Abstract
Heavy metal pollution of natural and cultivated habitats may be caused by agricultural, industrial, and human activities. Fish living in these habitats easily accumulate metals in their organs; for food safety and human health, the heavy metals in fish flesh are of major concern due to the harmful nature of these pollutants even in low quantities. In this study, metals (Iron, Fe; Lead, Pb; Cadmium, Cd; Nickel, Ni; Copper, Cu and Zinc, Zn) in the muscle, liver, intestine, and gill of gilthead seabream (Sparus aurata, Denis is local name) were monitored to determine the contamination levels and to investigate the protective impact of cooking methods on the reduction or mitigation of metal levels. Although the Denis samples exhibited relatively low Pb and Cd levels, most fish samples had elevated levels of Fe and Zn. The examined metals accumulated at the highest level in the liver and gills compared to the other organs. Results showed that cooking methods had a considerable effect on concentrations of metals. However, the levels of metal in S. aurata from various sources were reduced significantly (P < 0.05) by frying, microwave, and grilling cooking, which was ordered in the following sequence as microwave cooking < grilling < frying. The consumption of Denis fish from different sources (wild and cultured) has no negative effects on health, according to a study of health hazards based on indices of carcinogenic and non-carcinogenic. The metal results indicated that different fish sources (wild and cultured) could be acceptable for human consumption. Data hypothesized a positive impact of awareness among the native community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal pollution of aquatic habitats is a major environmental concern worldwide, especially in Egypt. Heavy metals are present in the aquatic environment in a variety of ways, including through untreated or insufficiently treated agricultural, domestic, and industrial effluent [1]. Metals are ingested by aquatic organisms in low concentration through water uptake and in higher concentration through biomagnification of prey; however, consumers can ingest metals through the food chain, which can have acute and long-term health effects [2]. Among aquatic species, the Denis, Sparus aurata receives HMs from the sediments and water in which it lives. According to Hadj Taieb et al. [3], it is opportunistic and carnivorous, which allows it to accumulate metals through the biomagnification process (via the food chain). The S. aurata, is an economically important demersal species inhabiting Egypt’s Mediterranean region and is one of the most important species in Egyptian marine aquaculture [4]. The major Egyptian fisheries resource for seabream is the Bardawil Lake, which is a shallow body of water with high salinity and is regarded as one of the most important sources of Egyptian fisheries [5]. Metal accumulation in Denis tissues has been observed in various scientific studies [6,7,8].
Heavy metals, including Fe, Cu, Co, Ni, Mn, and Zn, are necessary for biological life; but become poisonous at higher concentrations [9]. However, Mercury, Cadmium, Arsenic, and Lead are toxic and, even at low concentrations, can be hazardous [10]. According to the classifications of heavy metals, consuming them in low or high quantities could pose a serious risk. Ingestion of toxic metals at low levels over an extended period can be extremely hazardous to human health. Additionally, ingesting significant essential metal levels may be hazardous to human health. [11]. Depending on the nutritional habits, seafood might be prepared in various ways using different cooking strategies such as boiling, frying, baking, and grilling [12]. Different cooking strategies can affect the heavy metal content of fish [13]. Consequently, it is important to determine their concentrations in raw and cooked Denis fish in order to evaluate the possible risks of consumption for humans. Therefore, the risks to human health from metal pollution can be reduced when fish consumers are aware of the most effective cooking strategies to reduce metal pollution.
This study aims to evaluate some trace elements such as Fe, Pb, Cd, Ni, Cu, and Zn levels in the muscle, liver, intestine, and gills of both cultured and wild Denis fish (S. aurata) to calculate the contamination level and investigate the protective effect of cooking strategies (frying, microwave cooking, and grilling) on the mitigation or reduction of metal levels. It also aims to identify the health risks to fish consumers and to ensure public health safety by raising awareness of possible health hazards related to fish intake.
Material and Methods
Collection of Denis Fish Samples
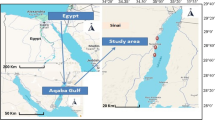
Eighty samples of Denis fish were collected by fishermen from two separate locations in Egypt: the first collection was from the Bardawil Lake (wild source), located at latitude of 31° 11′ 25.74″ N and longitude of 33° 09′ 44.03″ E, while the second one was from private fish farm waters (cultured source) situated in Ezbet Elborg, Domietta province, at latitude of 31° 24′ 59.33″ N and longitude of 31° 48′ 47.95″ E, both sources supplied saline-water from Mediterranean sea. Sampling was bought by local fishermen between July, to October 2022 (Fig. 1). The Denis samples were stored in an icebox after collection and transported to the marine biology laboratory for further analysis.
Cooking Methods of Denis Fish Muscles
The samples of Denis fish were washed, cleaned, and filleted in the laboratory after being measured for weight and length. Denis fish weights and lengths were 470±23 g and 25.52 ± 0.88 cm, respectively for the cultured source and 393.02 ± 44.40 g and 23.79 ± 1.19 cm, respectively for the wild source. Filleted Denis fish were divided into four groups (10 samples from each group). The first group was uncooked fish used as references (raw), the second group was cooked through frying (Fish was fried in fresh sunflower oil for 8 min), the third group was cooked through microwave, and the fourth group was cooked through grilling (Fish was grilled in oven for 20 min).
Heavy Metal Level Measurements
The Digestion of Denis Fish Samples
The Denis fish organs (raw; muscle, liver, intestine, and gill) and cooked fish (grilling, microwave cooking, and frying) samples were investigated for measurements of heavy metal level (HML). The samples (raw and cooked) were dried at 105°C for 24 h in the lab oven. About 0.5 g of dried samples were placed in 50 mL digestion vessels with ultrapure HNO3 (65%, 5 mL), and H2O2 (30%, 1 mL) was added. The mixture was warmed until completely digested on the hot plate. The digested samples were allowed to cool at room temperature, moved to volumetric flasks, and then mixed with HNO3 (1%), resulting in a final volume of 25 mL. The diluted solutions were then tested [14].
