger, H., Domnik, N. J., & Fisher, J. T. (2013). Recent advances and contraversies on the role of pulmonary neuroepithelial bodies as airway sensors. Seminars in Cell & Developmental Biology, 24(1), 40–50.
Dahl, E. (1973). The fine structure of the pancreatic nerves of the domestic fowl. Zeitschrift für Zellforschung und mikroskopische Anatomie, 136(4), 501–510.
Deshpande, M., & Rodal, A. A. (2016). The crossroads of synaptic growth signaling, membrane traffic and neurological disease: Insights from Drosophila. Traffic, 17(2), 87–101.
Desmond, N. L., & Levy, W. B. (1983). Synaptic correlates of associative potentiation/depression: An ultrastructural study in the hippocampus. Brain Research, 265(1), 21–30.
DeVries, S. H., Li, W., & Saszik, S. (2006). Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron, 50(5), 735–748.
Dilly, P. N., Gray, E. G., & Young, J. Z. (1963). Electron microscopy of optic nerves and optic lobes of Octopus and Eledone. Proceedings of the Royal Society of London, Series B: Biological Sciences, 158, 446–456.
Dolder, H. (1975). An ultrastructural and cytochemical study of neuromuscular junctions in echinoderms. Histochemistry, 44(4), 313–322.
Dowling, J. E., & Boycott, B. B. (1966). Organization of the primate retina: Electron microscopy. Proceedings of the Royal Society of London, Series B: Biological Sciences, 166(1002), 80–111.
Dowling, J. E., & Werblin, F. S. (1969). Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. Journal of Neurophysiology, 32(3), 315–338.
Doyle, W. L. (1967). Vesiculated axons in haemal vessels of an holothurian Cucumaria frondosa. Biological Bulletin, 132(3), 329–336.
Ducros, C. (1972). Ultrastructural study of the innervation of the posterior salivary glands in Octopus vulgaris. II. Muscle innervation in the ducts and the glands. Zeitschrift für Zellforschung und mikroskopische Anatomie, 132(1), 51–65.
Duvert, M., & Barets, A. L. (1983). Ultrastructural studies of neuromuscular junctions in visceral and skeletal muscles of the chaetognath Sagitta setosa. Cell and Tissue Research, 233(3), 657–669.
Duvert, M., & Savineau, J. P. (1986). Ultrastructural and physiological studies of the contraction of the trunk musculature of Sagitta setosa (chaetognath). Tissue and Cell, 18(6), 937–952.
Eakin, R. M., & Brandenburger, J. L. (1971). Fine structure of the eyes of jumping spiders. Journal of Ultrastructure Research, 37(5), 618–663.
Edwards, G. A. (1959). The fine structure of a multiterminal innervation of an insect muscle. The Journal of Biophysical and Biochemical Cytology, 5(2), 241–244.
Edwards, G. A., Ruska, H., & De Harven, E. (1958a). Electron microscopy of peripheral nerves and neuromuscular junctions in the wasp leg. The Journal of Biophysical and Biochemical Cytology, 4(1), 107–114.
Edwards, G. A., Ruska, H., & De Harven, E. (1958b). Neuromuscular junctions in flight and tymbal muscles of the cicada. The Journal of Biophysical and Biochemical Cytology, 4(3), 251–256.
Elekes, K., & Ude, J. (1994). Peripheral connections of FMRFamide-like immunoreactive neurons in the snail, Helix pomatia—An immunogold electron-microscopic study. Journal of Neurocytology, 23(12), 758–769.
Eliasieh, K., Liets, L. C., & Chalupa, L. M. (2007). Cellular reorganization in the human retina during normal aging. Investigative Ophthalmology & Visual Science, 48(6), 2824–2830.
Ellisman, M. H., Rash, J. E., Staehelin, L. A., & Porter, K. R. (1976). Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. Journal of Cell Biology, 68(3), 752–774.
Endeman, D., Fahrenfort, I., Sjoerdsma, T., Steijaert, M., Ten Eikelder, H., & Kamermans, M. (2012). Chloride currents in cones modify feedback from horizontal cells to cones in goldfish retina. Journal of Physiology, 590(22), 5581–5595.
Engel, A. G., & Santa, T. (1971). Histometric analysis of the ultrastructure of the neuromuscular junction in myasthenia gravis and in the myasthenic syndrome. Annals of the New York Academy of Sciences, 183, 46–63.
Esterhuizen, A. C., Spriggs, T. L., & Lever, J. D. (1968). Nature of islet-cell innervation in the cat pancreas. Diabetes, 17(1), 33–36.
Fahim, M. A., & Robbins, N. (1982). Ultrastructural studies of young and old mouse neuromuscular junctions. Journal of Neurocytology, 11(4), 641–656.
Fahrenbach, W. H. (1994). Microscopic anatomy of Pycnogonida: I. Cuticle, epidermis, and muscle. Journal of Morphology, 222(1), 33–48.
Fariss, R. N., Li, Z. Y., & Milam, A. H. (2000). Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. American Journal of Ophthalmology, 129(2), 215–223.
Farnesi, R. M., & Vagnetti, D. (1975). The fine structure of the myoneural junctions in the body wall muscles in Branchiobdella pentodonta Whit. (Annelida, Oligochaeta). Anatomical Record, 182(1), 91–102.
Fedorenko, G. M., & Uzdensky, A. B. (2009). Ultrastructure of neuroglial contacts in crayfish stretch receptor. Cell and Tissue Research, 337(3), 477–490.
Fernand, V. S., & Hess, A. (1969). The occurrence, structure and innervation of slow and twitch muscle fibres in the tensor tympani and stapedius of the cat. Journal of Physiology, 200(2), 547–554.
Filimonova, S. A., & Amosova, L. I. (2015). Peculiar salivary glands in a silk-producing mite Bakericheyla chanayi (Cheyletidae). Journal of Morphology, 276(7), 772–786.
Fischer, F. P. (1992). Quantitative analysis of the innervation of the chicken basilar papilla. Hearing Research, 61(1–2), 167–178.
Fisher, S. K., & Boycott, B. B. (1974). Synaptic connections made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proceedings of the Royal Society of London, Series B: Biological Sciences, 186(1085), 317–331.
Florey, E. (1969). Ultrastructure and function of cephalopod chromatophores. American Zoologist, 9(2), 429–442.
Fourtner, C. R., & Sherman, R. G. (1973). Chelicerate skeletal neuromuscular systems. American Zoologist, 13(2), 271–289.
Freifeld, L., Clark, D. A., Schnitzer, M. J., Horowitz, M. A., & Clandinin, T. R. (2013). GABAergic lateral interactions tune the early stages of visual processing in Drosophila. Neuron, 78(6), 1075–1089.
Frotscher, M., & Léránth, C. (1986). The cholinergic innervation of the rat fascia dentata: Identification of target structures on granule cells by combining choline acetyltransferase immunocytochemistry and Golgi impregnation. Journal of Comparative Neurology, 243(1), 58–70.
Fuentes-Medel, Y., Ashley, J., Barria, R., Maloney, R., Freeman, M., & Budnik, V. (2012). Integration of a retrograde signal during synapse formation by glia-secreted TGF-beta ligand. Current Biology, 22(19), 1831–1838.
Fuentes-Medel, Y., Logan, M. A., Ashley, J., Ataman, B., Budnik, V., & Freeman, M. R. (2009). Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biology, 7(8), e1000184. (1000181–1000115).
Gardner, C. L., Jones, J. R., Baer, S. M., & Crook, S. M. (2015). Drift-diffusion simulation of the ephaptic effect in the triad synapse of the retina. Journal of Computational Neuroscience, 38(1), 129–142.
Glantz, R. M., & Bartels, A. (1994). The spatiotemporal transfer function of crayfish lamina monopolar neurons. Journal of Neurophysiology, 71(6), 2168–2182.
Glantz, R. M., Miller, C. S., & Nässel, D. R. (2000). Tachykinin-related peptide and GABA-mediated presynaptic inhibition of crayfish photoreceptors. Journal of Neuroscience, 20(5), 1780–1790.
Goniakowska-Witalinska, L., Lauweryns, J. M., Zaccone, G., Fasulo, S., & Tagliafierro, G. (1992). Ultrastructure and immunocytochemistry of the neuroepithelial bodies in the lung of the tiger salamander, Ambystoma tigrinum (Urodela, Amphibia). Anatomical Record, 234(3), 419–431.
