Abstract
Diabetic foot ulcer (DFU) is a complication from incomplete or prolonged wound healing, at times requires amputation, putting substantial health and socioeconomic burden. Wound healing is a dynamic overlapping process that can be regulated by arrays of molecular factors showing redundancy in function. However, dysregulation in the mechanism of angiogenesis, extra cellular matrix (ECM) formation and immune modulation are the major causes for impair wound healing in hyperglycaemic patients. Despite development of wound care research, there is a lack of well-accepted targeted therapy with multidisciplinary approach for DFU treatment. Stem cell therapy holds a promising outcome both in preclinical and clinical trials because of its ability to promote healing via regeneration and specialized tissue differentiation. Among different types of stem cells, regenerative potential of mesenchymal stem cell (MSC) is well demonstrated in both experimental and clinical trial. Still there is a huge knowledge gap among medical practitioners for deciding the best stem cell source, administration route, and safety. This review strengthens the fact that why stem cell therapy is a promising candidate to treat DFU and cited multiple tissue engineering and biomaterial-based approaches for delivering stem cells and their aftermath paracrine events. Based on the pre-clinical and clinical studies, the review tried to come up with optimum stem cell source and delivery route for the treatment of DFU. At last, the review glances on possible direction to enhance therapeutics strategy for the same, including different approaches like: phytocompounds, exosomes, scaffold geometry, cell preconditioning and licensing etc.
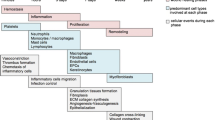
Graphical Abstract







Similar content being viewed by others
Data availability
Not applicable
Code availability
Not applicable
References
Aumiller, W. D., & Dollahite, H. A. (2015). Pathogenesis and management of diabetic foot ulcers. JAAPA: Official Journal of the American Academy of Physician Assistants, 28(5), 28–34. https://doi.org/10.1097/01.JAA.0000464276.44117.b1
Volmer-Thole, M., & Lobmann, R. (2016). Neuropathy and Diabetic Foot Syndrome. International Journal of Molecular Sciences, 17(6), 917. https://doi.org/10.3390/ijms17060917
Al-Rubeaan, K., Al Derwish, M., Ouizi, S., Youssef, A. M., Subhani, S. N., Ibrahim, H. M., & Alamri, B. N. (2015). Diabetic foot complications and their risk factors from a large retrospective cohort study. PloS One, 10(5), e0124446. https://doi.org/10.1371/journal.pone.0124446
Lim, J. Z. M., Ng, N. S. L., & Thomas, C. (2017). Prevention and treatment of diabetic foot ulcers. Journal of the Royal Society of Medicine, 110(3), 104–109. https://doi.org/10.1177/0141076816688346
Khanolkar, M. P., Bain, S. C., & Stephens, J. W. (2008). The diabetic foot. QJM, 101(9), 685–695. https://doi.org/10.1093/qjmed/hcn027
Jeffcoate, W. J., & Harding, K. G. (2003). Diabetic foot ulcers. Lancet (London, England), 361(9368), 1545–1551. https://doi.org/10.1016/S0140-6736(03)13169-8
Azhar, A., Basheer, M., Abdelgawad, M. S., Roshdi, H., & Kamel, M. F. (2021). Prevalence of peripheral arterial disease in diabetic foot ulcer patients and its impact in limb salvage. The International Journal of Lower Extremity Wounds, 15347346211027063. https://doi.org/10.1177/15347346211027063
Bandyk, D. F. (2018). The diabetic foot: pathophysiology, evaluation, and treatment. Seminars in Vascular Surgery, 31(2–4), 43–48. https://doi.org/10.1053/j.semvascsurg.2019.02.001
Andrews, K. L., Houdek, M. T., & Kiemele, L. J. (2015). Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthetics and Orthotics International, 39(1), 29–39. https://doi.org/10.1177/0309364614534296
Bader, M. S. (2008). Diabetic foot infection. American Family Physician, 78(1), 71–79.
Saeed, K., Esposito, S., Akram, A., Ascione, T., Bal, A. M., Bassetti, M., … Yalcin, A. N. (2020). Hot topics in diabetic foot infection. International Journal of Antimicrobial Agents, 55(6), 105942. https://doi.org/10.1016/j.ijantimicag.2020.105942
Mavrogenis, A. F., Megaloikonomos, P. D., Antoniadou, T., Igoumenou, V. G., Panagopoulos, G. N., Dimopoulos, L., … Lazaris, A. (2018). Current concepts for the evaluation and management of diabetic foot ulcers. EFORT open reviews, 3(9), 513–525. https://doi.org/10.1302/2058-5241.3.180010
Monteiro-Soares, M., Boyko, E. J., Jeffcoate, W., Mills, J. L., Russell, D., Morbach, S., & Game, F. (2020). Diabetic foot ulcer classifications: A critical review. Diabetes/Metabolism Research and Reviews, 36(Suppl 1), e3272. https://doi.org/10.1002/dmrr.3272
Schaper, N. C. (2004). Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes/Metabolism Research and Reviews, 20(S1), S90–S95. https://doi.org/10.1002/dmrr.464
Wang, X., Yuan, C.-X., Xu, B., & Yu, Z. (2022). Diabetic foot ulcers: Classification, risk factors and management. World Journal of Diabetes, 13(12), 1049–1065. https://doi.org/10.4239/wjd.v13.i12.1049
Wagner, F. W. (1981). The dysvascular foot: a system for diagnosis and treatment. Foot & Ankle, 2(2), 64–122. https://doi.org/10.1177/107110078100200202
Lavery, L. A., Armstrong, D. G., Wunderlich, R. P., Mohler, M. J., Wendel, C. S., & Lipsky, B. A. (2006). Risk factors for foot infections in individuals with diabetes. Diabetes Care, 29(6), 1288–1293. https://doi.org/10.2337/dc05-2425
Armstrong, D. G., Lavery, L. A., & Harkless, L. B. (1998). Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care, 21(5), 855–859. https://doi.org/10.2337/diacare.21.5.855
American Diabetes Association. (2014). Standards of Medical Care in Diabetes—2014. Diabetes Care, 37(Supplement 1), S14–S80. https://doi.org/10.2337/dc14-S014
Ince, P., Abbas, Z. G., Lutale, J. K., Basit, A., Ali, S. M., Chohan, F., … Jeffcoate, W. J. (2008). Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents. Diabetes Care, 31(5), 964–967. https://doi.org/10.2337/dc07-2367
Clayton, W., & Elasy, T. A. (2009). A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clinical Diabetes, 27(2), 52–58. https://doi.org/10.2337/diaclin.27.2.52
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., … Williams, R. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice, 157, 107843. https://doi.org/10.1016/j.diabres.2019.107843
Hoffstad, O., Mitra, N., Walsh, J., & Margolis, D. J. (2015). Diabetes, lower-extremity amputation, and death. Diabetes Care, 38(10), 1852–1857. https://doi.org/10.2337/dc15-0536
Edmonds, M., Manu, C., & Vas, P. (2021). The current burden of diabetic foot disease. Journal of Clinical Orthopaedics and Trauma, 17, 88–93. https://doi.org/10.1016/j.jcot.2021.01.017
