Abstract
Elements accumulation in crayfish is proportional to the increase in bioavailability (direct contact) with the surrounding water, sediment, and feeding. Five heavy metals (Cu, Cr, Mn, Ni, and Ag) and lithium (Li) were analyzed in the sediment, water, and crayfish tissues. Elements (heavy metals and lithium) concentrations in sediment, water, and crayfish tissues showed significant differences between the two sampling stations (El-Qanatir and El-Rahawi drain). However, the levels of elements in crayfish tissues were arranged in declining order as hepatopancreas > gills > exoskeleton > muscles for Cu and Cr; hepatopancreas > exoskeleton > gills > muscles for Ni and Ag; and exoskeleton > gills > hepatopancreas > muscles for Li and Mn. The human health hazard evaluation of heavy metals and lithium exposure via edible tissue consumption was assessed for both children and adult consumers. The target hazard quotient THQ values of crayfish edible tissues (less than 1) will not impose any health implications for consumers who ingest edible tissues in sufficient quantities. Furthermore, the hazard index (HI) values reported for children and adult consumers were lower than one, indicating non-carcinogenic and carcinogenic hazards, suggesting that crayfish edible tissues are safe for human ingestion. This evidence also found that Procambarus clarkii could be a good bio-indicator organism for monitoring potentially metals in aquatic systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Nile River basin is one of the most significant features of Africa’s northeastern basin section, extending for about 6825 km. In the delta, the Nile River is divided into two branches: Rosetta and Damietta. The Rosetta branch receives a variety of pollution categories from many sources, including sewage, domestic, industrial, and agricultural waste effluents from El-Rahawai drains, which total more than 5 × 108 m3 per day [1, 2].
HMs pollution is becoming more of a hazard worldwide, particularly in Egypt. HMs levels widely absorbed and accumulated in tissue; they pose a risk to human health when ingested through contaminated food [3]. HMs levels are mainly correlated with sediments compared to water layers due to the presence of a variety of various, i.e., organic compounds, clay minerals, and metal oxides in the sediment layers [4]. Aquatic species accumulate trace metals in minor amounts via absorption from the water column and in higher quantities via biomagnification from their prey, whereas in humans, they might be ingested through the food chain, leading to acute and chronic health impacts [5]. Among aquatic species, the freshwater crayfish, Procambarus clarkii, receives HMs from the sediments and water in which it lives. Metal accumulation in crayfish tissues has been observed in various scientific studies [6, 7]. Apart from that, exogenous and endogenous variables regulate the bioaccumulation of metals in aquatic species. Environmental parameters such as metal bioactivity, temperatures, and alkalinity of ambient aquatic habitats reflect exogenous variables, whereas endogenous variables include lifetime, environment, size, gender, ecology, physiological operation, and feeding habits [8]. The bio-sedimentation factor is defined as the ratio of heavy metal concentration in the body of organisms to that in the sediment. It makes it possible to evaluate the effectiveness of the bioaccumulation of heavy metals in the organism and give an idea of the speed of substance absorption and excretion by a living organism [9, 10]. Pollutants can pose a significant risk to aquatic fauna through exposed, bioaccumulation, and biomagnification processes [11].
Potential health hazards related to elements exposure have been recognized in recent decades. According to recent studies [12], elements are either carcinogenic or non-carcinogenic to humans. According to their indecomposable and protracted nature inside the visceral organ categories of consumers, HMs pose health risks such as kidney failure, skeletal deformation, and liver failure [13]. As a result, it is critical to examine the potential risks to human health linked to the ingestion of contaminated food. The goals of this study were: (1) to compare heavy metals and lithium concentrations in crayfish tissues, sediment, and water from El-Qanatir and El-Rahawi stations; (2) to determine the bioaccumulation and biosedimentation factors of heavy metals and lithium; and (3) to evaluate the possible human health hazards associated with ingestion of crayfish muscle.