Govind, C. K. (1992). Age-related remodeling of lobster neuromuscular terminals. Experimental Gerontology, 27(1), 63–74.
Govind, C. K., Atwood, H. L., & Pearce, J. (1995). Inhibitory axoaxonal and neuromuscular synapses in the crayfish opener muscle: Membrane definition and ultrastructure. Journal of Comparative Neurology, 351(3), 476–488.
Govind, C. K., & Derosa, R. A. (1983). Fine structure of comparable synapses in a mature and larval lobster muscle. Tissue and Cell, 15(1), 97–106.
Govind, C. K., & Pearce, J. (1981). Remodeling of multiterminal innervation by nerve terminal sprouting in an identifiable lobster motoneuron. Science, 212(4502), 1522–1524.
Govind, C. K., Stephens, P. J., & Eisen, J. S. (1985). Polyneuronal innervation of an adult and embryonic lobster muscle. Journal of Embryology and Experimental Morphology, 87, 13–26.
Graziadei, P. (1966). The ultrastructure of the motor nerve endings in the muscles of cephalopods. Journal of Ultrastructure Research, 15(1), 1–13.
Greb, H., Hermann, S., Dirks, P., Ommen, G., Kretschmer, V., Schultz, K., et al. (2017). Complexity of gap junctions between horizontal cells of the carp retina. Neuroscience, 340, 8–22.
Greferath, U., Grünert, U., Müller, F., & Wässle, H. (1994). Localization of GABAA receptors in the rabbit retina. Cell and Tissue Research, 276(2), 295–307.
Gur, M., Purple, R. L., & Whitehead, R. (1972). Ultrastructure within the lateral plexus of the Limulus eye. Journal of General Physiology, 59(3), 285–304.
Hafner, G. S. (1974). The ultrastructure of retinula cell endings in the compound eye of the crayfish. Journal of Neurocytology, 3(3), 295–311.
Haghighat, N., Cohen, R. S., & Pappas, G. D. (1984). Fine structure of squid (Loligo pealei) optic lobe synapses. Neuroscience, 13(2), 527–546.
Hama, K. (1961). Some observations on the fine structure of the giant fibers of the crayfishes (Cambarus virilus and Cambarus clarkii) with special reference to the submicroscopic organization of the synapses. Anatomical Record, 141, 275–293.
Hámori, J., & Horridge, G. A. (1966). Lobster optic lamina. 2. Types of synapse. Journal of Cell Science, 1(2), 257–270.
Hand, A. R. (1970). Nerve-acinar cell relationships in the rat parotid gland. Journal of Cell Biology, 47(2), 540–543.
Hanson, J., & Lowy, J. (1957). Structure of smooth muscles. Nature, 180(4592), 906–909.
Häring, P., Stähli, C., Schoch, P., Takács, B., Staehelin, T., & Möhler, H. (1985). Monoclonal-antibodies reveal structural homogeneity of gamma-aminobutyric acid benzodiazepine receptors in different brain-areas. Proceedings of the National Academy of Sciences USA, 82(14), 4837–4841.
Hart, R. J., Beadle, D. J., & Botham, R. P. (1980). The ultrastructure of somatic neuromuscular junctions in the tick Amblyomma variegatum (Fabr.). Cell and Tissue Research, 206(3), 505–508.
Harvey, D. M., & Calkins, D. J. (2002). Localization of kainate receptors to the presynaptic active zone of the rod photoreceptor in primate retina. Visual Neuroscience, 19(5), 681–692.
Haverkamp, S., Grünert, U., & Wässle, H. (2000). The cone pedicle, a complex synapse in the retina. Neuron, 27(1), 85–95.
He, S., Weiler, R., & Vaney, D. I. (2000). Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. Journal of Comparative Neurology, 418(1), 33–40.
Heitler, W. J., Cobb, J. L., & Fraser, K. (1985). Ultrastructure of the segmental giant neuron of crayfish. Journal of Neurocytology, 14(6), 921–941.
Hering, H., Lin, C. C., & Sheng, M. (2003). Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. Journal of Neuroscience, 23(8), 3262–3271.
Hernandez-Nicaise, M. L. (1973). The nervous system of ctenophores. III. Ultrastructure of synapses. Journal of Neurocytology, 2(3), 249–263.
Hernandez-Nicaise, M. L., Mackie, G. O., & Meech, R. W. (1980). Giant smooth muscle cells of Beroe. Ultrastructure, innervation, and electrical properties. Journal of General Physiology, 75(1), 79–105.
Hess, A. (1965). The sarcoplasmic reticulum, the T system, and the motor terminals of slow and twitch muscle fibers in the garter snake. Journal of Cell Biology, 26(2), 467–476.
Hess, A. (1967). The structure of vertebrate slow and twitch muscle fibers. Investigative Ophthalmology, 6(3), 217–228.
Hess, A. (1970). Vertebrate slow muscle fibers. Physiological Reviews, 50(1), 40–62.
Heuser, J. E., & Reese, T. S. (1973). Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. Journal of Cell Biology, 57(2), 315–344.
Hill, R. B., & Sanger, J. W. (1974). Anatomy of innervation and neuromuscular-junctions of radular protractor muscle of whelk, Busycon canaliculatum (L). Biological Bulletin, 147(2), 369–385.
Hinojosa, R. (1973). Synaptic ultrastructure in the tangential nucleus of the goldfish (Carassius auratus). American Journal of Anatomy, 137(2), 159–186.
Hinojosa, R., & Robertson, J. D. (1967). Ultrastructure of the spoon type synaptic endings in the nucleus vestibularis tangentialis of the chick. Journal of Cell Biology, 34(2), 421–430.
Hirano, H. (1967). Ultrastructural study on the morphogenesis of the neuromuscular junction in the skeletal muscle of the chick. Zeitschrift für Zellforschung und mikroskopische Anatomie, 79(2), 198–208.
Hirano, A. A., Brandstätter, J. H., & Brecha, N. C. (2005). Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina. Journal of Comparative Neurology, 488(1), 70–81.
Hirano, A. A., Liu, X., Boulter, J., Grove, J., de Sevilla, Perez, Müller, L., et al. (2016). Targeted deletion of vesicular GABA transporter from retinal horizontal cells eliminates feedback modulation of photoreceptor calcium channels. eNeuro, 3(2), 1–13.
Hirano, H., & Ogawa, K. (1967). Ultrastructural localization of cholinesterase activity in nerve endings in the guinea pig heart. Journal of Electron Microscopy (Tokyo), 16(4), 313–321.
Holmberg, K. (1970). The hagfish retina: Fine structure of retinal cells in Myxine glutinosa, L., with special reference to receptor and epithelial cells. Zeitschrift für Zellforschung und mikroskopische Anatomie, 111(4), 519–538.
Holmberg, K. (1971). Hagfish retina—Electron microscopic study comparing receptor and epithelial cells in pacific hagfish, Polistotrema stouti, with those in Atlantic hagfish, Myxine glutinosa. Zeitschrift für Zellforschung und mikroskopische Anatomie, 121(2), 249–269.
Holmberg, K., & Öhman, P. (1976). Fine-structure of retinal synaptic organelles in lamprey and hagfish photoreceptors. Vision Research, 16(3), 237–239.
Holtmann, M., & Thurm, U. (2001a). Variations of concentric hair cells in a cnidarian sensory epithelium (Coryne tubulosa). Journal of Comparative Neurology, 432(4), 550–563.
Holtmann, M., & Thurm, U. (2001b). Mono- and oligo-vesicular synapses and their connectivity in a Cnidarian sensory epithelium (Coryne tubulosa). Journal of Comparative Neurology, 432(4), 537–549.
Hombach, S., Janssen-Bienhold, U., Söhl, G., Schubert, T., Büssow, H., Ott, T., et al. (2004). Functional expression of connexin57 in horizontal cells of the mouse retina. European Journal of Neuroscience, 19(10), 2633–2640.
Hong, T., & Shaw, R. M. (2017). Cardiac T-Tubule Microanatomy and Function. Physiological Reviews, 97(1), 227–252.
Horridge, G. A. (1965). Non-motile sensory cilia and neuromuscular junctions in a ctenophore independent effector organ. Proceedings of the Royal Society Series B-Biological Sciences, 162(988), 333–337.