Zhang, P., Lu, J., Jing, Y., Tang, S., Zhu, D., & Bi, Y. (2017). Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Annals of Medicine, 49(2), 106–116. https://doi.org/10.1080/07853890.2016.1231932
Mishra, S. C., Chhatbar, K. C., Kashikar, A., & Mehndiratta, A. (2017). Diabetic foot. BMJ (Clinical Research ed.), 359, j5064. https://doi.org/10.1136/bmj.j5064
Nourian Dehkordi, A., Mirahmadi Babaheydari, F., Chehelgerdi, M., & Raeisi Dehkordi, S. (2019). Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Research & Therapy, 10(1), 111. https://doi.org/10.1186/s13287-019-1212-2
Blakytny, R., & Jude, E. B. (2009). Altered molecular mechanisms of diabetic foot ulcers. The International Journal of Lower Extremity Wounds, 8(2), 95–104. https://doi.org/10.1177/1534734609337151
Spampinato, S. F., Caruso, G. I., De Pasquale, R., Sortino, M. A., & Merlo, S. (2020). The treatment of impaired wound healing in diabetes: looking among old drugs. Pharmaceuticals, 13(4), 60. https://doi.org/10.3390/ph13040060
Galkowska, H., Wojewodzka, U., & Olszewski, W. L. (2006). Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair and Regeneration, 14(5), 558–565. https://doi.org/10.1111/j.1743-6109.2006.00155.x
Martin, A., Komada, M. R., & Sane, D. C. (2003). Abnormal angiogenesis in diabetes mellitus. Medicinal Research Reviews, 23(2), 117–145. https://doi.org/10.1002/med.10024
Hickey, M. M., & Simon, M. C. (2006). Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Current Topics in Developmental Biology, 76, 217–257. https://doi.org/10.1016/S0070-2153(06)76007-0
Catrina, S.-B., Okamoto, K., Pereira, T., Brismar, K., & Poellinger, L. (2004). Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes, 53(12), 3226–3232. https://doi.org/10.2337/diabetes.53.12.3226
Boeringer, T., Gould, L. J., & Koria, P. (2020). Protease-resistant growth factor formulations for the healing of chronic wounds. Advances in Wound Care, 9(11), 612–622. https://doi.org/10.1089/wound.2019.1043
Yasuda, H., Terada, M., Maeda, K., Kogawa, S., Sanada, M., Haneda, M., … Kikkawa, R. (2003). Diabetic neuropathy and nerve regeneration. Progress in Neurobiology, 69(4), 229–285. https://doi.org/10.1016/s0301-0082(03)00034-0
Rando, T. A. (2006). Stem cells, ageing and the quest for immortality. Nature, 441(7097), 1080–1086. https://doi.org/10.1038/nature04958
Perez-Favila, A., Martinez-Fierro, M. L., Rodriguez-Lazalde, J. G., Cid-Baez, M. A., Zamudio-Osuna, M. de J., Martinez-Blanco, Ma. del R., … Garza-Veloz, I. (2019). Current Therapeutic Strategies in Diabetic Foot Ulcers. Medicina, 55(11), 714. https://doi.org/10.3390/medicina55110714
Liu, R., Li, L., Yang, M., Boden, G., & Yang, G. (2013). Systematic review of the effectiveness of hyperbaric oxygenation therapy in the management of chronic diabetic foot ulcers. Mayo Clinic Proceedings, 88(2), 166–175. https://doi.org/10.1016/j.mayocp.2012.10.021
Alexiadou, K., & Doupis, J. (2012). Management of diabetic foot ulcers. Diabetes Therapy, 3(1), 4. https://doi.org/10.1007/s13300-012-0004-9
Cychosz, C. C., Phisitkul, P., Belatti, D. A., & Wukich, D. K. (2016). Preventive and therapeutic strategies for diabetic foot ulcers. Foot & Ankle International, 37(3), 334–343. https://doi.org/10.1177/1071100715611951
Everett, E., & Mathioudakis, N. (2018). Update on management of diabetic foot ulcers: diabetic foot ulcers. Annals of the New York Academy of Sciences, 1411(1), 153–165. https://doi.org/10.1111/nyas.13569
Moura, L. I. F., Dias, A. M. A., Carvalho, E., & de Sousa, H. C. (2013). Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomaterialia, 9(7), 7093–7114. https://doi.org/10.1016/j.actbio.2013.03.033
Téllez, G. A., & Castaño, J. C. (2010). Péptidos antimicrobianos. Infectio, 14(1), 55–67. https://doi.org/10.1016/S0123-9392(10)70093-X
Raghuram, A. C., Yu, R. P., Lo, A. Y., Sung, C. J., Bircan, M., Thompson, H. J., & Wong, A. K. (2020). Role of stem cell therapies in treating chronic wounds: A systematic review. World Journal of Stem Cells, 12(7), 659–675. https://doi.org/10.4252/wjsc.v12.i7.659
Izadi, M., Kheirjou, R., Mohammadpour, R., Aliyoldashi, M. H., Moghadam, S. J., Khorvash, F., … khalili, N. (2019). Efficacy of comprehensive ozone therapy in diabetic foot ulcer healing. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 13(1), 822–825. https://doi.org/10.1016/j.dsx.2018.11.060
Hotkar, M. S., Avachat, A. M., Bhosale, S. S., & Oswal, Y. M. (2015). Preliminary investigation of topical nitroglycerin formulations containing natural wound healing agent in diabetes-induced foot ulcer. International Wound Journal, 12(2), 210–217. https://doi.org/10.1111/iwj.12084
Li, N., Zhan, A., Jiang, Y., & Liu, H. (2022). A novel matrix metalloproteinases-cleavable hydrogel loading deferoxamine accelerates diabetic wound healing. International Journal of Biological Macromolecules, 222, 1551–1559. https://doi.org/10.1016/j.ijbiomac.2022.09.185
Gasca-Lozano, L. E., Lucano-Landeros, S., Ruiz-Mercado, H., Salazar-Montes, A., Sandoval-Rodríguez, A., Garcia-Bañuelos, J., … Armendariz-Borunda, J. (2017). Pirfenidone Accelerates Wound Healing in Chronic Diabetic Foot Ulcers: A Randomized, Double-Blind Controlled Trial. Journal of Diabetes Research, 2017, 1–12. https://doi.org/10.1155/2017/3159798
Ramirez-Acuña, J. M., Cardenas-Cadena, S. A., Marquez-Salas, P. A., Garza-Veloz, I., Perez-Favila, A., Cid-Baez, M. A., … Martinez-Fierro, M. L. (2019). Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics (Basel, Switzerland), 8(4), 193. https://doi.org/10.3390/antibiotics8040193
Forsythe, R. O., Brownrigg, J., & Hinchliffe, R. J. (2015). Peripheral arterial disease and revascularization of the diabetic foot. Diabetes, Obesity and Metabolism, 17(5), 435–444. https://doi.org/10.1111/dom.12422
Mao, A. S., & Mooney, D. J. (2015). Regenerative medicine: Current therapies and future directions. Proceedings of the National Academy of Sciences, 112(47), 14452–14459. https://doi.org/10.1073/pnas.1508520112
Gurtner, G. C., & Chapman, M. A. (2016). Regenerative medicine: charting a new course in wound healing. Advances in Wound Care, 5(7), 314–328. https://doi.org/10.1089/wound.2015.0663
Farahani, M., & Shafiee, A. (2021). Wound healing: from passive to smart dressings. Advanced Healthcare Materials, 10(16), 2100477. https://doi.org/10.1002/adhm.202100477
Yu, Q., Qiao, G., Wang, M., Yu, L., Sun, Y., Shi, H., & Ma, T. (2022). Stem cell-based therapy for diabetic foot ulcers. Frontiers in Cell and Developmental Biology, 10, 812262. https://doi.org/10.3389/fcell.2022.812262
Chen, M., Przyborowski, M., & Berthiaume, F. (2009). stem cells for skin tissue engineering and wound healing. Critical Reviews™ in Biomedical Engineering, 37(4–5), 399–421. https://doi.org/10.1615/CritRevBiomedEng.v37.i4-5.50