Analysis of HML in Denis Fish Organs
Levels of HM were detected in diluted solutions of Denis organs. An inductively coupled plasma optical emission spectrophotometer (Perkin Elmer, ICP-OES, 4300 DV, Shelton, USA) was employed to quantify the HML in the serial dilutions (specimens, n=5). The specimens treated with four calibration standards were made up of a stock solution (1 μg l−1) of each HM mixed in 5% (v/v) nitric acid at levels of 0, 50, 100, 200, 400 μg/L to calculate the calibration plot to determine the level of each HM in the digested mixtures. The quality control (QC) sample was checked every ten samples to ensure that both the instrument drift and calibration curve were within tolerable limits. To determine the strategy detection limit, duplicate blank specimens from each analytical group were performed in a randomized order. The calculated correlation coefficient (R2) for all calibration curves of the metals analyzed was from 0.992 to 0.999. The validation parameters of the analytical method are given in Table 1S. The recovery percentage varied from 95.36 to 98.65%. On a dry weight basis (dw-b), the contents of metals in the fish samples were assessed in μg/g dw-b [2].
Environmental Hazard Assessment
The levels of metal pollution in aquatic species are estimated using a variety of indices [15]. In this study, the contamination status of HM in the organs of wild and cultured Denis fish was assessed using several frequently used index values, including the contamination factor (CF-HML) and the pollution index (MPI-HML) to evaluate the contamination degree of HML in Denis fish captured from different sources.
Contamination Factor (CF-HML)
Using metal levels in Denis fish samples, the contamination factor (CF-HML) for metals was derived as the following equation:
where CHML stands for the HML Denis fish samples (μg/g dw-b), and CBL stands for background level of metals (Pb, Fe, Cd, Cu, Ni, and Zn). The values of CF-HML ≤ 1 denote a minimal limit of contamination, 1< CF-HML ≤ 2 is denoted as a low contamination degree, 2 < CF-HML ≤ 3 is moderate contamination, and CF-HML > 3 represented by a high degree of contamination [15].
Pollution Index (MPI-HML)
The pollution index (MPI-HML) is an integrated approach to assess heavy metal pollution. This equation was used to estimate the MPI-HML [15]:
where HML1 is the first metal level, HML2 is the second metal level, HML3 is the third metal level, n is the number of examined metals and HMLx is the xth metal level (μg/g dw-b) in the Denis fish organs. The contamination level is safe degree when the MPI-HML value is less than 1, the MPI-HML is between 1.0 and 2.0, conditions are categorized as slightly contaminated, 2.0 to 3.0, moderately to severely contaminated, 3.0 to 5.0, severely contaminated, and > 10 heavily contaminated.
Health Hazard Assessment
We employed a technique established by the USEPA [16] to evaluate the risk to human health of HML consumed by ingestion of the muscles of the investigated fish. The estimated daily intake (EDI- HML), non-carcinogenic and carcinogenic indexes of HML were all performed by detecting the levels of HM in the raw muscles, fried, microwaved cooking, and grilled samples.
Estimated Daily Intake (EDI)
The EDI-HML (the daily average ingestion of a specific metal during the lifespan) was used to calculate the exposure dose caused by direct human consumption of some metals observed in edible organs. The EDI-HML was calculated using the following formula and represented as mg/kg/day [17].
where the EP refers to the lifespan of exposure time, which is estimated to be 70 years old; the IR needs to account again for the daily ingestion of fish intake was calculated as kilograms per day, or 41 g per day for adults and 27 g per day for children. C-HML stands for the metal levels in raw muscles, fried, microwaved cooking, and grilled samples (μg/g wet wt.), Fish wet weight was converted to dry weight using a conversion coefficient of 4.8 [18]; ER means standing for exposure rate (365 days year-1); BW refers to the body weight, which was previously defined as 70 per kg in adults and 30 per kg children; The average lifetime is AT (70 years × 365 days per year).
Non-carcinogenic Index
Target hazard quotient or THQ-HML
The THQ, a non-cancer evaluation of harmful health effects associated with ingesting certain HML pollutants in edible fish flesh, was established to assess human risk. The ratio of EDI-HML (average daily dosage) to the oral reference dose (mg/kg/day, ORD-HML) was used to calculate THQ-HML as:
According to recommendations made by the USEPA in 2018 for Pb, Cu, Cd, Ni, Fe, and Zn, the ORD-HML should be 0.00357, 0.04, 0.001, 0.02, and 0.3 mg/kg/day, respectively [16].
Hazard index (HI-HML)
The HI-HML is another mathematical formula that, according to Cui et al. [19], reflects the impact of non-carcinogenic risks by the sum of the THQ-HML values for the metals under study:
Carcinogenic index (CI-HML)
The carcinogenic index (CI-HML) of heavy metal exposure lifetime was established as the incremental risk of an individual acquiring cancer depending on the carcinogenic slope factor (CSF-HML for Ni, Cd, and Pb were 0.00084, 6.3, and 0.0042 mg/kg/day, respectively). This equation was applied to calculate the CI-HML [20]:
Statistical Analysis
The SPSS, a statistical program (Version 22; software, USA), was used to perform statistical analyses. To determine a normal distribution and homogeneity of variance, Levene’s test was applied. To determine the statistically significant differences between the impacts of different cooking strategies on the level of metals, the results were statistically evaluated using analysis of variance (one-way ANOVA), and Post hoc Tukey analyses were performed when differences occurred. Additionally, to investigate the statistical differences between wild and cultured sources of each metal in Denis fish, the independent-sample T-test was employed. However, the correlations between the metal levels in samples of wild and cultivated Denis fish were assessed using Pearson's correlation coefficient. The statistics are presented in tables as means±standard deviation. Statistical significance, however, was represented at p < 0.05.