Hoyle, G., & Mcneill, P. A. (1968). Correlated physiological and ultrastructural studies on specialised muscles. Ic. Neuromuscular junctions in eyestalk levator muscles of Podophthalmus vigil (Weber). Journal of Experimental Zoology, 167(4), 523–550.
Ichiki, M., Nakagaki, I., Konishi, A., & Fukami, Y. (1976). The innervation of muscle spindles in the snake, Elaphe quadrivirgata. Journal of Anatomy, 122(Pt 1), 141–167.
Jahromi, S. S., & Atwood, H. L. (1974). Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. Journal of Cell Biology, 63(2 Pt 1), 599–613.
James, R. E., Hoover, K. M., Bulgari, D., McLaughlin, C. N., Wilson, C. G., Wharton, K. A., et al. (2014). Crimpy enables discrimination of presynaptic and postsynaptic pools of a BMP at the Drosophila neuromuscular junction. Developmental Cell, 31(5), 586–598.
Jansen, J., Andersen, P., & Jansen, J. K. S. (1963). On the structure and innervation of the parietal muscle of the hagfish (Myxine glutinosa). Acta Morphologica Neerlando-Scandinavica, 5, 329–338.
Jensen, H., Holtet, L., & Hoen, R. (1978). Nerve-Purkinje fiber relationship in the moderator band of bovine and caprine heart. Cell and Tissue Research, 188(1), 11–18.
Jerusalem, F., Engel, A. G., & Gomez, M. R. (1974). Duchenne dystrophy. II. Morphometric study of motor end-plate fine structure. Brain, 97(1), 123–130.
Jhaveri, S., & Morest, D. K. (1982). Sequential alterations of neuronal architecture in nucleus magnocellularis of the developing chicken: An electron microscope study. Neuroscience, 7(4), 855–870.
Johnston, J. B. (1902). The brain of Petromyzon. Journal of Comparative Neurology, 12(1), 1–82.
Jones, E. G., & Powell, T. P. (1969). Morphological variations in the dendritic spines of the neocortex. Journal of Cell Science, 5(2), 509–529.
Jørgensen, J. M. (2005). Morphology of electroreceptive sensory organs. In T. H. Bullock, C. D. Hopkins, A. N. Popper, & R. R. Fay (Eds.), Electroreception (pp. 47–67). New York: Springer.
Ju, G., & Zhang, X. (1990). An electron-microscopic study on substance P-like immunoreactive nerve-fibers in the pars-distalis of the adenohypophysis in the dog. Neuroscience, 38(2), 503–513.
Ju, G., & Zhang, X. (1992). An electron-microscopic study of calcitonin gene-related peptide-like immunoreactive innervation of the anterior-pituitary in the dog. Journal of Comparative Neurology, 326(1), 101–111.
Jung, H. H., Han, S. H., Nam, S. Y., Kim, Y. H., & Kim, J. L. (2004). Myosin heavy chain composition of rat middle ear muscles. Acta Oto-Laryngologica, 124(5), 569–573.
Kamermans, M., & Fahrenfort, I. (2004). Ephaptic interactions within a chemical synapse: Hemichannel-mediated ephaptic inhibition in the retina. Current Opinion in Neurobiology, 14(5), 531–541.
Kamimura, K., Ueno, K., Nakagawa, J., Hamada, R., Saitoe, M., & Maeda, N. (2013). Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction. Journal of Cell Biology, 200(2), 219–233.
Katz, B. (1961). Terminations of afferent nerve fibre in muscle spindle of frog. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 243(703), 221–240.
Kerr, K. S., Fuentes-Medel, Y., Brewer, C., Barria, R., Ashley, J., Abruzzi, K. C., et al. (2014). Glial wingless/Wnt regulates glutamate receptor clustering and synaptic physiology at the Drosophila neuromuscular junction. Journal of Neuroscience, 34(8), 2910–2920.
King, M. J., Atwood, H. L., & Govind, C. K. (1996). Structural features of crayfish phasic and tonic neuromuscular terminals. Journal of Comparative Neurology, 372(4), 618–626.
Klaassen, L. J., Sun, Z., Steijaert, M. N., Bolte, P., Fahrenfort, I., Sjoerdsma, T., et al. (2011). Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biology, 9(7), 1–17.
Kleitman, N., & Holzwarth, M. A. (1985). Catecholaminergic innervation of the rat adrenal-cortex. Cell and Tissue Research, 241(1), 139–147.
Klooster, J., & Kamermans, M. (2016). An ultrastructural and immunohistochemical analysis of the outer plexiform layer of the retina of the European Silver Eel (Anguilla anguilla L). PLoS ONE, 11(3), e0152967.
Klooster, J., Nunes Cardozo, B., Yazulla, S., & Kamermans, M. (2004). Postsynaptic localization of gamma-aminobutyric acid transporters and receptors in the outer plexiform layer of the goldfish retina: An ultrastructural study. Journal of Comparative Neurology, 474(1), 58–74.
Kobayashi, K. (1966). Electron microscope studies of the Langerhans Islets in the toad pancreas. Archivum Histologicum Japonicum, 26(5), 439–482.
Kobayashi, S., & Fujita, T. (1969). Fine structure of mammalian and avian pancreatic islets with special reference to D cells and nervous elements. Zeitschrift für Zellforschung und mikroskopische Anatomie, 100(3), 340–363.
Kolb, H. (1977). The organization of the outer plexiform layer in the retina of the cat: Electron microscopic observations. Journal of Neurocytology, 6(2), 131–153.
Kolb, H., & Jones, J. (1984). Synaptic organization of the outer plexiform layer of the turtle retina: An electron microscope study of serial sections. Journal of Neurocytology, 13(4), 567–591.
Kordylewski, L. (1974). The anatomy and the fine structure of extraocular muscles of the gudgeon, Gobio gobio (Linnaeus). Acta Anatomica (Basel), 87(4), 597–614.
Korkut, C., Ataman, B., Ramachandran, P., Ashley, J., Barria, R., Gherbesi, N., et al. (2009). Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell, 139(2), 393–404.
Korkut, C., Li, Y., Koles, K., Brewer, C., Ashley, J., Yoshihara, M., et al. (2013). Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron, 77(6), 1039–1046.
Korn, H., Sotelo, C., & Bennett, M. V. L. (1977). Lateral vestibular nucleus of toadfish Opsanus tau—Ultrastructural and electrophysiological observations with special reference to electrotonic transmission. Neuroscience, 2(6), 851–884.
Korn, H., Sotelo, C., & Crepel, F. (1973). Electronic coupling between neurons in the rat lateral vestibular nucleus. Experimental Brain Research, 16(3), 255–275.
Korneliussen, H. (1973a). Ultrastructure of motor nerve terminals on different types of muscle fibers in the Atlantic hagfish (Myxine glutinosa, L.). Occurrence of round and elongated profiles of synaptic vesicles and dense-core vesicles. Zeitschrift für Zellforschung und mikroskopische Anatomie, 147(1), 87–105.
Korneliussen, H. (1973b). Dense-core vesicles in motor nerve terminals. Monoaminergic innervation of slow non-twitch muscle fibers in the Atlantic hagfish (Myxine glutinosa, L.)? Zeitschrift für Zellforschung und mikroskopische Anatomie, 140(3), 425–432.
Korneliussen, H. (1973c). Ultrastructure of myotendinous junctions in Myxine and rat. Specializations between the plasma membrane and the lamina densa. Z Anat Entwicklungsgesch, 142(1), 91–101.
Kramer, R. H., & Davenport, C. M. (2015). Lateral inhibition in the vertebrate retina: The case of the missing neurotransmitter. PLoS Biology, 13(12), 1–8.
Kryvi, H. (1977). Ultrastructure of the different fibre types in axial muscles of the sharks Etmopterus spinax and Galeus melastomus. Cell and Tissue Research, 184(3), 287–300.
Kucera, J. (1984). Ultrastructure of extrafusal and intrafusal terminals of a (dynamic) skeletofusimotor axon in cat tenuissimus muscle. Brain Research, 298(1), 181–186.
Kucera, J., & Walro, J. M. (1986). Factors that determine the form of neuromuscular junctions of intrafusal fibers in the cat. American Journal of Anatomy, 176(1), 97–117.