Materials and Methods
Collection of Water, Sediment, and Crayfish Samples
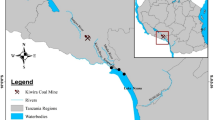
Sediment and water samples were collected from two locations: the first is upstream (El-Qanatir station) on the Nile River, and the second is the El-Rahawi drain, which discharges into the Nile near Rosetta Branch in El-Qalyubia Province (Fig. 1). Specimens were taken between the spring and summer of 2021. Fifty (50) crayfish, Procambarus clarkii, individuals were collected from each studied location by fisherman. Crayfish, P. clarkii, samples were obtained at the same time as water and sediment samples and transported to the laboratory of Environmental Physiology, Faculty of Science, Al-Azhar University, Egypt.
In the lab, crayfish were identified. Crayfish samples (individuals’ lengths ranged from 8.2 to 12.85 cm, with an average of 10.33 ± 1.69 cm, while their total weights ranged from 23.18 to 47.86 g, with an average of 38.52 ± 4.67 g) were re-washed carefully with potable water, then beheaded, skinned, and dissected (using plastic tools) to obtain exoskeleton, muscles, hepatopancreas, and gills for elements analysis.
Elements Levels Measurement
Elements (manganese, Mn; copper, Cu; lithium, Li; nickel, Ni; chromium, Cr; and silver, Ag) were measured in water, sediment, and crayfish tissues (exoskeleton, muscles, hepatopancreas, and gills; n = 5) Heavy metals and lithium were determined in water samples using an acid digestion procedure according to [14]. However, sediment samples were air-dried, sieved (100 mesh). In a 50-mL digesting container, processed samples (0.5 g) were digested with HNO3 (65%, 5 mL), HF (40%, 2 mL), and HClO4 (40%, 1 mL) [14]. Furthermore, 0.5 g of crayfish tissues was dried, then digested in a flask with 2 mL ultrapure HNO3 (65%) and 1 mL H2O2 (30%). The mixture was heated to remove acid until the solutions were completely digested [15].
Finally, the digested mixtures of water, sediment, and tissues were transferred to a volumetric flask and diluted with de-ionized water. An inductively coupled plasma optical emission spectrophotometer (ICP-OES, Model 4300 DV, Perkin Elmer, Shelton, CT, USA) was used to measure the heavy metals and lithium in diluted water, tissues, and sediment solutions (n = 5). To estimate the ppm of each analyst in the diluted solution, samples were treated to a multi-element standard curve. The concentrations of elements in the water were reported in µg/L, whereas those in the crayfish tissues and sediment were expressed in µg/g on a dry weight basis.
Bioaconcentration Factor Calculation
The bioaccumulation factor (Bio-AF) was calculated using the following formula [16] as: Bio-AF = C-crayfish / C-water, where C-crayfish is the concentration of elements in crayfish tissues (µg/kg) and C-water is the concentration of elements in water (µg/L). However, the biosedimentation factor (Bio-SF) was calculated using the following formula [17] as: Bio-SF = C-crayfish / C-sediment, where, C-crayfish, the concentration of elements in crayfish tissues (µg/g), and C-sediment, the concentration of elements in sediments (µg/g).