Results and discussion
HML in Organs of Denis Fish
Some HMLs can be found in the environment naturally. For example, essential HMLs such as Zn, Cu, and Fe have biological roles for aquatic species, but above certain threshold levels, they are potentially toxic to aquatic biota. However, other non-essential HMLs, such as Pb, As, Hg, and Cd, have no known biological role and are often toxic even at low levels [21, 22]. The levels of Pb, Fe, Cu, Ni, Cd, and Zn in the different sources-specific Denis organs, i.e., intestine, gill, muscle, and liver, revealed that there was a significant possibility of HML in the organs of Denis fish (Table 1). Likewise, the present study mentioned that the HML in the organs of wild and cultured Denis fish (gill, intestine, muscle, and liver) showed more Iron (Fe) than any studied HML, while Cadmium (Cd) was at the lowest end, and the HML ranged in this order: Fe > Cu > Zn > Ni > Pb > Cd for the studied organs (intestine, muscle, and liver). In contrast, the HML of gills is arranged in this sequencing: Fe > Zn > Cu > Ni > Pb > Cd. The most abundant of the HML studied was Iron, while Cd showed alternating levels of accumulation in Denis fish organs. This observation is in accordance with Al-Halani et al. [23] who revealed that the maximum level of HM in wild fish organs, Dicentrarchus labrax, occurred for Fe and the minimum level was detected for Cadmium. Moreover, The HML in the wild and cultured Denis organs exhibited the minimum values recorded for muscle. The findings of the present study also confirmed the results reported by Begum et al. [24], Liu et al. [25] Abbas et al. [26] and disagreed with Zhao et al. [27], Liu et al. [28] and Liu et al. [29] [30], whom reported that the muscles of marine fish recorded the highest levels of heavy metals.
In comparison to FAO standards, the Iron levels in the wild organs of Denis fish ranged from 36.21±3.54 to 86.83±10.32 g/g, dw-b, which were lower than the acceptable limits. In the cultured organs, however, it fluctuated between 77.88±8.52 and 118.50±7.54 μg/g, dw-b. Cultured origins of muscles and intestine were lower than the permissible limit, whereas gills and liver were higher than the acceptable limits. According to FAO [31], the Zinc levels in the wild and cultured organs of Denis fish were below the permissible levels, except for the gills (45.85±1.65 and 49.85±2.01 μg/g, dw-b, respectively). The lowest levels of Pb in the organs of wild and cultured Denis fish were 0.84±0.07 and 0.74±0.24 μg/g, dw-b, respectively, and the highest levels were 1.69±0.88 and 1.59±0.42 μg/g, dw-b, respectively. All four organs of the Denis fish tested for Pb levels were below the acceptable limits estimated by the FAO [31]. Copper levels in the wild and cultured organs of Denis fish ranged between 21.79±1.24 to 39.04±3.21 μg/g, dw-b in the former origin and 23.77±2.02 to 43.04±2.65 μg/g, dw-b in the second one. Cu levels in the muscles and intestine were lower than the permissible limit, whereas the gills and liver were higher than the acceptable limits estimated by the FAO [31]. The minimal levels of Nickel in the organs of wild and cultured Denis fish were 1.44±0.85 and 2.09±0.64 μg/g, dw-b, respectively and the maximal levels were 8.39±0.84 and 12.04±0.48 μg/g, dw-b, respectively. All four organs of Denis fish from wild and cultured origins tested for Ni levels were below the acceptable limits estimated by the FAO [31]. Cadmium levels in the wild and cultured organs of Denis fish ranged between 0.35±0.03 to 0.69±0.07 μg/g, dw-b in the former origin and 0.25±0.02 to 0.59±0.04 μg/g, dw-b in the second one. Cd levels in the muscles and intestine were lower than the permissible limit, whereas gills and liver were higher than the acceptable limits estimated by the FAO [31].
In most cases, the essential metals exhibited higher levels in cultured Denis organs than in wild Denis organs; this may be attributed to the fact that these metals are required for different biological activities and thus supplied into fish diets [22, 26, 32], suggesting that cultured fish have higher levels [33,34,35].
This observation agrees with Yipel et al. [36], who reported that the wild fish, Sparus aurata, accumulate less Fe and Zn than the cultured ones. However, the level of toxic metals in wild Dines fish organs was significantly lower than in cultured fish organs (P < 0.05), which may be attributed to wild fish surviving over several years compared to cultured fish, which are captured within six months. Wild Denis organs can accumulate pollutants with prolonged biological lifespans, notably both Cadmium and Lead, over a longer lifetime compared to cultured Denis organs. According to Chatta et al. [37], the Lead and Cadmium absorbed in the cultured Labeo rohita and Cirrhinus mrigala were lower than in the wild ones.
The cadmium levels exhibited the lowest values in the organs of Denis fish (Sparus aurata) from both studied sources. Cadmium is extremely harmful due to its extremely potential toxicity even at low levels, its persistence in the environment, and its proclivity for bioaccumulation in aquatic biota. In the aquatic biota, Cd is not digested by the body, and it accumulates in the soft tissues and becomes poisonous, and as a direct consequence of their bioaccumulation, the food chain has become contaminated, affecting the entire ecological activity. Nowadays, global attention becomes more critical for African countries such as Egypt, where the pressure from exploding the population requires a lot of food supply [38,39,40,41,42]. Additionally, Perera et al. [43] stated that natural and anthropogenic activities can be identified as an important source of Cd to the biosphere. Natural emissions are mainly from the mobilization of naturally occurring Cd from the earth’s crust and mantle, e.g. volcanic eruptions and weathering of rocks. Anthropogenic sources are mainly from the mobilization of Cd impurities in raw materials (e.g., phosphate minerals, fossil fuels) and emissions from the manufacturing, use, disposal, recycling, reclamation, or incineration of products intentionally.