Kullberg, R. W., Lentz, T. L., & Cohen, M. W. (1977). Development of the myotomal neuromuscular junction in Xenopus laevis: An electrophysiological and fine-structural study. Development Biology, 60(1), 101–129.
Kwong, W. H., & Gauthier, G. F. (1987). Neuromuscular junctions in adult and developing fast and slow muscles. Anatomical Record, 219(4), 409–419.
Lacalli, T. C. (1990). Structure and organization of the nervous-system in the actinotroch larva of Phoronis vancouverensis. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 327(1244), 655–685.
Lacalli, T. C. (2002). The dorsal compartment locomotory control system in amphioxus larvae. Journal of Morphology, 252(3), 227–237.
Lane, B. P., & Rhodin, J. A. (1964). Cellular interrelationships and electrical activity in two types of smooth muscle. Journal of Ultrastructure Research, 10, 470–488.
Lang, F. (1972). Ultrastructure of neuromuscular junctions in Limulus heart. Zeitschrift für Zellforschung und mikroskopische Anatomie, 130(4), 481–488.
Lasansky, A. (1973). Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 265(872), 471–489.
Lauweryns, J. M., & Van Lommel, A. (1987). Ultrastructure of nerve endings and synaptic junctions in rabbit intrapulmonary neuroepithelial bodies: A single and serial section analysis. Journal of Anatomy, 151, 65–83.
Legg, P. G. (1967). Fine structure and innervation of beta and delta cells in Islet of Langerhans of cat. Zeitschrift für Zellforschung und mikroskopische Anatomie, 80(3), 307–321.
Leitch, B. (1992). Ultrastructure of electrical synapses: Review. Electron Microsc Rev, 5(2), 311–339.
Leitch, B., Cobb, J. L., Heitler, W. J., & Pitman, R. M. (1989). Post-embryonic development of rectifying electrical synapses in the crayfish: Ultrastructure. Journal of Neurocytology, 18(6), 749–761.
Leitch, B., Pitman, R. M., Heitler, W. J., & Cobb, J. L. (1992). Structural and functional post-embryonic development of a non-rectifying electrical synapse in the crayfish. Journal of Neurocytology, 21(2), 120–128.
Lemanski, L. F., Fitts, E. P., & Marx, B. S. (1975). Fine structure of the heart in the Japanese Medaka, Oryzias latipes. Journal of Ultrastructure Research, 53(1), 37–65.
Léránth, C., & Frotscher, M. (1986). Synaptic connections of cholecystokinin-immunoreactive neurons and terminals in the rat fascia dentata: A combined light and electron microscopic study. Journal of Comparative Neurology, 254(1), 51–64.
Li, Y. C., Li, Y. N., Cheng, C. X., Sakamoto, H., Kawate, T., Shimada, O., et al. (2005). Subsurface cisterna-lined axonal invaginations and double-walled vesicles at the axonal-myelin sheath interface. Neuroscience Research, 53(3), 298–303.
Li, W., Ochalski, P. A., Brimijoin, S., Jordan, L. M., & Nagy, J. I. (1995). C-terminals on motoneurons: Electron microscope localization of cholinergic markers in adult rats and antibody-induced depletion in neonates. Neuroscience, 65(3), 879–891.
Liets, L. C., Eliasieh, K., van der List, D. A., & Chalupa, L. M. (2006). Dendrites of rod bipolar cells sprout in normal aging retina. Proceedings of the National Academy of Sciences USA, 103(32), 12156–12160.
Linberg, K. A., & Fisher, S. K. (1986). An ultrastructural study of interplexiform cell synapses in the human retina. Journal of Comparative Neurology, 243(4), 561–576.
Linberg, K. A., & Fisher, S. K. (1988). Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. Journal of Comparative Neurology, 268(2), 281–297.
Lissmann, H. W., & Mullinger, A. M. (1968). Organization of ampullary electric receptors in Gymnotidae (Pisces). Proceedings of the Royal Society of London, Series B: Biological Sciences, 169(1017), 345–378.
Liu, X., Hirano, A. A., Sun, X., Brecha, N. C., & Barnes, S. (2013). Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. Journal of Physiology, 591(13), 3309–3324.
Liu, J., Zhao, J. W., Du, J. L., & Yang, X. L. (2005). Functional GABA(B) receptors are expressed at the cone photoreceptor terminals in bullfrog retina. Neuroscience, 132(1), 103–113.
Macrae, E. K. (1963). Observations on the fine structure of pharyngeal muscle in the planarian Dugesia tigrina. Journal of Cell Biology, 18, 651–662.
Mahadeva, B., Phillips, L. H., 2nd, & Juel, V. C. (2008). Autoimmune disorders of neuromuscular transmission. Seminars in Neurology, 28(2), 212–227.
Manni, L., Mackie, G. O., Caicci, F., Zaniolo, G., & Burighel, P. (2006). Coronal organ of ascidians and the evolutionary significance of secondary sensory cells in chordates. Journal of Comparative Neurology, 495(4), 363–373.
Marotte, L. R., & Mark, R. F. (1970). The mechanism of selective reinnervation of fish eye muscle. II. Evidence from electronmicroscopy of nerve endings. Brain Research, 19(1), 53–62.
Marques, M. J., Conchello, J. A., & Lichtman, J. W. (2000). From plaque to pretzel: Fold formation and acetylcholine receptor loss at the developing neuromuscular junction. Journal of Neuroscience, 20(10), 3663–3675.
Martin, A. R. (1994). Amplification of neuromuscular transmission by postjunctional folds. Proceedings of the Royal Society of London B: Biological Sciences, 258(1353), 321–326.
Matthews-Bellinger, J., & Salpeter, M. M. (1978). Distribution of acetylcholine receptors at frog neuromuscular-junctions with a discussion of some physiological implications. Journal of Physiology-London, 279, 197–213.
McCabe, B. D., Marques, G., Haghighi, A. P., Fetter, R. D., Crotty, M. L., Haerry, T. E., et al. (2003). The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron, 39(2), 241–254.
McKenna, O. C., & Rosenbluth, J. (1973). Myoneural and intermuscular junctions in a molluscan smooth muscle. Journal of Ultrastructure Research, 42(5), 434–450.
Melamed, J., & Trujillo-Cenóz, O. (1971). Innervation of the retinal muscles in wolf spiders (Araneae-Lycosidae). Journal of Ultrastructure Research, 35(3), 359–369.
Mescher, A. L. (2016). Junqueira’s Basic Histology Text and Atlas (14th ed.). New York: McGraw Hill Education.
Mill, P. J., & Knapp, M. F. (1970a). Neuromuscular junctions in the body wall muscles of the earthworm, Lumbricus terrestris Linn. Journal of Cell Science, 7(1), 263–271.
Mill, P. J., & Knapp, M. F. (1970b). The fine structure of obliquely striated body wall muscles in the earthworm, Lumbricus terrestris Linn. Journal of Cell Science, 7(1), 233–261.
Miller, T., & Adams, M. E. (1974). Ultrastructure and electrical properties of the hyperneural muscle of Periplaneta americana. Journal of Insect Physiology, 20(10), 1925–1936.
Mitchell, N., Petralia, R. S., Currier, D. G., Wang, Y. X., Kim, A., Mattson, M. P., et al. (2012). Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. Journal of Cell Science, 125(Pt 18), 4207–4213.
Mochet, M., Moravec, J., Guillemot, H., & Hatt, P. Y. (1975). The ultrastructure of rat conductive tissue; an electron microscopic study of the atrioventricular node and the bundle of His. Journal of Molecular and Cellular Cardiology, 7(12), 879–889.
Moraes, G. D., Achaval, M., Piva, M. M. D., Faccioni-Heuser, M. C., Wassermann, G. F., & Zancan, D. M. (2010). Ultrastructural analysis of the dorsal body gland of the terrestrial snail Megalobulimus abbreviatus (Becquaert, 1948). Brazilian Journal of Biology, 70(2), 341–350.
Morgan, D. L., & Proske, U. (1984). Vertebrate slow muscle: Its structure, pattern of innervation, and mechanical properties. Physiological Reviews, 64(1), 103–169.
Msghina, M., Govind, C. K., & Atwood, H. L. (1998). Synaptic structure and transmitter release in crustacean phasic and tonic motor neurons. Journal of Neuroscience, 18(4), 1374–1382.