Sun, Y., Zhao, J., Zhang, L., Li, Z., & Lei, S. (2022). Effectiveness and safety of stem cell therapy for diabetic foot: a meta-analysis update. Stem Cell Research & Therapy, 13(1), 416. https://doi.org/10.1186/s13287-022-03110-9
Gadelkarim, M., Abushouk, A. I., Ghanem, E., Hamaad, A. M., Saad, A. M., & Abdel-Daim, M. M. (2018). Adipose-derived stem cells: effectiveness and advances in delivery in diabetic wound healing. Biomedicine & Pharmacotherapy, 107, 625–633. https://doi.org/10.1016/j.biopha.2018.08.013
Yang, M., Sheng, L., Zhang, T. R., & Li, Q. (2013). Stem cell therapy for lower extremity diabetic ulcers: where do we stand? BioMed Research International, 2013, 1–8. https://doi.org/10.1155/2013/462179
Kamal, M. M., & Kassem, D. H. (2020). Therapeutic potential of wharton’s jelly mesenchymal stem cells for diabetes: achievements and challenges. Frontiers in Cell and Developmental Biology, 8, 16. https://doi.org/10.3389/fcell.2020.00016
Tan, S. T., Firmansyah, Y., & Elizabeth, J. (2020). New approach to deep diabetic foot ulcer (DFU) treatment-potential of secretome from Wharton’s jelly mesenchymal stem cell therapy. International Journal of Dermatology, Venereology and Leprosy Sciences, 3(2), 21–26. https://doi.org/10.33545/26649411.2020.v3.i2a.41
Amin, A. H., Abd Elmageed, Z. Y., Nair, D., Partyka, M. I., Kadowitz, P. J., Belmadani, S., & Matrougui, K. (2010). Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Laboratory Investigation, 90(7), 985–996. https://doi.org/10.1038/labinvest.2010.86
O’Loughlin, A., Kulkarni, M., Creane, M., Vaughan, E. E., Mooney, E., Shaw, G., … O’Brien, T. (2013). Topical Administration of Allogeneic Mesenchymal Stromal Cells Seeded in a Collagen Scaffold Augments Wound Healing and Increases Angiogenesis in the Diabetic Rabbit Ulcer. Diabetes, 62(7), 2588–2594. https://doi.org/10.2337/db12-1822
Shen, L., Zeng, W., Wu, Y.-X., Hou, C.-L., Chen, W., Yang, M.-C., … Zhu, C.-H. (2013). Neurotrophin-3 Accelerates Wound Healing in Diabetic Mice by Promoting a Paracrine Response in Mesenchymal Stem Cells. Cell Transplantation, 22(6), 1011–1021. https://doi.org/10.3727/096368912X657495
Kato, J., Kamiya, H., Himeno, T., Shibata, T., Kondo, M., Okawa, T., … Nakamura, J. (2014). Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. Journal of Diabetes and its Complications, 28(5), 588–595. https://doi.org/10.1016/j.jdiacomp.2014.05.003
Wan, J., Xia, L., Liang, W., Liu, Y., & Cai, Q. (2013). Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. Journal of Diabetes Research, 2013, 1–11. https://doi.org/10.1155/2013/647107
Uchiyama, A., Motegi, S., Sekiguchi, A., Fujiwara, C., Perera, B., Ogino, S., … Ishikawa, O. (2017). Mesenchymal stem cells-derived MFG-E8 accelerates diabetic cutaneous wound healing. Journal of Dermatological Science, 86(3), 187–197. https://doi.org/10.1016/j.jdermsci.2017.02.285
Lee, K.-B., Choi, J., Cho, S.-B., Chung, J.-Y., Moon, E.-S., Kim, N.-S., & Han, H.-J. (2011). Topical embryonic stem cells enhance wound healing in diabetic rats: effect of esc on diabetic wound healing. Journal of Orthopaedic Research, 29(10), 1554–1562. https://doi.org/10.1002/jor.21385
Loretelli, C., Ben Nasr, M., Giatsidis, G., Bassi, R., Lancerotto, L., D’Addio, F., … Fiorina, P. (2020). Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetologica, 57(7), 883–890. https://doi.org/10.1007/s00592-020-01500-0
Kuo, Y.-R., Wang, C.-T., Cheng, J.-T., Kao, G.-S., Chiang, Y.-C., & Wang, C.-J. (2016). Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplantation, 25(1), 71–81. https://doi.org/10.3727/096368915X687921
Kato, Y., Iwata, T., Morikawa, S., Yamato, M., Okano, T., & Uchigata, Y. (2015). Allogeneic transplantation of an adipose-derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes, 64(8), 2723–2734. https://doi.org/10.2337/db14-1133
Shi, R., Jin, Y., Cao, C., Han, S., Shao, X., Meng, L., … Li, M. (2016). Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Research & Therapy, 7(1), 155. https://doi.org/10.1186/s13287-016-0412-2
De Gregorio, C., Contador, D., Díaz, D., Cárcamo, C., Santapau, D., Lobos-Gonzalez, L., … Ezquer, F. (2020). Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Research & Therapy, 11(1), 168. https://doi.org/10.1186/s13287-020-01680-0
Maharlooei, M. K., Bagheri, M., Solhjou, Z., Jahromi, B. M., Akrami, M., Rohani, L., … Omrani, G. R. (2011). Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Research and Clinical Practice, 93(2), 228–234. https://doi.org/10.1016/j.diabres.2011.04.018
An, R., Zhang, Y., Qiao, Y., Song, L., Wang, H., & Dong, X. (2020). Adipose stem cells isolated from diabetic mice improve cutaneous wound healing in streptozotocin-induced diabetic mice. Stem Cell Research & Therapy, 11(1), 120. https://doi.org/10.1186/s13287-020-01621-x
Amos, P. J., Kapur, S. K., Stapor, P. C., Shang, H., Bekiranov, S., Khurgel, M., … Katz, A. J. (2010). Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Engineering Part A, 16(5), 1595–1606. https://doi.org/10.1089/ten.tea.2009.0616
Navone, S. E., Pascucci, L., Dossena, M., Ferri, A., Invernici, G., Acerbi, F., … Parati, E. A. (2014). Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Research & Therapy, 5(1), 7. https://doi.org/10.1186/scrt396
Zhao, Q.-S., Xia, N., Zhao, N., Li, M., Bi, C.-L., Zhu, Q., … Cheng, Z.-F. (2014). Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. International Journal of Biological Sciences, 10(1), 80–89. https://doi.org/10.7150/ijbs.7237
Zhang, C., Huang, L., Wang, X., Zhou, X., Zhang, X., Li, L., … Zhou, X. (2022). Topical and intravenous administration of human umbilical cord mesenchymal stem cells in patients with diabetic foot ulcer and peripheral arterial disease: a phase I pilot study with a 3-year follow-up. Stem Cell Research & Therapy, 13(1), 451. https://doi.org/10.1186/s13287-022-03143-0
Yue, C., Guo, Z., Luo, Y., Yuan, J., Wan, X., & Mo, Z. (2020). c-Jun overexpression accelerates wound healing in diabetic rats by human umbilical cord-derived mesenchymal stem cells. Stem Cells International, 2020, 1–10. https://doi.org/10.1155/2020/7430968
Shi, R., Lian, W., Jin, Y., Cao, C., Han, S., Yang, X., … Zhao, H. (2020). Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochimica et Biophysica Sinica, 52(6), 620–630. https://doi.org/10.1093/abbs/gmaa039
Zhang, Y., Pan, Y., Liu, Y., Li, X., Tang, L., Duan, M., … Zhang, G. (2021). Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Research & Therapy, 12(1), 434. https://doi.org/10.1186/s13287-021-02517-0
Wang, H., Chen, L., Liu, Y., Luo, B., Xie, N., Tan, T., … Luo, M. (2016). Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. American Journal of Translational Research, 8(11), 4912–4921.