Environmental Hazards Estimation
The contamination factor (Cf-HML), and metal pollution index (MPI-HML) were all applied to evaluate the contamination degree of HML in different organs of fish [15]. The evaluated Cf-HML values for the studied HML in wild Denis fish organs ranged from 0.05 to 1.38 μg g−1, and from 0.07 to 1.43 μg g−1 in cultured Denis fish organs (Fig. 2). The Cf-Pb and Cf-Cd values were higher in the wild fish organs compared with cultured Denis organs suggesting that their pollution level was relatively increased in organs of cultured Denis, in accordance with previous studies, Pb and Cd pose significant potential ecological risks [44, 45]. Cf-HML levels in Denis organs dropped in the following order: gills > liver > intestine > muscles for Cf-Fe, Cf-Pb, Cf-Ni, and Cf-Cd, whereas gills > liver > intestine > muscles for Cf-Zn and Cf-Cu. Moreover, the computed values of CF-HML in the Denis fish showed a low level of contamination (Cf-HML<1) detected in the intestines and muscles of two sources. Contrarily, Cf-Fe values in the gills and liver of cultured Denis fish had moderate contamination (1 ≥ CF ≤3), and Cf-Zn, Cf-Cu, and Cf-Cd in the gills and liver from different sources had moderate contamination. Additionally, Cf-HML values for Fe, Zn, Cu, and Ni were lower in the wild organs compared with cultured Denis organs, while they were higher for Pb and Cd. This indicates that cultured Denis may be highly contaminated with the essential metals (Fe, Zn, Cu, and Ni), while highly contaminated wild fish organs with non-essential metals were also found (Pb and Cd). The estimated values for MPI-HML in cultured sources of Denis organs were higher than the wild ones, suggesting a high pollution degree in cultured organs. However, the contamination degree based on HML in Denis fish organs can be classified as follows: Gills > liver > intestine > muscle for wild organs, while liver > gills > intestine > muscle for cultured organs, according to the estimated data resulting from MPI-HML (Fig. 2). Therefore, the high values in pollution indices of cultured Denis compared to wild sources raise concern for consumers' health due to metal contamination. Hence, it is possible to determine the potential impact on the reduction of HML by using cooking methods.
Cf-HML, and MPI-MHL (averages±SD) based on HML in the organs of Denis fish (Sparus aurata) from different sources; a one-way ANOVA, reveals that results from the same fish source having different alphabetic small letters (Wild Denis source) and capital letters (Cultured Denis source) are significantly different (p<0.05).
Pearson Correlation of HML in the Denis Fish Origin
Pearson correlation (r) was evaluated to calculate if some of these metals were interrelated with each other based on HML in the wild and cultured Denis organs (Table 2S). The positive correlation between the MHL indicates a similar input source of metal, while the negative correlation indicates a different source. In this study, Pearson correlation analysis based on HML in organs of wild Denis fish showed a significant positive correlation between Fe-Ni, Fe-Cd, Pb-Ni, Cu-Ni, and Cu-Cd, whereas a significant negative relationship was revealed between Cd-Ni, Pb-Cd, and Pb-Cu. In the cultured Denis fish, however, correlation analysis showed a significant positive correlation between essential metals with each other (Fe, Ni, Cu, and Zn), while a significant negative correlation was shown between Cd and the studied HML, except Zn; Lead-Copper, and Lead-Nickel.
Effect of Cooking Strategies on HML
Cooking the wild and cultured Denis muscle identifies any changes in HML, providing a significantly (P < 0.05) accurate representation of the potential human consumption of HML. Every day, humans cook using various cooking strategies of their choice, and fish is almost never consumed uncooked, especially in Egypt. This research chose frying, grilling, and microwave cooking as examples of cooking strategies on the HML in the muscle of Denis fish (Table 2). Reduction percentages of HML in cooked Denis fish from different sources were represented in Table 1S. The grilling of wild and cultured Denis samples resulted in the reduction of Lead (56.60 and 63.87%, respectively), Cadmium (53.46 and 55.96%, respectively), Nickel (45.13 and 31.10%, respectively), Iron (11.74 and 5.46%, respectively), Copper (7.57 and 6.94%, respectively), and Zinc (23.36 and 21.06%, respectively). In accordance with our findings, Abd-Elghany et al. [46] revealed that the levels of Lead (10%) and Arsenic (50 %) in raw shrimps were lowered by 10 % and 24 %, respectively in grilled shrimp, and by 27 % and 36 %, respectively in crabs caught in the Mediterranean Sea and cooked on the grill. Additionally, Ersoy et al. [47] found lower levels of Cr, As, and Pb and higher levels of Nickel in grilled-farmed seabass caught in Turkey. Abu-Raya et al. [48] mentioned that the Copper, Lead, and Cd levels increased after grilling Bolti fish compared to raw samples, while the Zn level declined. In contrast to our observations, an increment in the levels of Hg, Cu, Se, Mn, Zn, As, and Sr by 10–55% and a lowering in Iron and Cadmium levels by 27–66% after grilling techniques for cultivated Meagre fish were observed in Portugal [49]. According to Kalogeropoulos et al. [50], grilled anchovy have higher levels of Cd, Zn, Hg, Fe, Pb, Ni, Cu, and Cr than their raw flesh.
Frying of wild and cultured Denis samples exhibited a significant minimization of Lead (45.28 and 50.97%, respectively), Cadmium (12.56 and 17.51%, respectively), Nickel (24.99 and 17.22%, respectively), Iron (9.28 and 4.31%, respectively), Copper (6.24 and 5.72%, respectively), and Zinc (18.46 and 16.65%, respectively). The minimization of HML after frying may be attributed to moisture loss and fat increase during the frying strategy. The outcomes are consistent with the investigation on seabass conducted by Hosseini et al. [51], who determined that the frying method reduced Iron and Zinc levels, whereas increasing Manganese and Copper levels, and Arisekar et al. [52] who calculated that the levels of HML in Penaeus vannamei varied mainly due to moisture loss and uptake of oil. Pb and Ni levels were significantly lower (P < 0.05) than in the muscle of Thunnus tonggol, longtail tuna, after the frying strategy [53]. The levels of Hg, Zn, Fe, Cu, Cd, Pb, Cr, and Ni in hake, anchovy, bogue, picarel, sand smelt, sardine, stripped mullet, Mediterranean mussel, squid, and shrimp were significantly higher after domestic pan-frying [50]. Ersoy et al. [47] recorded that the Lead levels in raw and fried fish were 0.278, and 0.277 mg kg−1, respectively. The minimization of Fe, Cd, Ni, Mn, Zn, As, Se, and Cu after shrimp frying was evaluated as 96.4, 49.1, 67.3, 19.8, 95.3, 99.2, 89.6, and 75.3 %, respectively. Copper and Zinc levels declined after frying in Rainbow trout muscles [54]. A minimization trend in Cadmium and Zinc was observed in sardines fishes [55]. However, Abu-Raya et al 2007 revealed that Copper, and Cadmium levels after frying of Bolti fish were higher compared to raw samples, while the Zinc and Lead levels declined. However, Lead levels were not significantly lowered after the frying of sea bass [47].