Mugnaini, E., Walberg, F., & Brodal, A. (1967). Mode of termination of primary vestibular fibres in the lateral vestibular nucleus. An experimental electron microscopical study in the cat. Experimental Brain Research, 4(3), 187–211.
Mullinger, A. M. (1969). The organization of ampullary sense organs in the electric fish, Gymnarchus niloticus. Tissue Cell, 1(1), 31–52.
Murakami, M., Shimoda, Y., Nakatani, K., Miyachi, E., & Watanabe, S. (1982). GABA-mediated negative feedback from horizontal cells to cones in carp retina. Japanese Journal of Physiology, 32(6), 911–926.
Murray, M., Zimmer, J., & Raisman, G. (1979). Quantitative electron-microscopic evidence for re-Innervation in the adult-rat interpeduncular nucleus after lesions of the fasciculus retroflexus. Journal of Comparative Neurology, 187(2), 447–468.
Nagel, A., Lehmann-Horn, F., & Engel, A. G. (1990). Neuromuscular transmission in the mdx mouse. Muscle and Nerve, 13(8), 742–749.
Nägerl, U. V., Willig, K. I., Hein, B., Hell, S. W., & Bonhoeffer, T. (2008). Live-cell imaging of dendritic spines by STED microscopy. Proceedings of the National Academy of Sciences USA, 105(48), 18982–18987.
Nagy, J. I., Bautista, W., Blakley, B., & Rash, J. E. (2013). Morphologically mixed chemical-electrical synapses formed by primary afferents in rodent vestibular nuclei as revealed by immunofluorescence detection of connexin36 and vesicular glutamate transporter-1. Neuroscience, 252, 468–488.
Nakajima, Y. (1969). Fine structure of red and white muscle fibers and their neuromuscular junctions in the snake fish (Ophiocephalus argus). Tissue and Cell, 1(2), 229–246.
Neises, G. R., Mattox, D. E., & Gulley, R. L. (1982). The maturation of the end bulb of Held in the rat anteroventral cochlear nucleus. Anatomical Record, 204(3), 271–279.
Nguyen, C. T., & Stewart, B. A. (2016). The influence of postsynaptic structure on missing quanta at the Drosophila neuromuscular junction. BMC Neuroscience, 17, 53. (51-11).
Nunzi, M. G., & Franzini Armstrong, C. (1981). The structure of smooth and striated portions of the adductor muscle of the valves in a scallop. Journal of Ultrastructure Research, 76(2), 134–148.
O’Connor, A. K., O’Brien, R. D., & Salpeter, M. M. (1965). Pharmacology and fine structure of peripheral muscle innervation in the cockroach Periplaneta americana. Journal of Insect Physiology, 11(10), 1351–1358.
Oberdorfer, M. D. (1977). The neural organization of the first optic ganglion of the principle eyes of jumping spiders (Salticidae). Journal of Comparative Neurology, 174(1), 95–118.
Ohkawa, T., Satake, S., Yokoi, N., Miyazaki, Y., Ohshita, T., Sobue, G., et al. (2014). Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. Journal of Neuroscience, 34(24), 8151–8163.
Økland, S. (1980). The heart ultrastructure of Lepidopleurus asellus (Spengler) and Tonicella marmorea (Fabricius) (Mollusca, Polyplacophora). Zoomorphology, 96(1–2), 1–19.
Oleskevich, S., Youssoufian, M., & Walmsley, B. (2004). Presynaptic plasticity at two giant auditory synapses in normal and deaf mice. Journal of Physiology, 560(Pt 3), 709–719.
Omiya, Y., Uchigashima, M., Konno, K., Yamasaki, M., Miyazaki, T., Yoshida, T., et al. (2015). VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. Journal of Neuroscience, 35(10), 4215–4228.
Osborne, M. P. (1967). Fine Structure of Neuromuscular Junctions in Segmental Muscles of Blowfly Larva. Journal of Insect Physiology, 13(6), 827–833.
Ovalle, W. K., Dow, P. R., & Nahirney, P. C. (1999). Structure, distribution and innervation of muscle spindles in avian fast and slow skeletal muscle. Journal of Anatomy, 194(Pt 3), 381–394.
Padykula, H. A., & Gauthier, G. F. (1970). The ultrastructure of the neuromuscular junctions of mammalian red, white, and intermediate skeletal muscle fibers. Journal of Cell Biology, 46(1), 27–41.
Page, S. G. (1965). A comparison of fine structures of frog slow and twitch muscle fibres. Journal of Cell Biology, 26(2), 477–497.
Page, S. (1968). Structure of the sarcoplasmic reticulum in vertebrate muscle. British Medical Bulletin, 24(2), 170–173.
Pan, S. C. (1980). The fine structure of the miracidium of Schistosoma mansoni. Journal of Invertebrate Pathology, 36(3), 307–372.
Parks, T. N. (1981). Morphology of axosomatic endings in an avian cochlear nucleus: Nucleus magnocellularis of the chicken. Journal of Comparative Neurology, 203(3), 425–440.
Pattnaik, B., Jellali, A., Sahel, J., Dreyfus, H., & Picaud, S. (2000). GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. Journal of Neuroscience, 20(18), 6789–6796.
Pavans de Ceccatty, M. (1966). Ultrastructures et rapports des cellules mesenchymateuses de type nerveux de l’eponge Tethya lyncurium Link. Annales des Sciences Naturelles—Zoologie et Biologie Animale, 8, 577–614.
Pearce, J., Govind, C. K., & Meiss, D. E. (1985). Growth-related features of lobster neuromuscular terminals. Brain Research, 353(2), 215–228.
Peichl, L., & González-Soriano, J. (1994). Morphological types of horizontal cell in rodent retinae: A comparison of rat, mouse, gerbil, and guinea pig. Visual Neuroscience, 11(3), 501–517.
Pentreath, V. W., & Cobb, J. L. (1972). Neurobiology of echinodermata. Biological Reviews of the Cambridge Philosophical Society, 47(3), 363–392.
Peters, A., & Kaiserman-Abramof, I. R. (1970). The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. American Journal of Anatomy, 127(4), 321–356.
Petralia, R. S., Mattson, M. P., & Yao, P. J. (2014). Communication breakdown: The impact of ageing on synapse structure. Ageing Research Reviews, 14, 31–42.
Petralia, R. S., & Peusner, K. D. (1990). Ultrastructural study of synapses at the time of neuronal migration and early differentiation in the tangential vestibular nucleus of the chick embryo in vivo. Journal of Comparative Neurology, 292(2), 231–245.
Petralia, R. S., Wang, Y. X., Mattson, M. P., & Yao, P. J. (2015). Structure, distribution, and function of neuronal/synaptic spinules and related invaginating projections. Neuromolecular Medicine, 17(3), 211–240.
Petralia, R. S., Wang, Y. X., Mattson, M. P., & Yao, P. J. (2016). The diversity of spine synapses in animals. NeuroMolecular Medicine, 18, 497–539.
Pette, D., & Staron, R. S. (1997). Mammalian skeletal muscle fiber type transitions. International Review of Cytology, 170, 143–223.
Peusner, K. D. (1984). The development of synapses and “spoon” synaptic terminal space in the tangential vestibular nucleus: A quantitative electron microscope study. Journal of Comparative Neurology, 230(3), 372–385.
Peusner, K. D., & Giaume, C. (1994). The first developing “mixed” synapses between vestibular sensory neurons mediate glutamate chemical transmission. Neuroscience, 58(1), 99–113.
Piscopo, S., Moccia, F., Di Cristo, C., Caputi, L., Di Cosmo, A., & Brown, E. R. (2007). Pre- and postsynaptic excitation and inhibition at octopus optic lobe photoreceptor terminals; implications for the function of the ‘presynaptic bags’. European Journal of Neuroscience, 26(8), 2196–2203.
Popova, E. (2014). Role of dopamine in distal retina. Journal of Comparative Physiology A, 200(5), 333–358.
Prokop, A. (1999). Integrating bits and pieces: Synapse structure and formation in Drosophila embryos. Cell and Tissue Research, 297(2), 169–186.
Prokop, A. (2006). Organization of the efferent system and structure of neuromuscular junctions in Drosophila. International Review of Neurobiology, 75, 71–90.