Francki, A., Labazzo, K., He, S., Baum, E. Z., Abbot, S. E., Herzberg, U., … Lamensdorf, I. (2016). Angiogenic properties of human placenta-derived adherent cells and efficacy in hindlimb ischemia. Journal of Vascular Surgery, 64(3), 746-756.e1. https://doi.org/10.1016/j.jvs.2015.04.387
Yoon, B. S., Moon, J.-H., Jun, E. K., Kim, J., Maeng, I., Kim, J. S., … You, S. (2010). Secretory profiles and wound healing effects of human amniotic fluid–derived mesenchymal stem cells. Stem Cells and Development, 19(6), 887–902. https://doi.org/10.1089/scd.2009.0138
Zhao, L., Guo, Z., Chen, K., Yang, W., Wan, X., Zeng, P., … Mo, Z. (2020). Combined transplantation of mesenchymal stem cells and endothelial colony-forming cells accelerates refractory diabetic foot ulcer healing. Stem Cells International, 2020, 1–13. https://doi.org/10.1155/2020/8863649
Di Nicola, M., Carlo-Stella, C., Magni, M., Milanesi, M., Longoni, P. D., Matteucci, P., … Gianni, A. M. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood, 99(10), 3838–3843. https://doi.org/10.1182/blood.V99.10.3838
Le Blanc, K. (2003). Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy, 5(6), 485–489. https://doi.org/10.1080/14653240310003611
Kim, K. H., Blasco-Morente, G., Cuende, N., & Arias-Santiago, S. (2017). Mesenchymal stromal cells: properties and role in management of cutaneous diseases. Journal of the European Academy of Dermatology and Venereology, 31(3), 414–423. https://doi.org/10.1111/jdv.13934
Al-Khaldi, A., Al-Sabti, H., Galipeau, J., & Lachapelle, K. (2003). Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. The Annals of Thoracic Surgery, 75(1), 204–209. https://doi.org/10.1016/S0003-4975(02)04291-1
Vojtassák, J., Danisovic, L., Kubes, M., Bakos, D., Jarábek, L., Ulicná, M., & Blasko, M. (2006). Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinology Letters, 27(Suppl 2), 134–137.
Lopes, L., Setia, O., Aurshina, A., Liu, S., Hu, H., Isaji, T., … Dardik, A. (2018). Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Research & Therapy, 9(1), 188. https://doi.org/10.1186/s13287-018-0938-6
Dash, S. N., Dash, N. R., Guru, B., & Mohapatra, P. C. (2014). Towards reaching the target: clinical application of mesenchymal stem cells for diabetic foot ulcers. Rejuvenation Research, 17(1), 40–53. https://doi.org/10.1089/rej.2013.1467
Mansilla, E., Marín, G. H., Berges, M., Scafatti, S., Rivas, J., Núñez, A., … Tarditti, A. (2015). Cadaveric bone marrow mesenchymal stem cells: first experience treating a patient with large severe burns. Burns & Trauma, 3, s41038-015-0018–4. https://doi.org/10.1186/s41038-015-0018-4
de Mayo, T., Conget, P., Becerra-Bayona, S., Sossa, C. L., Galvis, V., & Arango-Rodríguez, M. L. (2017). The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. PLoS One, 12(6), e0177533. https://doi.org/10.1371/journal.pone.0177533
Becerra-Bayona, S. M., Solarte-David, V. A., Sossa, C. L., Mateus, L. C., Villamil, M., Pereira, J., & Arango-Rodríguez, M. L. (2020). Mesenchymal stem cells derivatives as a novel and potential therapeutic approach to treat diabetic foot ulcers. Endocrinology, Diabetes & Metabolism Case Reports, 2020. https://doi.org/10.1530/EDM-19-0164
Blumberg, S. N., Berger, A., Hwang, L., Pastar, I., Warren, S. M., & Chen, W. (2012). The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Research and Clinical Practice, 96(1), 1–9. https://doi.org/10.1016/j.diabres.2011.10.032
Falanga, V. (2005). Wound healing and its impairment in the diabetic foot. The Lancet, 366(9498), 1736–1743. https://doi.org/10.1016/S0140-6736(05)67700-8
Shafiee, S., Heidarpour, M., Sabbagh, S., Amini, E., Saffari, H., Dolati, S., & Meamar, R. (2021). Stem cell transplantation therapy for diabetic foot ulcer: a narrative review. Asian Biomedicine, 15(1), 3–18. https://doi.org/10.2478/abm-2021-0002
El Hage, R., Knippschild, U., Arnold, T., & Hinterseher, I. (2022). Stem cell-based therapy: a promising treatment for diabetic foot ulcer. Biomedicines, 10(7), 1507. https://doi.org/10.3390/biomedicines10071507
Cao, Y., Gang, X., Sun, C., & Wang, G. (2017). Mesenchymal stem cells improve healing of diabetic foot ulcer. Journal of Diabetes Research, 2017, 1–10. https://doi.org/10.1155/2017/9328347
Li, M., Zhao, Y., Hao, H., Dai, H., Han, Q., Tong, C., … Fu, X. (2015). Mesenchymal stem cell–conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. The International Journal of Lower Extremity Wounds, 14(1), 73–86. https://doi.org/10.1177/1534734615569053
Wu, Y., Zhao, R. C. H., & Tredget, E. E. (2010). Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells, 28(5), 905–915. https://doi.org/10.1002/stem.420
Kim, S.-W., Zhang, H.-Z., Guo, L., Kim, J.-M., & Kim, M. H. (2012). Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS One, 7(7), e41105. https://doi.org/10.1371/journal.pone.0041105
Massagué, J. (1999). Wounding smad. Nature Cell Biology, 1(5), E117–E119. https://doi.org/10.1038/12944
Subhan, B. S., Kwong, J., Kuhn, J. F., Monas, A., Sharma, S., & Rabbani, P. S. (2021). Amniotic fluid-derived multipotent stromal cells drive diabetic wound healing through modulation of macrophages. Journal of Translational Medicine, 19(1), 16. https://doi.org/10.1186/s12967-020-02674-5
Bourin, P., Bunnell, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., … Gimble, J. M. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy, 15(6), 641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Li, P., & Guo, X. (2018). A review: therapeutic potential of adipose-derived stem cells in cutaneous wound healing and regeneration. Stem Cell Research & Therapy, 9(1), 302. https://doi.org/10.1186/s13287-018-1044-5
Zhu, X., Du, J., & Liu, G. (2012). The comparison of multilineage differentiation of bone marrow and adipose-derived mesenchymal stem cells. Clinical Laboratory, 58(9–10), 897–903.