The microwave cooking of wild and cultured Denis muscles exhibited a significant reduction in HML levels by 81.74, 92.51% for Pb; 66.14, 77.27% for Cd; 65.96, 45.45% for Ni; 27.48, 12.78% for Fe; 18.13, 16.62% for Cu and 43.69, 36.10% for Zn. Also, the higher in HML minimization observed after microwave cooking than the processes of grilling and frying, Arisekar et al. [52] reported that after microwave cooking, Fe, Cr, Ni, Zn, and Cu levels in shrimp tissue decreased by 25.7, 47.5, 11.5, 32.5, and 57.7 percent, respectively. The reductions in Zn, Fe, Cu, Pb, and Cr levels were observed after the microwave strategy of rainbow trout [56], sea bass [51] and catfish [47]. Previous studies mentioned that the higher levels of HML after the microwave strategy are related to oil decline/uptake and water loss [55, 57]. This minimization in HML may be due to the denaturation of proteins. Microwave heating consistently results in a higher level of protein denature than traditional methods of cooking [58], and it also leaches away proteins that bind Iron, Chromium, and Copper [59]. However, microwaved cooking of catfish resulted in no changes in the Lead level [56].
According to our findings, the HML in the muscles of the Denis fish was significantly (P < 0.05) affected by the cooking techniques. Cooking techniques, including microwaving, grilling, and frying, showed a significant (P< 0.05) reduction in the studied HML levels (Ni, Zn, Pb, Cu, Cd, and Fe) observed in the muscle of wild and cultivated Denis. HML was found in raw and cooked samples in the following order: raw > fried > grilled > microwaved samples. These outcomes might be the result of changes in moisture and fat content that happened during microwave, grilling, and frying cooking. The reduction of HML in fish cooked to different temperatures varies according to the physico-chemical features of HML and their chemical variation, the sulfhydryl link between the protein and the HML, the species, size, and the cooking variables, such as the duration, temperature, and cooking condition [50, 57]. It would be significantly (P < 0.05) more accurate to assess the potential health effects of ingestion by tracking changes in HML in cooked Denis fish.
Health Hazards Estimation
Consumers’ daily exposure to HML through eating foods high in HML was employed to avoid any detrimental effects on humans during their lifespan [60]. EDI (mg kg−1day−1) for HML in the raw and cooked muscles of both cultured and wild Denis fish are represented in Table 4S and Fig. 3. The EDI-HML values of Fe, Zn, Cu, Ni, Cd, and Pb for consumers (children and adults) were lower than the PTDI (permissible tolerable daily intake). The PTDI values of Fe, Zn, Cu, Ni, Cd, and Pb are 50, 70, 50, 4E-02, 3E-03, and 3E-02 mg/kg/day, respectively [61]. The recorded EDI-HML of both groups was compared to that for 70 kg of body weight [61], and it was concluded that the mean EDI values of the metals for consumers (children and adults) do not exceed the PTDI values. The EDI-HML values for the essential metals in the raw and cooked Denis muscles of cultivated origin were higher than those of wild origin, indicating that consumers (children and adults) had a higher rate of exposure when consuming the cultivated fish than those of wild origin. On the other hand, the EDI-HML values for the non-essential metals in the raw and cooked Denis muscles of cultivated origin were lower in comparison to those of wild origin, which suggested that consumers (children and adults) had a higher rate of exposure when consuming wild fish than those cultivated.
Target hazard quotient (THQ-HML) for Cu, Zn, Fe, Ni, Cd, and Pb in raw and cooked muscles of both cultured and wild fish are illustrated in Table 4S and Fig. 4. The allowable threshold level of THQ-HML is one [16]. The THQ-HML values determined in edible Denis fish were under 1, suggesting that eating muscles won't have any adverse health effects for consumers (children and adults) who consumed the studied Denis fish. Furthermore, the hazard index (HI-HML) values for both adults and children through consumption of the two fish sources were evaluated based on the THQ-HML values; if the HI-HML value was less than one (HI-HML ≤1), the effects on humans would be adverse, HI-HML > 1 most probably had a negative impact; and HI-HML >10 strong or chronic of acute implications, as recommended by [62]. The HI-HML values in raw and cooked muscles of both cultured and wild Denis fish for both consumers (children and adults) were less than one, suggesting no hazard for human consumption occurred (Table 5S and Fig. 5).
The carcinogenic index (CI-HML) values for Cd, Ni, and Pb in the raw and cooked muscles of both cultured and wild Denis fish were determined for both adult and child eaters, and the results are presented in Table 5S and Fig. 5. The CI-Pb and CI-Ni values in the raw and cooked muscles of Denis fish were lower than 1E−6 for children as well as adult consumers, meaning that the carcinogenic hazard caused by Pb and Ni was safe [63]. However, Cd poses a carcinogenic risk (CI-Cd) to adult as well as children’s consumers of Denis fish, as the values of CI-Cd were higher than the acceptable value of 1E−6 [60].
Non-carcinogenic indexes (THQ and HI) and Nickel carcinogenic index (CI-Ni) of metals for both consumers (children and adults) were higher in cultured muscles of raw and cooked Denis fish compared to wild Denis fish. However, the carcinogenic indexes of Cadmium and Lead (CI-Cd and CI-Pb) for both consumers (children and adults) were lower in cultured muscles of raw and cooked Denis fish compared to wild Denis fish. These results agree with Tahity et al. [15].
Conclusion
Cooking strategies (grilling, microwave cooking, and frying) were applied to determine whether the metal levels in cultured and wild Denis muscles may be declined to a safe level or avoided it. The levels of essential HML were a significantly decreased (P < 0.05) in the wild Denis organs compared to cultured ones. Non-essential HML, concentrations increased significantly (P < 0.05) in wild Denis organs compared to cultured ones. Moreover, the slightly high values in pollution indices of cultured Denis compared to wild sources (Bardawil Lake) raise concern for consumers' health due to metal contamination. Hence, it is possible to detect the potential impact of heavy metals by using cooking methods. Results indicated that the levels of the examined metals declined in the sequence of frying > grilling > microwaved cooking. For more accuracy in evaluating the possible risks to human health from eating both wild and cultivated Denis muscles, it was confirmed that the cooking processes gave consumers a clear view of the potential hazards and showed that there was no non-carcinogenic hazard, as well as no carcinogenic risk for Nickel and Lead, except Cadmium, which poses a carcinogenic hazard to adult and children consumers of wild and cultured Denis cooked muscles. These findings show that it is vital to apply the cooking strategies for muscles of wild and cultured Denis to minimize possible health hazards. Therefore, a long-term management strategy and biomonitoring of these HM in Lake Bardawil as Egyptian vision of 2030 and around fish farm waters are required. This will reduce the amount of pollution in the aquatic ecosystem, which represents a health risk to humans who consume polluted fish with HMs.