Prokop, A., & Meinertzhagen, I. A. (2006). Development and structure of synaptic contacts in Drosophila. Seminars in Cell & Developmental Biology, 17(1), 20–30.
Pucci, I., & Afzelius, B. A. (1962). Electron microscope study of sarcotubules and related structures in the leech muscle. Journal of Ultrastructure Research, 7, 210–224.
Puthussery, T., Percival, K. A., Venkataramani, S., Gayet-Primo, J., Grünert, U., & Taylor, W. R. (2014). Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina. Journal of Neuroscience, 34(22), 7611–7621.
Raviola, E., & Gilula, N. B. (1975). Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. Journal of Cell Biology, 65(1), 192–222.
Reger, J. F. (1961). The fine structure of neuromuscular junctions and the sarcoplasmic reticulum of extrinsic eye muscles of Fundulus heteroclitus. The Journal of Biophysical and Biochemical Cytology, 10(4), 111–121.
Reger, J. F. (1965). Fine Structure of Neuromuscular Junctions and Contact Zones between Body Wall Muscle Cells of Ascaris lumbricoides (Var Suum). Zeitschrift für Zellforschung und mikroskopische Anatomie, 67(2), 196–210.
Reger, J. F. (1969). Studies on the fine structure of muscle fibres and contained crystalloids in basal socket muscle of the entoproct, Barentsia gracilis. Journal of Cell Science, 4(2), 305–325.
Rheuben, M. B. (1985). Quantitative comparison of the structural features of slow and fast neuromuscular junctions in Manduca. Journal of Neuroscience, 5(7), 1704–1716.
Rheuben, M. B., & Reese, T. S. (1978). Three-dimensional structure and membrane specializations of moth excitatory neuromuscular synapse. Journal of Ultrastructure Research, 65(2), 95–111.
Richardson, K. C. (1964). The fine structure of the albino rabbit iris with special reference to the identification of adrenergic and cholinergic nerves and nerve endings in its intrinsic muscles. American Journal of Anatomy, 114, 173–205.
Rieger, R. M., & Rieger, G. E. (1975). Fine structure of the pharyngeal bulb in Trilobodrilus and its phylogenetic significance within Archiannelida. Tissue and Cell, 7(2), 267–279.
Rivlin, P. K., St Clair, R. M., Vilinsky, I., & Deitcher, D. L. (2004). Morphology and molecular organization of the adult neuromuscular junction of Drosophila. Journal of Comparative Neurology, 468(4), 596–613.
Robertson, J. D. (1956). The ultrastructure of a reptilian myoneural junction. The Journal of Biophysical and Biochemical Cytology, 2(4), 381–394.
Robinson, P. M., Perry, R. A., Hardy, K. J., Coghlan, J. P., & Scoggins, B. A. (1977). Innervation of adrenal-cortex in sheep, Ovis ovis. Journal of Anatomy, 124, 117–129.
Roelandse, M., Welman, A., Wagner, U., Hagmann, J., & Matus, A. (2003). Focal motility determines the geometry of dendritic spines. Neuroscience, 121(1), 39–49.
Rogers, D. C. (1969). Fine structure of smooth muscle and neuromuscular junctions in the foot of Helix aspersa. Zeitschrift für Zellforschung und mikroskopische Anatomie, 99(3), 315–335.
Rogers, D. C., & Burnstock, G. (1966). Muliaxonal autonomic junctions in intestinal smooth muscle of the toad (Bufo marinus). Journal of Comparative Neurology, 126(4), 625–652.
Ronnevi, L. O. (1977). Spontaneous phagocytosis of boutons on spinal motoneurons during early postnatal-development—Electron microscopical study in cat. Journal of Neurocytology, 6(5), 487–504.
Ronnevi, L. O. (1979). Spontaneous phagocytosis of C-type synaptic terminals by spinal alpha-motoneurons in newborn kittens. An electron microscopic study. Brain Research, 162(2), 189–199.
Rosenbluth, J. (1965). Ultrastructure of somatic muscle cells in Ascaris lumbricoides. II. Intermuscular junctions, neuromuscular junctions, and glycogen stores. Journal of Cell Biology, 26(2), 579–591.
Rosenbluth, J. (1972). Myoneural junctions of two ultrastructurally distinct types in earthworm body wall muscle. Journal of Cell Biology, 54(3), 566–579.
Rosenbluth, J. (1973). Postjunctional membrane specialization at cholinergic myoneural junctions in the leech. Journal of Comparative Neurology, 151(4), 399–406.
Rosser, B. W. C., George, J. C., & Frombach, S. K. (1987). Architecture of the pectoralis-muscle of the Japanese-Quail (Coturnix japonica)—Histochemical and ultrastructural characterization, and distribution of muscle-fiber types. Canadian Journal of Zoology-Revue Canadienne De Zoologie, 65(1), 63–71.
Roth, A., & Tscharntke, H. (1976). Ultrastructure of the ampullary electroreceptors in lungfish and Brachiopterygii. Cell and Tissue Research, 173(1), 95–108.
Ryugo, D. K., Montey, K. L., Wright, A. L., Bennett, M. L., & Pongstaporn, T. (2006). Postnatal development of a large auditory nerve terminal: The endbulb of Held in cats. Hearing Research, 216–217, 100–115.
Saetersdal, T. S., Justesen, N. P., & Krohnstad, A. W. (1974). Ultrastructure and innervation of the teleostean atrium. Journal of Molecular and Cellular Cardiology, 6(5), 415–437.
Sakai, H., & Naka, K. I. (1983). Synaptic organization involving receptor, horizontal and on- and off-center bipolar cells in the catfish retina. Vision Research, 23(4), 339–351.
Sakai, H. M., & Naka, K. (1986). Synaptic organization of the cone horizontal cells in the catfish retina. Journal of Comparative Neurology, 245(1), 107–115.
Samuel, M. A., Zhang, Y., Meister, M., & Sanes, J. R. (2011). Age-related alterations in neurons of the mouse retina. Journal of Neuroscience, 31(44), 16033–16044.
Sanes, J. R., & Lichtman, J. W. (1999). Development of the vertebrate neuromuscular junction. Annual Review of Neuroscience, 22, 389–442.
Santa, T., Engel, A. G., & Lambert, E. G. (1972). Histometric study of neuromuscular junction ultrastructure. II. Myasthenic syndrome. Neurology, 22(4), 370–376.
Schiaffino, S., & Reggiani, C. (2011). Fiber types in mammalian skeletal muscles. Physiological Reviews, 91(4), 1447–1531.
Sethi, C. S., Lewis, G. P., Fisher, S. K., Leitner, W. P., Mann, D. L., Luthert, P. J., et al. (2005). Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Investigative Ophthalmology & Visual Science, 46(1), 329–342.
Shao, M., Hirsch, J. C., Giaume, C., & Peusner, K. D. (2004). Spontaneous synaptic activity in chick vestibular nucleus neurons during the perinatal period. Neuroscience, 127(1), 81–90.
Shaw, S. R. (1975). Retinal resistance barriers and electrical lateral inhibition. Nature, 255(5508), 480–483.
Shepherd, G. M. (2004). The Synaptic Organization of the Brain (5th ed.). New York: Oxford University Press.
Sherman, R. G. (1973). Ultrastructural features of cardiac muscle cells in a tarantula spider. Journal of Morphology, 140(2), 215–241.
Sherman, R. G., & Atwood, H. L. (1972). Correlated electrophysiological and ultrastructural studies of a crustacean motor unit. Journal of General Physiology, 59(5), 586–615.
Sherman, R. G., & Fourtner, C. R. (1972). Ultrastructural features of synaptic regions in walking leg muscles of the horseshoe crab, Limulus polyphemus (L.). Journal of Ultrastructure Research, 40(1), 44–54.
Sherman, R. G., & Luff, A. R. (1971). Structural features of tarsal claw muscles of spider Eurypelma marxi Simon. Canadian Journal of Zoology, 49(12), 1549–1556.
Shi, L., Fu, A. K. Y., & Ip, N. Y. (2012). Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends in Neurosciences, 35(7), 441–453.
Slater, C. R. (2008). Structural factors influencing the efficacy of neuromuscular transmission. Myasthenia Gravis and Related Disorders: 11th International Conference, 1132, 1–12.