McIntosh, K., Zvonic, S., Garrett, S., Mitchell, J. B., Floyd, Z. E., Hammill, L., … Gimble, J. M. (2006). The Immunogenicity of Human Adipose‐Derived Cells: Temporal Changes In Vitro. STEM CELLS, 24(5), 1246–1253. https://doi.org/10.1634/stemcells.2005-0235
Hassanshahi, A., Hassanshahi, M., Khabbazi, S., Hosseini-Khah, Z., Peymanfar, Y., Ghalamkari, S., … Xian, C. J. (2019). Adipose-derived stem cells for wound healing. Journal of Cellular Physiology, 234(6), 7903–7914. https://doi.org/10.1002/jcp.27922
Hassan, W. U., Greiser, U., & Wang, W. (2014). Role of adipose-derived stem cells in wound healing: Role of ASCs in wound healing. Wound Repair and Regeneration, 22(3), 313–325. https://doi.org/10.1111/wrr.12173
Chen, L., Zheng, Q., Liu, Y., Li, L., Chen, X., Wang, L., & Wang, L. (2020). Adipose-derived stem cells promote diabetic wound healing via the recruitment and differentiation of endothelial progenitor cells into endothelial cells mediated by the VEGF-PLCγ-ERK pathway. Archives of Biochemistry and Biophysics, 692, 108531. https://doi.org/10.1016/j.abb.2020.108531
Álvaro-Afonso, F. J., Sanz-Corbalán, I., Lázaro-Martínez, J. L., Kakagia, D., & Papanas, N. (2020). Adipose-derived mesenchymal stem cells in the treatment of diabetic foot ulcers: a review of preclinical and clinical studies. Angiology, 71(9), 853–863. https://doi.org/10.1177/0003319720939467
Nambu, M., Kishimoto, S., Nakamura, S., Mizuno, H., Yanagibayashi, S., Yamamoto, N., … Kanatani, Y. (2009). Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Annals of Plastic Surgery, 62(3), 317–321. https://doi.org/10.1097/SAP.0b013e31817f01b6
Pakyari, M., Farrokhi, A., Maharlooei, M. K., & Ghahary, A. (2013). Critical role of transforming growth factor beta in different phases of wound healing. Advances in Wound Care, 2(5), 215–224. https://doi.org/10.1089/wound.2012.0406
Yun, I. S., Jeon, Y. R., Lee, W. J., Lee, J. W., Rah, D. K., Tark, K. C., & Lew, D. H. (2012). Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatologic Surgery, 38(10), 1678–1688. https://doi.org/10.1111/j.1524-4725.2012.02495.x
Hu, L., Wang, J., Zhou, X., Xiong, Z., Zhao, J., Yu, R., … Chen, L. (2016). Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Scientific Reports, 6(1), 32993. https://doi.org/10.1038/srep32993
Marino, G., Moraci, M., Armenia, E., Orabona, C., Sergio, R., De Sena, G., … Barbarisi, A. (2013). Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. Journal of Surgical Research, 185(1), 36–44. https://doi.org/10.1016/j.jss.2013.05.024
Jaing, T.-H. (2014). Umbilical cord blood: a trustworthy source of multipotent stem cells for regenerative medicine. Cell Transplantation, 23(4–5), 493–496. https://doi.org/10.3727/096368914X678300
Rizk, M., Aziz, J., Shorr, R., & Allan, D. S. (2017). Cell-based therapy using umbilical cord blood for novel indications in regenerative therapy and immune modulation: an updated systematic scoping review of the literature. Biology of Blood and Marrow Transplantation, 23(10), 1607–1613. https://doi.org/10.1016/j.bbmt.2017.05.032
Gang, E. J., Jeong, J. A., Han, S., Yan, Q., Jeon, C.-J., & Kim, H. (2006). In vitro endothelial potential of human UC blood-derived mesenchymal stem cells. Cytotherapy, 8(3), 215–227. https://doi.org/10.1080/14653240600735933
Allan, D. S. (2020). Using umbilical cord blood for regenerative therapy: Proof or promise? Stem Cells, 38(5), 590–595. https://doi.org/10.1002/stem.3150
Saleh, R., & Reza, H. M. (2017). Short review on human umbilical cord lining epithelial cells and their potential clinical applications. Stem Cell Research & Therapy, 8(1), 222. https://doi.org/10.1186/s13287-017-0679-y
Shrestha, C., Zhao, L., Chen, K., He, H., & Mo, Z. (2013). Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. International Journal of Endocrinology, 2013, 1–10. https://doi.org/10.1155/2013/592454
Xie, Q., Liu, R., Jiang, J., Peng, J., Yang, C., Zhang, W., … Song, J. (2020). What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Research & Therapy, 11(1), 519. https://doi.org/10.1186/s13287-020-02011-z
Jung, J.-A., Yoon, Y.-D., Lee, H.-W., Kang, S.-R., & Han, S.-K. (2018). Comparison of human umbilical cord blood-derived mesenchymal stem cells with healthy fibroblasts on wound-healing activity of diabetic fibroblasts: Umbilical cord blood-derived mesenchymal stromal cells. International Wound Journal, 15(1), 133–139. https://doi.org/10.1111/iwj.12849
Yang, J., Chen, Z., Pan, D., Li, H., & Shen, J. (2020). Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic f127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. International Journal of Nanomedicine, 15, 5911–5926. https://doi.org/10.2147/IJN.S249129