Data Availability
The data sets in this study are available from the corresponding author upon reasonable request.
References
Yipel M, Tekeli IO, Dikmen B et al (2021) Distribution and ecotoxicological risk assessment of heavy metals in streams of Amanos Mountains from Southern Turkey. Bull Environ Contam Toxicol 107:895–903. https://doi.org/10.1007/s00128-021-03316-2
Abbas MMM, Abd El-Aziz ME, Hassan AM, El-Naggar HA et al (2022) Bioaccumulation, biosedimentation, and health hazards of elements in crayfish, Procambarus clarkii from El-Rahawi Drain and El-Qanatir in the River Nile, Egypt. Biol Trace Elem Res 201:3050–3059. https://doi.org/10.1007/s12011-022-03380-7
Hadj Taieb A, Sley A, Ghorbel M, Jarboui O (2013) Feeding habits of Sparus aurata (Sparidae) from the Gulf of Gabes (central Mediterranean). Cah Biol Mar 54:263–270
GAFRD (General Authority For Fish resources Development) (2012) Report of General Authority for Fish Resources Development on Bardwell Lake. https://www.gafrd.org/posts/450754
Said TO (2022) An overview of the status of Bardawil Lake environment: contemporary constraints, current government policy and proposed sustainable actions. Egypt J Aquatic Res 48(3):181–190. https://doi.org/10.1016/j.ejar.2022.08.002
Cretì P, Trinchella F, Scudiero R (2010 Jun) Heavy metal bioaccumulation and metallothionein content in tissues of the sea bream Sparus aurata from three different fish farming systems. Environ Monit Assess 165(1-4):321–329. https://doi.org/10.1007/s10661-009-0948-z
Döndü M, Özdemir N, Demirak A, Keskin F, Zeynalova N (2023) Bioaccumulation and human health risk assessment of some heavy metals in sediments, Sparus aurata and Salicornia europaea in Güllük Lagoon, the south of Aegean Sea. Environ Sci Pollut Res Int. 30(7):18227–18243. https://doi.org/10.1007/s11356-022-23463-1 Epub 2022 Oct 8
Yeşilbudak, B. (2023). Health risk assessment of heavy metals in seabream (Sparus aurata) sampled from a public market in Türkiye . Eskişehir Teknik Üniversitesi Bilim ve Teknoloji Dergisi - C Yaşam Bilimleri Ve Biyoteknoloji , 12 (1) , 30-41 https://doi.org/10.18036/estubtdc.1199123
Maret W (2016) The metals in the biological periodic system of the elements: concepts and conjectures. Int J Mol Sci 17(1):66. https://doi.org/10.3390/ijms17010066
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) HMs levels toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Celik U, Oehlenschlager J (2004) Determination of zinc and copper in fish samples collected from northeast Atlantic by DPSAV. Food Chem 873:343–347. https://doi.org/10.1016/j.foodchem.2003.11.018
Aljabryn DH (2022) Heavy metals in some commercially fishery products marketed in Saudi Arabia. Food Sci. Technol. https://doi.org/10.1590/fst.34222
Kucukgulmez A, Çelik M, Yanar Y, Ersoy B, Çikrikci M (2006) Effects of different cooking methods on the proximate composition and mineral contents of sea bass Dicentrarchus labrax. Adv Food Sci 284:223–227
AOAC., Association of Official Analytical Chemists (2012) Official methods of analysis, 15th edn. Association of official analytical chemists Inc, Washington, DC USA, p 478
Tahity T, Islam MRU, Bhuiyan NZ, Choudhury TR, Yu J, Noman MA, Hosen MM, Quraishi SB, Paray BA, Arai T (2022) Heavy metals accumulation in organs of wild and farmed Barramundi from the Northern Bay of Bengal Coast, and its estimated human health risks. Toxics 10:410. https://doi.org/10.3390/toxics10080410
USEPA., United States Environmental Protection Agency, 2018 US EPA regional screening levels RSLs, Dataset- California open data. https://data.ca.gov/dataset/us-epa-regional-screening-levels-rsls-november-2018
Mwakalapa EB, Simukoko CK, Mmochi AJ, Mdegela RH, Berg V, Bjorge Muller MH, Polder A, Polder A (2019) HMs levels in cultivated and wild milkfish Chanos chanos and wild mullet Mugil cephalus along the coasts of Tanzania and associated health risk for humans and fish. Chemosphere 224:176–186. https://doi.org/10.1016/jchemosphere201902063
Rahman MS, Molla AH, Saha N, Rahman A (2012) Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Dhaka, Bangladesh. Food Chem 134:1847–1854
Cui L, Ge J, Zhu Y, Yang Y, Wang J (2015) Concentrations, bioaccumulation, and human health risk assessment of organochlorine pesticides and heavy metals in edible fish from Wuhan, China. Environ Sci Pollut Res Int 22:15866–15879
Varol M, Kaya GK, Alp A (2017) Heavy metals and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the firat (euphrates) river: Risk-based consumption advisories. Sci Total Environ 599:1288–1296. https://doi.org/10.1016/j.scitotenv.2017.05.052
Abdel-Aziz ME, Hassan AM, El-Naggar HA, Abbas MMM et al (2022) Potential carcinogenic and non-carcinogenic health risks of heavy metals ingestion from consumption of the crayfish, Procambarus clarkii in El-Rahawy Drain and ElKanater in the River Nile, Egypt. Egypt J Aquat Biol Fish 26:667–686 https://doi.org/10.21608/ejabf.2022.244364
Radwan M, Abbas MMM, Mohammadein A, Al-Malki JS, Elraey SMA, Magdy M (2022) Growth performance, immune response, antioxidative status, and antiparasitic and antibacterial capacity of the Nile Tilapia Oreochromis niloticus after dietary supplementation with bottle gourd Lagenaria siceraria, Molina Seed Powder. Front Mar Sci 9:901439. https://doi.org/10.3389/fmars.2022.901439