Smith, D. S. (1960). Innervation of the fibrillar flight muscle of an insect: Tenebrio molitor (Coleoptera). The Journal of Biophysical and Biochemical Cytology, 8(2), 447–466.
Smith, D. S. (1966). The organization and function of the sarcoplasmic reticulum and T-system of muscle cells. Progress in Biophysics and Molecular Biology, 16, 107–142.
Smith, D. S. (1971). On the significance of cross-bridges between microtubules and synaptic vesicles. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 261(839), 395–405.
Sommer, J. R., & Johnson, E. A. (1968). Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. Journal of Cell Biology, 36(3), 497–526.
Sommer, J. R., & Johnson, E. A. (1969). Cardiac muscle. A comparative ultrastructural study with special reference to frog and chicken hearts. Zeitschrift für Zellforschung und mikroskopische Anatomie, 98(3), 437–468.
Sotelo, C., & Palay, S. L. (1968). The fine structure of the lateral vestibular nucleus in the rat. I. Neurons and neuroglial cells. Journal of Cell Biology, 36(1), 151–179.
Sotelo, C., & Palay, S. L. (1970). The fine structure of the later vestibular nucleus in the rat. II. Synaptic organization. Brain Research, 18(1), 93–115.
Spencer, A. N. (1979). Neurobiology of Polyorchis. II. Structure of effector systems. Journal of Neurobiology, 10(2), 95–117.
Stefanelli, A. (1937). Il sistema statico dei Petromizonti (sistema laterale, sistema vestibolare, cervelletto). I: Centri nervosi e vie centrali. Archivio Zoologico italiano, 24, 209–273.
Stefanelli, A., & Caravita, S. (1970). Ultrastructural features of the synaptic complex of the vestibular nuclei of Lampetra planeri (Bloch). Zeitschrift für Zellforschung und mikroskopische Anatomie, 108(2), 282–296.
Steinmetz, P. R., Kraus, J. E., Larroux, C., Hammel, J. U., Amon-Hassenzahl, A., Houliston, E., et al. (2012). Independent evolution of striated muscles in cnidarians and bilaterians. Nature, 487(7406), 231–234.
Sterling, P., & Demb, J. B. (2004). Retina. In G. M. Shepherd (Ed.), The Synaptic Organization of the Brain (5th ed., pp. 217–269). New York: Oxford University Press.
Sterling, P., & Matthews, G. (2005). Structure and function of ribbon synapses. Trends in Neurosciences, 28(1), 20–29.
Sulbarán, G., Alamo, L., Pinto, A., Márquez, G., Méndez, F., Padrón, R., et al. (2015). An invertebrate smooth muscle with striated muscle myosin filaments. Proceedings of the National Academy of Sciences USA, 112(42), E5660–E5668.
Sullivan, R. K., Woldemussie, E., & Pow, D. V. (2007). Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Investigative Ophthalmology & Visual Science, 48(6), 2782–2791.
Suwa, H., Gilland, E., & Baker, R. (1999). Otolith ocular reflex function of the tangential nucleus in teleost fish. Annals of the New York Academy of Sciences, 871, 1–14.
Suzuki, H., & Tasaki, K. (1983). Inhibitory retinal efferents from dopaminergic cells in the optic lobe of the octopus. Vision Research, 23(4), 451–457.
Szamier, R. B., & Bennett, M. V. (1973). Rapid degeneration of ampullary electroreceptor organs after denervation. Journal of Cell Biology, 56(2), 466–477.
Szamier, R. B., & Bennett, M. V. L. (1980). Ampullary electroreceptors in the fresh-water ray. Potamotrygon. Journal of Comparative Physiology, 138(3), 225–230.
Szamier, R. B., & Wachtel, A. W. (1969). Special cutaneous receptor organs of fish.3. Ampullary organs of Eigenmannia. Journal of Morphology, 128(3), 261–290.
Szpir, M. R., Sento, S., & Ryugo, D. K. (1990). Central projections of cochlear nerve fibers in the alligator lizard. Journal of Comparative Neurology, 295(4), 530–547.
Takasaka, T., & Smith, C. A. (1971). The structure and innervation of the pigeon’s basilar papilla. Journal of Ultrastructure Research, 35(1), 20–65.
Tan, X., Beurg, M., Hackney, C., Mahendrasingam, S., & Fettiplace, R. (2013). Electrical tuning and transduction in short hair cells of the chicken auditory papilla. Journal of Neurophysiology, 109(8), 2007–2020.
Tan, C. K., & Wong, W. C. (1980). Innervation of the muscularis externa in the stomach of a coral fish, Chelmon rostratus Cuvier. Journal of Anatomy, 130(Pt 2), 289–303.
Tatsukawa, T., Hirasawa, H., Kaneko, A., & Kaneda, M. (2005). GABA-mediated component in the feedback response of turtle retinal cones. Visual Neuroscience, 22(3), 317–324.
Taxi, J. (1964). Electron microscope study of the innervation of intestinal smooth muscle, compared to that of some other mammalian smooth muscles. Archives de Biologie (Liege), 75, 301–328.
Teeter, J. H., Szamier, R. B., & Bennett, M. V. L. (1980). Ampullary electroreceptors in the sturgeon Scaphirhynchus platorynchus (Rafinesque). Journal of Comparative Physiology, 138(3), 213–223.
Teräväinen, H. (1968a). Electron microscopic and histochemical observations on different types of nerve endings in the extraocular muscles of the rat. Zeitschrift für Zellforschung und mikroskopische Anatomie, 90(3), 372–388.
Teräväinen, H. (1968b). Development of the myoneural junction in the rat. Zeitschrift für Zellforschung und mikroskopische Anatomie, 87(2), 249–265.
Teräväinen, H. (1971). Anatomical and physiological studies on muscles of lamprey. Journal of Neurophysiology, 34(6), 954–973.
Terzibasi, E., Calamusa, M., Novelli, E., Domenici, L., Strettoi, E., & Cellerino, A. (2009). Age-dependent remodelling of retinal circuitry. Neurobiology of Aging, 30(5), 819–828.
Teuchert, G. (1977). Ultrastructure of marine gastrotrich Turbanella cornuta Remane (Macrodasyoidea) and its functional and phylogenetical importance. Zoomorphologie, 88(3), 189–246.
Thaemert, J. C. (1966). Ultrastructure of cardiac muscle and nerve contiguities. Journal of Cell Biology, 29(1), 156–162.
Thoreson, W. B., Babai, N., & Bartoletti, T. M. (2008). Feedback from horizontal cells to rod photoreceptors in vertebrate retina. Journal of Neuroscience, 28(22), 5691–5695.
Thoreson, W. B., & Mangel, S. C. (2012). Lateral interactions in the outer retina. Progress in Retinal and Eye Research, 31(5), 407–441.
Titmus, M. J. (1981). Ultrastructure of identified fast excitatory, slow excitatory and inhibitory neuromuscular junctions in the locust. Journal of Neurocytology, 10(3), 363–385.
Torres, L. F., & Duchen, L. W. (1987). The mutant mdx: Inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain, 110(Pt 2), 269–299.
Torroja, L., Packard, M., Gorczyca, M., White, K., & Budnik, V. (1999). The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. Journal of Neuroscience, 19(18), 7793–7803.
Trautwein, W., & Uchizono, K. (1963). Electron microscopic and electrophysiologic study of the pacemaker in the sino-atrial node of the rabbit heart. Zeitschrift für Zellforschung und mikroskopische Anatomie, 61, 96–109.
Trujillo-Cenóz, O., & Melamed, J. (1967). The fine structure o the visual system of Lycosa (Araneae: Lycosidae). II. Primary visual centers. Zeitschrift für Zellforschung und mikroskopische Anatomie, 76(3), 377–388.
Unsicker, K. (1971). Innervation of mammalian endocrine glands (anterior pituitary and parathyroids). Zeitschrift für Zellforschung und mikroskopische Anatomie, 121(2), 283–291.
Van den Berge, H., & Wirtz, P. (1989). Detailed morphology of the stapedius muscle of the rat. An integrated light microscopical, morphometrical, histochemical, immunohistochemical and electron microscopical study in relation to function. Journal of Anatomy, 166, 157–169.