Zhang, B., Wu, X., Zhang, X., Sun, Y., Yan, Y., Shi, H., … Xu, W. (2015). Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Translational Medicine, 4(5), 513–522. https://doi.org/10.5966/sctm.2014-0267
Qin, H., Zhu, X., Zhang, B., Zhou, L., & Wang, W. (2016). Clinical evaluation of human umbilical cord mesenchymal stem cell transplantation after angioplasty for diabetic foot. Experimental and Clinical Endocrinology & Diabetes, 124(08), 497–503. https://doi.org/10.1055/s-0042-103684
Han, Y., Sun, T., Han, Y., Lin, L., Liu, C., Liu, J., … Tao, R. (2019). Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. European Journal of Medical Research, 24(1), 10. https://doi.org/10.1186/s40001-019-0366-9
Montanucci, P., di Pasquali, C., Ferri, I., Pescara, T., Pennoni, I., Siccu, P., … Calafiore, R. (2017). Human umbilical cord wharton jelly-derived adult mesenchymal stem cells, in biohybrid scaffolds, for experimental skin regeneration. Stem Cells International, 2017, 1–13. https://doi.org/10.1155/2017/1472642
Niu, Y., Cao, X., Song, F., Xie, T., Ji, X., Miao, M., … Lu, S. (2012). Reduced dermis thickness and age accumulation in diabetic abdominal skin. The International Journal of Lower Extremity Wounds, 11(3), 224–230. https://doi.org/10.1177/1534734612457570
Ramasamy, R., Yan, S. F., & Schmidt, A. M. (2012). The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascular Pharmacology, 57(5–6), 160–167. https://doi.org/10.1016/j.vph.2012.06.004
Han, Y., Sun, T., Tao, R., Han, Y., & Liu, J. (2017). Clinical application prospect of umbilical cord-derived mesenchymal stem cells on clearance of advanced glycation end products through autophagy on diabetic wound. European Journal of Medical Research, 22(1), 11. https://doi.org/10.1186/s40001-017-0253-1
Li, L., Sima, Y., Wang, Y., Zhou, J., Wang, L., & Chen, Y. (2020). The cytotoxicity of advanced glycation end products was attenuated by UCMSCs in human vaginal wall fibroblasts by inhibition of an inflammatory response and activation of PI3K/AKT/PTEN. BioScience Trends, 14(4), 263–270. https://doi.org/10.5582/bst.2020.03125
Oliveira, M. S. (2015). Placental-derived stem cells: Culture, differentiation and challenges. World Journal of Stem Cells, 7(4), 769. https://doi.org/10.4252/wjsc.v7.i4.769
Meamar, R., Ghasemi-Mobarakeh, L., Norouzi, M.-R., Siavash, M., Hamblin, M. R., & Fesharaki, M. (2021). Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: a randomized clinical trial. International Immunopharmacology, 101, 108282. https://doi.org/10.1016/j.intimp.2021.108282
Wu, S. C., Pollak, R., Frykberg, R. G., Zhou, W., Karnoub, M., Jankovic, V., … Chitkara, D. (2017). Safety and efficacy of intramuscular human placenta-derived mesenchymal stromal-like cells (cenplacel [PDA-002]) in patients who have a diabetic foot ulcer with peripheral arterial disease: Safety of cenplacel (PDA-002) in diabetic foot ulcer. International Wound Journal, 14(5), 823–829. https://doi.org/10.1111/iwj.12715
Wang, J., Zeng, X.-X., Cai, W., Han, Z.-B., Zhu, L.-Y., Liu, J.-Y., & Xu, J.-X. (2021). Safety and efficacy of placenta-derived mesenchymal stem cell treatment for diabetic patients with critical limb ischemia: a pilot study. Experimental and Clinical Endocrinology & Diabetes, 129(07), 542–548. https://doi.org/10.1055/a-0978-4972
Kinzer, M., Hingerl, K., König, J., Reinisch, A., Strunk, D., Huppertz, B., & Lang, I. (2014). Mesenchymal stromal cells from the human placenta promote neovascularization in a mouse model in vivo. Placenta, 35(7), 517–519. https://doi.org/10.1016/j.placenta.2014.04.004
Zeng, X., Tang, Y., Hu, K., Jiao, W., Ying, L., Zhu, L., … Xu, J. (2017). Three-week topical treatment with placenta-derived mesenchymal stem cells hydrogel in a patient with diabetic foot ulcer: A case report. Medicine, 96(51), e9212. https://doi.org/10.1097/MD.0000000000009212
Mathew, S. A., Naik, C., Cahill, P. A., & Bhonde, R. R. (2020). Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cellular and Molecular Life Sciences, 77(2), 253–265. https://doi.org/10.1007/s00018-019-03268-1
Xu, L., & Li, G. (2014). Circulating mesenchymal stem cells and their clinical implications. Journal of Orthopaedic Translation, 2(1), 1–7. https://doi.org/10.1016/j.jot.2013.11.002
Lindner, U., Kramer, J., Rohwedel, J., & Schlenke, P. (2010). Mesenchymal stem or stromal cells: toward a better understanding of their biology? Transfusion Medicine and Hemotherapy, 37(2), 75–83. https://doi.org/10.1159/000290897
Malek, A., & Bersinger, N. A. (2011). Human placental stem cells: biomedical potential and clinical relevance. Journal of Stem Cells, 6(2), 75–92.