Al-Halani AA, Solima AM, Shady SHH (2021) The seasonal assessment of heavy metals bioaccumulation in European Seabass Dicentrarchus labrax inhabiting Damietta Fishing Harbor Egypt. 255:607–625. https://doi.org/10.21608/ejabf.2021.205025
Begum A, Mustafa AI, Amin MN, Chowdhury TR, Quraishi SB, Banu N (2013) Levels of heavy metals in tissues of shingi fish (Heteropneustes fossilis) from Buriganga River, Bangladesh. Environ Monit. Assess 185:5461–5469
Liu Q, Xu X, Zeng J, Shi X, Liao Y, Du P, Tang Y, Huang W, Chen Q, Shou L (2019) Heavy metal concentrations in commercial marine organisms from Xiangshan Bay, China, and the potential health risks. Marine Poll Bull 141:215–226
Abbas MMM, Afifi MAM, Darweesh KF, El-Sharkawy MA, Farrag DMG, Radwan M (2023) Parasitological indicators, haemato-biochemical alternations, and environmental risks of heavy metals in cultivated and wild freshwater catfish, Egypt. Egypt J Aquat Biol Fish 27(4):1085–1205. https://doi.org/10.21608/EJABF.2023.314426
Zhao S, Feng C, Quan W, Chen X, Niu J, Shen Z (2012) Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Marine PollBull 64:1163–1171
Liu Q, Liao Y, Xu X et al (2020) Heavy metal concentrations in tissues of marine fish and crab collected from the middle coast of Zhejiang Province, China. Environ Monit Assess 192:285. https://doi.org/10.1007/s10661-020-8234-1
Liu JL, Xu XR, Ding ZH, Peng JX, Jin MH, Wang YS, Hong YG, Yue WZ (2015) Heavy metals in wild marine fish from South China Sea: levels, tissue- and species-specific accumulation and potential risk to humans. Ecotoxicol 24:1583–1592
El-Gaar DM, Abdelaziz GS, Abbas MMM, Anees F, Genina RME, Ghannam HE, Talab AS (2022) Biochemical alterations of Nile tilapia fish in El-Manzala Lake as an indicator of pollution impacts. Egypt J Aquat Biol Fish 26(3):711–723. https://doi.org/10.21608/ejabf.2022.246093
FAO, Food and Agriculture Organization (2016) Fishery Information Data and Statistics. Unit FISHSTAT, Databases and Statistics Food and Agriculture Tissueization of the United Nation, Rome Italy
Simukoko CK, Mwakalapa EB, Bwalya P, Muzandu K, Berg V, Mutoloki S et al (2022) Assessment of HMs levels in wild and cultivated tilapia (Oreochromis niloticus) on Lake Kariba, Zambia: implications for human and fish health. Food Addit Contam Part A 39(1):74–91. https://doi.org/10.1080/19440049.2021.1975830
Burridge L, Weis JS, Cabello F, Pizarro J, Bostick K (2010) Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture 3061:7–23. https://doi.org/10.1016/jaquaculture201005020
Oumar D, Flibert G, Tidjani A, Rirabe N, Patcha M, Bakary T et al (2018) Risks assessments of heavy metals bioaccumulation in water and Tilapia nilotica fish from Maguite Island of Fitri Lake. Curr J Appl Sci Technol. 26:1–9. https://doi.org/10.9734/cjast/2018/39384
Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, Lawrence R (2008) Aquaculture practices and potential human health risks: current knowledge and future priorities. Environ Int 348:1215–1226. https://doi.org/10.1016/j.envint.2008.04.009
Yipel M, Turk E, Tekeli IO, Oguz H (2016) Heavy metal levels in farmed and wild fishes of Aegean sea and assessment of potential risks to human health. Kafkas U ̈niversitesi Veteriner Faku ̈ltesi Dergisi, pp 22–26. https://doi.org/10.9775/kvfd.2016.15576
Chatta AM, Khan MN, Mirza ZS, Ali A (2016) Heavy metal cadmium, lead, and chromium contamination in farmed fish: a potential risk for consumers’ health. Turk J Zool 40(2):1–9. https://doi.org/10.3906/zoo-1506-1
Abdel-Kader HH, Mourad MH (2023) Estimation of cadmium in muscles of five freshwater fish species from Manzalah Lake, and possible human risk assessment of fish consumption (Egypt). Biol Trace Elem Res 201:937–945. https://doi.org/10.1007/s12011-022-03188-5
Elarabany N, Bahnasawy M (2019) Comparative and interactive biochemical effects of sub-lethal concentrations of cadmium and lead on some tissues of the African catfish (Clarias gariepinus). Toxicol Res 35(3):249–255. https://doi.org/10.5487/TR.2019.35.3.249
Helmy NA, Hassan MA, Hassanien FS, Maarouf AA (2018) Detection of heavy metals residues in fish and shellfish. Benha Veterinary Med J 34(2):255–264
Mahjoub M, Fadlaoui S, El Maadoudi M, Smiri Y (2021) Mercury, lead, and cadmium in the muscles of five fish species from the mechraâ-hammadi dam in morocco and health risks for their consumers. J Toxicol 8865869. https://doi.org/10.1155/2021/8865869
Panigrahi AK, Bakshi A, Pattanayak S (2021) A comprehensive review on the uptake by and accumulation of some heavy metals in fresh water fishes. Biosc Biotech Res Comm 14(1). https://doi.org/10.21786/bbrc/14.1/55
Perera PACT, Kodithuwakku SP, Sundarabarathy TV, Edirisinghe U (2015) Bioaccumulation of cadmium in freshwater fish: an environmental perspective. Insight Ecol 4(1):1–12. https://doi.org/10.5567/ECOLOGYIK.2015.1.12
Hao Y, Miao X, Song M, Zhang H (2022) The bioaccumulation and health risk assessment of metals among two most consumed species of angling fish Cyprinus carpio and Pseudohemiculter dispar in Liuzhou China: winter should be treated as a suitable season for fish angling. Int J Environ Res Public Health 19:1519. https://doi.org/10.3390/ijerph19031519
Miao X, Hao Y, Liu H, Xie Z, Miao D, He X (2021) Effects of heavy metals speciations in sediments on their bioaccumulation in wild fish in rivers in Liuzhou—a typical karst catchment in southwest China. Ecotoxicol Environ Saf 214:112099