Van Vactor, D., & Sigrist, S. J. (2017). Presynaptic morphogenesis, active zone organization and structural plasticity in Drosophila. Current Opinion in Neurobiology, 43, 119–129.
Vardi, N., Morigiwa, K., Wang, T. L., Shi, Y. J., & Sterling, P. (1998). Neurochemistry of the mammalian cone ‘synaptic complex’. Vision Research, 38(10), 1359–1369.
Vardi, N., & Sterling, P. (1994). Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Research, 34(10), 1235–1246.
Varela, C., Igartua, I., De la Rosa, E. J., & De la Villa, P. (2003). Functional modifications in rod bipolar cells in a mouse model of retinitis pigmentosa. Vision Research, 43(8), 879–885.
Veggetti, A., Mascarello, F., & Carpene, E. (1982). A comparative histochemical study of fibre types in middle ear muscles. Journal of Anatomy, 135(Pt 2), 333–352.
Wagner, N., Laugks, U., Heckmann, M., Asan, E., & Neuser, K. (2015). Aging Drosophila melanogaster display altered pre- and postsynaptic ultrastructure at adult neuromuscular junctions. Journal of Comparative Neurology, 523(16), 2457–2475.
Walrond, J. P., & Reese, T. S. (1985). Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. Journal of Neuroscience, 5(5), 1118–1131.
Wang, Y. X., Wenthold, R. J., Ottersen, O. P., & Petralia, R. S. (1998). Endbulb synapses in the anteroventral cochlear nucleus express a specific subset of AMPA-type glutamate receptor subunits. Journal of Neuroscience, 18(3), 1148–1160.
Watanabe, T., & Yasuda, M. (1977). Electron microscopic study on the innervation of the pancreas of the domestic fowl. Cell and Tissue Research, 180(4), 453–465.
Watari, N. (1968). Fine structure of nervous elements in the pancreas of some vertebrates. Zeitschrift für Zellforschung und mikroskopische Anatomie, 85(3), 291–314.
Weckström, M., & Laughlin, S. (2010). Extracellular potentials modify the transfer of information at photoreceptor output synapses in the blowfly compound eye. Journal of Neuroscience, 30(28), 9557–9566.
Weiler, R., & Wagner, H. J. (1984). Light-dependent change of cone-horizontal cell-Interactions in carp retina. Brain Research, 298(1), 1–9.
Westfall, J. A., Elliott, C. F., & Carlin, R. W. (2002). Ultrastructural evidence for two-cell and three-cell neural pathways in the tentacle epidermis of the sea anemone Aiptasia pallida. Journal of Morphology, 251(1), 83–92.
Westfall, J. A., Kinnamon, J. C., & Sims, D. E. (1980). Neuro-epitheliomuscular cell and neuro-neuronal gap junctions in Hydra. Journal of Neurocytology, 9(6), 725–732.
Westfall, J. A., Yamataka, S., & Enos, P. D. (1971). Ultrastructural evidence of polarized synapses in the nerve net of Hydra. Journal of Cell Biology, 51(1), 318–323.
Wilson, V. J., & Wylie, R. M. (1970). A short-latency labyrinthine input to the vestibular nuclei in the pigeon. Science, 168(3927), 124–127.
Wolf, H., & Harzsch, S. (2002). Evolution of the arthropod neuromuscular system. 2. Inhibitory innervation of the walking legs of a scorpion: Vaejovis spinigerus (Wood, 1863), Vaejovidae, Scorpiones, Arachnida. Arthropod Structure & Development, 31(3), 203–215.
Wong, W. C. (1977). Ultrastructural localization of adrenergic nerve terminals in the circular muscle layer and muscularis mucosae of the rat duodenum after acute treatment with 6-hydroxydopamine. Journal of Anatomy, 124(Pt 3), 637–642.
Woods, R. I. (1970). The innervation of the frog’s heart. 3. Electronmicroscopy of the autonomic nerve fibres and their vesicles. Proceedings of the Royal Society of London, Series B: Biological Sciences, 176(1042), 63–68.
Yaksta-Sauerland, B. A., & Coggeshall, R. E. (1973). Neuromuscular junctions in the leech. Journal of Comparative Neurology, 151(1), 85–100.
Yamauchi, A., & Burnstock, G. (1968). An electron microscopic study on the innervation of the trout heart. Journal of Comparative Neurology, 132(4), 567–588.
Yazulla, S., Studholme, K. M., Vitorica, J., & de Blas, A. L. (1989). Immunocytochemical localization of GABAA receptors in goldfish and chicken retinas. Journal of Comparative Neurology, 280(1), 15–26.
Ylä-Anttila, P., Vihinen, H., Jokitalo, E., & Eskelinen, E. L. (2009). Monitoring autophagy by electron microscopy in Mammalian cells. Methods in Enzymology, 452, 143–164.
Yoshida, T., Uchigashima, M., Yamasaki, M., Katona, I., Yamazaki, M., Sakimura, K., et al. (2011). Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proceedings of the National Academy of Sciences USA, 108(7), 3059–3064.
Zebe, E., & Rathmayer, W. (1968). Elektronenmikroskopische untersuchungen an spinnenmuskeln. Zeitschrift für Zellforschung und mikroskopische Anatomie, 92, 377–387.
Zeni, C., & Zaffagnini, F. (1988). Occurrence of innervation in labral glands of Daphnia obtusa (Crustacea, Cladocera). Journal of Morphology, 198(1), 43–48.
Acknowledgements
This work was supported by the Intramural Research Programs of NIH/NIDCD and NIH/NIA. The code and animal protocol for the Advanced Imaging Core of NIDCD is ZIC DC000081 and 1167-16, respectively.
Author information
Authors and Affiliations
Corresponding author
Glossary
- Active zone
-
The area of the presynaptic membrane where presynaptic vesicles release neurotransmitter. In the structures described in this review, the active zones are located in the portion of the presynaptic terminal that lies within the postsynaptic invagination
- Gutter
-
A postsynaptic indention or shallow invagination on the surface of some muscle fibers; in these cases, the presynaptic terminal runs parallel to the surface of the muscle cell and forms the neuromuscular junction along the gutter (e.g., Fig. 10a)
- Indenting terminals
-
Presynaptic terminals that are enclosed partially within a postsynaptic invagination (indention); see definitions of invaginating/invaginated terminals and postsynaptic invagination
- Invaginating/invaginated terminals
-
Presynaptic terminals that are enclosed only partially or entirely within a postsynaptic invagination; if only partially, then the postsynaptic structure can be described as indented. The term “invaginate” is based here on standard definitions, including “to fold up or enclose in a sheath-like or pouch-like structure” (Wiktionary) and “to insert or receive, as into a sheath” (Dictionary.com)
- Motor ending
-
Presynaptic terminal junctions formed on muscle or gland cells
- Postsynaptic invagination
-
An infolding of the postsynaptic membrane to form a cavity (pouch, sheath) that contains the invaginated structure; it can contain the entire presynaptic terminal, or part of the terminal (postsynaptic indention) or an invaginated projection or protrusion from the presynaptic terminal
- Protrusions
-
The Merriam–Webster Dictionary (online) describes extensions beyond the normal surface of a structure as projections, protrusions, protuberances, and bulges. A protrusion is an extension that seems to be a deformity that is thrust out from the surface. More specifically for this review, we define typically wide, invaginating extensions of presynaptic terminals, containing active zones within the extensions, as types of invaginating protrusions (see Fig. 1b3)
- Sarcoplasmic reticulum (SR) and subsynaptic reticulum (SSR)
-
SR denotes the endoplasmic reticulum (ER) found in the sarcoplasm, i.e., the cytoplasm of a muscle cell. SSR refers to SR, often elaborate, associated with the postsynaptic membrane in many arthropod neuromuscular junctions
- Subjunctional folds
-
Multiple postsynaptic invaginations, often deep and convoluted, found in many kinds of vertebrate neuromuscular junctions. Typically, these lack any extensions of the presynaptic membrane, but they do contain extensions of the basal lamina from the synaptic cleft
Rights and permissions
About this article
Cite this article
Petralia, R.S., Wang, YX., Mattson, M.P. et al. Invaginating Presynaptic Terminals in Neuromuscular Junctions, Photoreceptor Terminals, and Other Synapses of Animals. Neuromol Med 19, 193–240 (2017). https://doi.org/10.1007/s12017-017-8445-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-017-8445-y