He, Q., Wan, C., & Li, G. (2007). Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells, 25(1), 69–77. https://doi.org/10.1634/stemcells.2006-0335
Longhini, A. L. F., Salazar, T. E., Vieira, C., Trinh, T., Duan, Y., Pay, L. M., … Grant, M. B. (2019). Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS One, 14(3), e0212642. https://doi.org/10.1371/journal.pone.0212642
Xu, S.-M., & Liang, T. (2016). Clinical observation of the application of autologous peripheral blood stem cell transplantation for the treatment of diabetic foot gangrene. Experimental and Therapeutic Medicine, 11(1), 283–288. https://doi.org/10.3892/etm.2015.2888
Huang, P., Li, S., Han, M., Xiao, Z., Yang, R., & Han, Z. C. (2005). Autologous transplantation of granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care, 28(9), 2155–2160. https://doi.org/10.2337/diacare.28.9.2155
Fong, C.-Y., Chak, L.-L., Biswas, A., Tan, J.-H., Gauthaman, K., Chan, W.-K., & Bongso, A. (2011). Human wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Reviews and Reports, 7(1), 1–16. https://doi.org/10.1007/s12015-010-9166-x
Gauthaman, K., Fong, C.-Y., Suganya, C.-A., Subramanian, A., Biswas, A., Choolani, M., & Bongso, A. (2012). Extra-embryonic human Wharton’s jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reproductive BioMedicine Online, 24(2), 235–246. https://doi.org/10.1016/j.rbmo.2011.10.007
Rachakatla, R. S., Marini, F., Weiss, M. L., Tamura, M., & Troyer, D. (2007). Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Therapy, 14(10), 828–835. https://doi.org/10.1038/sj.cgt.7701077
Musiał-Wysocka, A., Kot, M., Sułkowski, M., Badyra, B., & Majka, M. (2019). Molecular and functional verification of wharton’s jelly mesenchymal stem cells (WJ-MSCs) pluripotency. International Journal of Molecular Sciences, 20(8), 1807. https://doi.org/10.3390/ijms20081807
Shetty, P., Cooper, K., & Viswanathan, C. (2010). Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian Journal of Transfusion Science, 4(1), 14. https://doi.org/10.4103/0973-6247.59386
Li, X., Bai, J., Ji, X., Li, R., Xuan, Y., & Wang, Y. (2014). Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. International Journal of Molecular Medicine, 34(3), 695–704. https://doi.org/10.3892/ijmm.2014.1821
Liau, L. L., Ruszymah, B. H. I., Ng, M. H., & Law, J. X. (2020). Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Current Research in Translational Medicine, 68(1), 5–16. https://doi.org/10.1016/j.retram.2019.09.001
Allickson, J. G. (2011). Recent studies assessing the proliferative capability of a novel adult stem cell identified in menstrual blood. The Open Stem Cell Journal, 3(1), 4–10. https://doi.org/10.2174/1876893801103010004
Meng, X., Ichim, T. E., Zhong, J., Rogers, A., Yin, Z., Jackson, J., … Riordan, N. H. (2007). Endometrial regenerative cells: a novel stem cell population. Journal of Translational Medicine, 5(1), 57. https://doi.org/10.1186/1479-5876-5-57
Chen, L., Qu, J., Mei, Q., Chen, X., Fang, Y., Chen, L., … Xiang, C. (2021). Small extracellular vesicles from menstrual blood-derived mesenchymal stem cells (MenSCs) as a novel therapeutic impetus in regenerative medicine. Stem Cell Research & Therapy, 12(1), 433. https://doi.org/10.1186/s13287-021-02511-6
Dalirfardouei, R., Jamialahmadi, K., Jafarian, A. H., & Mahdipour, E. (2019). Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. Journal of Tissue Engineering and Regenerative Medicine, 13(4), 555–568. https://doi.org/10.1002/term.2799
Hollands, P., Aboyeji, D., & Orcharton, M. (2018). Dental pulp stem cells in regenerative medicine. British Dental Journal, 224(9), 747–750. https://doi.org/10.1038/sj.bdj.2018.348
Malthiery, E., Chouaib, B., Hernandez-Lopez, A. M., Martin, M., Gergely, C., Torres, J.-H., … Collart-Dutilleul, P.-Y. (2021). Effects of green light photobiomodulation on Dental Pulp Stem Cells: enhanced proliferation and improved wound healing by cytoskeleton reorganization and cell softening. Lasers in Medical Science, 36(2), 437–445. https://doi.org/10.1007/s10103-020-03092-1
Omi, M., Hata, M., Nakamura, N., Miyabe, M., Ozawa, S., Nukada, H., … Naruse, K. (2017). Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Research & Therapy, 8(1), 279. https://doi.org/10.1186/s13287-017-0729-5
Greene, C., & Das, H. (2021). Development of cutaneous wound in diabetic immunocompromised mice and use of dental pulp–derived stem cell product for healing. In H. Das (Ed.), Wound Regeneration (Vol. 2193, pp. 23–30). New York, NY: Springer US. https://doi.org/10.1007/978-1-0716-0845-6_3
Nishino, Y., Ebisawa, K., Yamada, Y., Okabe, K., Kamei, Y., & Ueda, M. (2011). Human deciduous teeth dental pulp cells with basic fibroblast growth factor enhance wound healing of skin defect. Journal of Craniofacial Surgery, 22(2), 438–442. https://doi.org/10.1097/SCS.0b013e318207b507
Mousaei Ghasroldasht, M., Seok, J., Park, H.-S., Liakath Ali, F. B., & Al-Hendy, A. (2022). Stem cell therapy: from idea to clinical practice. International Journal of Molecular Sciences, 23(5), 2850. https://doi.org/10.3390/ijms23052850
Chiang, K.-J., Chiu, L.-C., Kang, Y.-N., & Chen, C. (2021). Autologous stem cell therapy for chronic lower extremity wounds: a meta-analysis of randomized controlled trials. Cells, 10(12), 3307. https://doi.org/10.3390/cells10123307
Braid, L. R., Wood, C. A., Wiese, D. M., & Ford, B. N. (2018). Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy, 20(2), 232–244. https://doi.org/10.1016/j.jcyt.2017.09.013
Lee, D. E., Ayoub, N., & Agrawal, D. K. (2016). Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Research & Therapy, 7(1), 37. https://doi.org/10.1186/s13287-016-0303-6
Kirby, G. T. S., Mills, S. J., Cowin, A. J., & Smith, L. E. (2015). Stem cells for cutaneous wound healing. BioMed Research International, 2015, 1–11. https://doi.org/10.1155/2015/285869
Pleguezuelos-Beltrán, P., Gálvez-Martín, P., Nieto-García, D., Marchal, J. A., & López-Ruiz, E. (2022). Advances in spray products for skin regeneration. Bioactive Materials, 16, 187–203. https://doi.org/10.1016/j.bioactmat.2022.02.023
Stolzing, A., Jones, E., McGonagle, D., & Scutt, A. (2008). Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mechanisms of Ageing and Development, 129(3), 163–173. https://doi.org/10.1016/j.mad.2007.12.002
Cheng, C.-H., Chen, L.-R., & Chen, K.-H. (2022). Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. International Journal of Molecular Sciences, 23(3), 1376. https://doi.org/10.3390/ijms23031376
Rezabakhsh, A., Cheraghi, O., Nourazarian, A., Hassanpour, M., Kazemi, M., Ghaderi, S., … Garjani, A. (2017). Type 2 diabetes inhibited human mesenchymal stem cells angiogenic response by over‐activity of the autophagic pathway. Journal of Cellular Biochemistry, 118(6), 1518–1530. https://doi.org/10.1002/jcb.25814
Mastrolia, I., Foppiani, E. M., Murgia, A., Candini, O., Samarelli, A. V., Grisendi, G., … Dominici, M. (2019). Challenges in clinical development of mesenchymal stromal/stem cells: concise review. Stem Cells Translational Medicine, 8(11), 1135–1148. https://doi.org/10.1002/sctm.19-0044