Abd-Elghany SM, Zaher HA, Elgazzar MM, Sallam KI (2020) Effect of boiling and grilling on some heavy metal residues in crabs and shrimps from the Mediterranean Coast at Damietta region with their probabilistic health risk assessment. J Food Compos Anal 93:103–106
Ersoy B, Yanar Y, Küçükgülmez A, Çelik M (2006) Effects of four cooking methods on the heavy metal concentrations of sea bass fillets Dicentrarchus labrax Linne, 1785. Food Chem 994:748–751
Abou-Raya MAMT, Shalaby AM, Kassem Amalika D, El-Dahshan F, Ibrahim Y (2007) Effect of cooking methods on heavy metals content in bolti fish from different enviroments. J Agric Sci Mansoura Univ 32(7):5413–5420
Costa S, Afonso C, Bandarra NM, Gueifão S, Castanheira I, Carvalho ML, Cardoso C, Nunes ML (2013) The emerging farmed fish species meagre Argyrosomus regius: how culinary treatment affects nutrients and contaminants concentration and associated benefit-risk balance. Food Chem Toxicol 60:277–285
Kalogeropoulos N, Karavoltsos S, Sakellari A, Avramidou S, Dassenakis M, Scoullos M (2012) Heavy metals in raw, fried and grilled Mediterranean finfish and shellfish. Food Chem Toxicol 50(10):3702–3708. https://doi.org/10.1016/jfct201207012
Hosseini H, Mahmoudzadeh M, Rezaei M, Mahmoudzadeh L, Khaksar R, Khosroshahi NK, Babakhani A (2014) Effect of different cooking methods on minerals, vitamins and nutritional quality indices of kutum roach Rutilus frisii kutum. Food Chem 148:86–91
Arisekar U, Jeya SR, Shalini R, Jeyasekaran G, Padmavathy P, Sukumar D (2022) Effect of different thermal processing methods on potentially toxic metals in the seafood, Penaeus vannamei, and the related human health risk assessment. J Food Compos Anal 105:104259. https://doi.org/10.1016/jjfca2021104259
Kazemi A, Esmaeilbeigi M, Ansari A, Asl AG, Mohammadzadeh B (2022) Alterations and health risk assessment of the environmental concentration of heavy metals in the edible tissue of marine fish Thunnus tonggol consumed by different cooking methods. Reg Stud Marine Sci 53:102361. https://doi.org/10.1016/jrsma2022102361
Khosroshahi NK, Hosseini H, Rezaei M, Khaksar R, Mahmoudzadeh M (2016) Effect of different cooking methods on minerals, vitamins, and nutritional quality indices of rainbow trout (Oncorhynchus mykiss). Int J Food Prop 19(11):2471–2480. https://doi.org/10.1080/10942912.2015.1039028
Perello G, Martí-Cid R, Llobet JM, Domingo JL (2008) Effects of various cooking processes on the concentrations of arsenic, cadmium, mercury, and lead in foods. J Agric Food Chem 56(23):11262–11269
Ersoy B (2011) Effects of cooking methods on the heavy metal concentrations of the African Catfish Clarias Gariepinus. J Food Biochem 352:351–356
Alia TTN, Hing LS, Sim SF, Pradit S, Ahmad A, Ong MC (2020) Comparative study of raw and cooked farmed sea bass Lates calcarifer in relation to metal content and its estimated human health risk. Mar Pollut Bull 153:111009
Fan H, Fan D, Huang J, Zhao J, Yan B, Ma S, Zhou W, Zhang H (2020) Cooking evaluation of crayfish Procambarus clarkia subjected to microwave and conduction heating: a visualized strategy to understand the heat-induced quality changes of food Innovative. Food Sci Emerg Technol 62:102368. https://doi.org/10.1016/j.ifset.2020.102368
Turan MD, Sari Z, Miller JD (2017) Leaching of blended Copper slag in microwave oven. Trans Nonferrous Met Soc China 27(6):1404–1410
Keshavarzi B, Hassanaghaei M, Moore F, Meh MR, Soltanian S, Lahijanzadeh AZ, Sorooshian A (2018) Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Mar Pollut Bull 129:245–252
FAO/WHO (2004) Summary of evaluations performed by the Joint FAO/WHO Expert Committee on Food Additives JECFA 1956–2003. First through sixtyfirst meetings ILSI Press International Life Sciences Institute
Lei M, Tie BQ, Song ZG, Liao BH, Lepo JE, Huang YZ (2015) Heavy metal pollution and potential health risk assessment of white rice around mine areas in Hunan Province China. Food Secur 7:45–54. https://doi.org/10.1007/s12571-014-0414-9
Wang X, Wu J, Yu B et al (2020) Heavy metals in aquatic products and the health risk assessment to population in China. Environ Sci Pollut Res 27:22708–22719. https://doi.org/10.1007/s11356-020-08685-5
Acknowledgments
Thanks are indebted to ALLAH, always and foremost, for his mercy guiding and helping. The author gratefully acknowledges the Department of Zoology, Faculty of Science, Al-Azhar University, Cairo, Egypt for providing the necessary support. Finally, we thank the anonymous reviewers of this article for their careful work and constructive suggestions.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The author declared that no funds, grants, or other support were received during the preparation of this manuscript. Open access funding is provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Dr. Mahmoud Mahrous M. Abbas designed the research idea, sampling, metal, and statistical Analyses as well as wrote the article and revised the finished version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The author followed all the valid national rules (guidelines) for the use and care of dead fish.
Consent for publication
Not applicable
Competing Interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbas, M.M.M. Heavy Metal Levels and Cancer Risk Assessments of the Commercial Denis, Sparus aurata Collected from Bardawil Lake and Private Fish Farm Waters as a Cultured Source, Egypt. Biol Trace Elem Res 202, 2864–2877 (2024). https://doi.org/10.1007/s12011-023-03880-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03880-0