Di Nardo, P., Singla, D., & Li, R.-K. (2012). The challenges of stem cell therapy. Canadian Journal of Physiology and Pharmacology, 90(3), 273–274. https://doi.org/10.1139/y2012-016
Sah, J. P. (2016). Challenges of stem cell therapy in developing country. Journal of Stem Cell Research & Therapeutics, 1(3). https://doi.org/10.15406/jsrt.2016.01.00018
Lee, S., Choi, E., Cha, M.-J., & Hwang, K.-C. (2015). Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxidative Medicine and Cellular Longevity, 2015, 1–9. https://doi.org/10.1155/2015/632902
Garg, R. K., Rennert, R. C., Duscher, D., Sorkin, M., Kosaraju, R., Auerbach, L. J., … Gurtner, G. C. (2014). Capillary Force Seeding of Hydrogels for Adipose-Derived Stem Cell Delivery in Wounds. Stem Cells Translational Medicine, 3(9), 1079–1089. https://doi.org/10.5966/sctm.2014-0007
Ullah, M., Liu, D. D., & Thakor, A. S. (2019). Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience, 15, 421–438. https://doi.org/10.1016/j.isci.2019.05.004
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676. https://doi.org/10.1016/j.cell.2006.07.024
Migdady, Y., Pang, Y., Kalsi, S. S., Childs, R., & Arai, S. (2022). Post–hematopoietic stem cell transplantation immune-mediated anemia: a literature review and novel therapeutics. Blood Advances, 6(8), 2707–2721. https://doi.org/10.1182/bloodadvances.2021006279
Herberts, C. A., Kwa, M. S., & Hermsen, H. P. (2011). Risk factors in the development of stem cell therapy. Journal of Translational Medicine, 9(1), 29. https://doi.org/10.1186/1479-5876-9-29
Amariglio, N., Hirshberg, A., Scheithauer, B. W., Cohen, Y., Loewenthal, R., Trakhtenbrot, L., … Rechavi, G. (2009). Donor-Derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Medicine, 6(2), e1000029. https://doi.org/10.1371/journal.pmed.1000029
Rodriguez, R., Rubio, R., Masip, M., Catalina, P., Nieto, A., de la Cueva, T., … García-Castro, J. (2009). Loss of p53 induces tumorigenesis in p21-deficient mesenchymal stem cells. Neoplasia, 11(4), 397-IN9. https://doi.org/10.1593/neo.81620
Li, H., Fan, X., Kovi, R. C., Jo, Y., Moquin, B., Konz, R., … Houghton, J. (2007). Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Research, 67(22), 10889–10898. https://doi.org/10.1158/0008-5472.CAN-07-2665
Kainer, M. A., Linden, J. V., Whaley, D. N., Holmes, H. T., Jarvis, W. R., Jernigan, D. B., & Archibald, L. K. (2004). Clostridium infections associated with musculoskeletal-tissue allografts. New England Journal of Medicine, 350(25), 2564–2571. https://doi.org/10.1056/NEJMoa023222
Martin, M. J., Muotri, A., Gage, F., & Varki, A. (2005). Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nature Medicine, 11(2), 228–232. https://doi.org/10.1038/nm1181
An, Y., Lin, S., Tan, X., Zhu, S., Nie, F., Zhen, Y., … Wu, J. (2021). Exosomes from adipose‐derived stem cells and application to skin wound healing. Cell Proliferation, 54(3), e12993. https://doi.org/10.1111/cpr.12993
Kucharzewski, M., Rojczyk, E., Wilemska-Kucharzewska, K., Wilk, R., Hudecki, J., & Los, M. J. (2019). Novel trends in application of stem cells in skin wound healing. European Journal of Pharmacology, 843, 307–315. https://doi.org/10.1016/j.ejphar.2018.12.012
Paschos, N. K., Brown, W. E., Eswaramoorthy, R., Hu, J. C., & Athanasiou, K. A. (2015). Advances in tissue engineering through stem cell-based co-culture: stem cell co-culture strategies in tissue engineering. Journal of Tissue Engineering and Regenerative Medicine, 9(5), 488–503. https://doi.org/10.1002/term.1870
Deschepper, M., Oudina, K., David, B., Myrtil, V., Collet, C., Bensidhoum, M., … Petite, H. (2011). Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. Journal of Cellular and Molecular Medicine, 15(7), 1505–1514. https://doi.org/10.1111/j.1582-4934.2010.01138.x
Blais, M., Lévesque, P., Bellenfant, S., & Berthod, F. (2013). Nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3 and glial-derived neurotrophic factor enhance angiogenesis in a tissue-engineered in vitro model. Tissue Engineering Part A, 19(15–16), 1655–1664. https://doi.org/10.1089/ten.tea.2012.0745
Wang, X., Shen, K., Wang, J., Liu, K., Wu, G., Li, Y., … Hu, D. (2020). Hypoxic preconditioning combined with curcumin promotes cell survival and mitochondrial quality of bone marrow mesenchymal stem cells, and accelerates cutaneous wound healing via PGC-1α/SIRT3/HIF-1α signaling. Free Radical Biology and Medicine, 159, 164–176. https://doi.org/10.1016/j.freeradbiomed.2020.07.023
Alagesan, S., Brady, J., Byrnes, D., Fandiño, J., Masterson, C., McCarthy, S., … O’Toole, D. (2022). Enhancement strategies for mesenchymal stem cells and related therapies. Stem Cell Research & Therapy, 13(1), 75. https://doi.org/10.1186/s13287-022-02747-w
Zhang, Y., Li, W., Laurent, T., & Ding, S. (2012). Small molecules, big roles – the chemical manipulation of stem cell fate and somatic cell reprogramming. Journal of Cell Science, 125(23), 5609–5620. https://doi.org/10.1242/jcs.096032
Cury, V., Moretti, A. I. S., Assis, L., Bossini, P., de Souza Crusca, J., Neto, C. B., … Parizotto, N. A. (2013). Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1α and MMP-2. Journal of Photochemistry and Photobiology B: Biology, 125, 164–170. https://doi.org/10.1016/j.jphotobiol.2013.06.004
Park, I.-S., Mondal, A., Chung, P.-S., & Ahn, J. C. (2015). Vascular regeneration effect of adipose-derived stem cells with light-emitting diode phototherapy in ischemic tissue. Lasers in Medical Science, 30(2), 533–541. https://doi.org/10.1007/s10103-014-1699-9
Bayat, M., & Chien, S. (2020). Combined adipose-derived mesenchymal stem cells and photobiomodulation could modulate the inflammatory response and treat infected diabetic foot ulcers. Photobiomodulation, Photomedicine, and Laser Surgery, 38(3), 135–137. https://doi.org/10.1089/photob.2019.4670
Udalamaththa, V. L., Jayasinghe, C. D., & Udagama, P. V. (2016). Potential role of herbal remedies in stem cell therapy: proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Research & Therapy, 7(1), 110. https://doi.org/10.1186/s13287-016-0366-4
Xing, F., Li, L., Zhou, C., Long, C., Wu, L., Lei, H., … Zhang, X. (2019). Regulation and directing stem cell fate by tissue engineering functional microenvironments: scaffold physical and chemical cues. Stem Cells International, 2019, 1–16. https://doi.org/10.1155/2019/2180925
Jeon, O., & Alsberg, E. (2013). Regulation of stem cell fate in a three-dimensional micropatterned dual-crosslinked hydrogel system. Advanced Functional Materials, 23(38), 4765–4775. https://doi.org/10.1002/adfm.201300529
Acknowledgement
The authors acknowledge Vellore Institute of Technology (VIT) for providing financial support as TRAship to the first author (DP) for pursuing PhD.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Debarchan Panda: Review of literature and manuscript preparation. Sunita Nayak: Framing and proofreading the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panda, D., Nayak, S. Stem Cell-Based Tissue Engineering Approaches for Diabetic Foot Ulcer: a Review from Mechanism to Clinical Trial. Stem Cell Rev and Rep 20, 88–123 (2024). https://doi.org/10.1007/s12015-023-10640-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10640-z