Health Risk Assessment
The estimated daily intake (EDI) was computed using the formula below in mg−1 kg−1 day−1 units [18] as: EDI = (CF × IR × ER × EP / BW × AT) × 10−3, where CF stands for the elements concentration in crayfish muscle (mg/kg); IR is the intake rate of crayfish ingested (kg/day), which in this investigation was 7.9 g/day for children and 20.1 g/day for adults; ER stands for the exposure rate (365 days/year); EP stands for the exposure time over a lifetime (assumed to be 70 years old); BW stands for body weight, which is 70 kg for adults and 52.5 kg for children 6–11 years old, which is the 95th percentile [19]; AT stands for an average lifetime (70 years, 365 days/year). However, the formula below, which was developed from the ratio of EDI to RfD (oral reference dose of HMs [20], was used to determine target hazard quotient (THQ) as: THQ = EDI / RfD, where RfD stands for elements oral reference doses (mg/kg/days). Moreover, the hazard index (HI) is a mathematical formula that calculates the non-carcinogenic hazard by summing the THQ values of metals under study [20] as follows: HI = THQ(Mn) + THQ(Cu) + THQ(Li) + THQ(Ni) + THQ(Cr) + THQ(Ag). Furthermore, the incremental likelihood of an individual developing cancer depends on the cancer slope factor (CSF), which is defined as the lifelong CR of exposure to HMs. The following formula was used to calculate the carcinogenic risk (CR) as: CR = (ER × EP × EDI × CSF × 10–3) / AT [21], where CSF refers to carcinogenic slope factor, and the CSF values for Ni and Cr are 8.4E − 4 and 4.1E − 2 mg kg−1 day−1, respectively [22].
Statistical Analysis
The statistical analyses were performed using IBM SPSS Statistics Version 22; SPSS Inc., IL, USA, and when significant differences of one-way analysis of variance (ANOVA) were found, multivariate, post hoc Tukey evaluations were utilized to quantify the statistical difference between the elements levels in various crayfish tissues for each element. On the other hand, the independent-samples t test was used to investigate the statistical differences between the two locations (El-Qanatir and El-Rahawi drain) based on elements levels in water and sediments. The Pearson’s correlation coefficient between elements levels in the sediment, water, and studied crayfish tissues was investigated. However, statistical significance was performed at p < 0.05.
Results and Discussion
Elements Levels in Water and Sediment Samples
Table 1 shows the levels of heavy metals and lithium in the water of the study stations (El-Qanatir and El-Rahawi stations). Except for silver, the levels of elements (Mn, Cu, Li, Ni, and Cr) in the water of the examined stations were significantly higher in El-Rahawi drain compared to El-Qanatir station (t test, p < 0.05). However, the highest value of elements (54.55 ± 7.00 µg/L) was recorded in the El-Rahawi drain station for copper, and the lowest value (0.1 ± 0.005 µg/L) was recorded in both stations for silver, with elements arranged as Cu > Mn > Li > Ni > Cr > Ag in the El-Rahawi drain, and Mn > Cu > Li > Ni > Cr > Ag in the El-Qanatir station.
In Egypt and other developing countries, where environmental protection laws have not been implemented, industrial and domestic wastes are dumped indiscriminately, entering water bodies. Toxic and hazardous chemicals, including metals, have been found in these wastes. Because of their toxicity, durability, and bioaccumulative nature, heavy metals poisoning of water resources is a major problem [23]. A comparison of the current study’s heavy metals levels in water and sediment with previous studies (Table 2). The Cr, Cu, Ni, and Mn levels in the water of the study stations were lower than WHO’s standard permissible values [24]. The level of Cr in the current study was lower than those recorded [25, 26]. The Cu level in the current study was within that mentioned [25, 27] . However, it was lower than that detected [28]. Moreover, it was higher than those recorded [26, 29]. The level of Ni in the current study was higher than that reported [29]. Moreover, it was lower than those determined [26,27,28]. The level of Mn in the current study was lower than that recorded by [25,26,27,28,29].
Sediment pollution is one of the most serious environmental issues affecting ecosystems, as sediments serve as both sinks and sources of toxins in aquatic systems. Sediment analysis is crucial for determining the degree of pollution in the environment [30]. Result reported that El-Rahawi drain station showed significantly higher levels of heavy metals and lithium in the sediment than El-Qanatir station (t test, p < 0.05, Table 1). The highest value of elements (255.69 ± 8.50 µg/g dw) was recorded in the El-Rahawi drain for manganese, and the lowest value (1.65 ± 0.68 µg/g dw) was noted in the El-Qanatir stations for silver, with elements arranged in the El-Rahawi drain as Mn > Li > Ni > Cu > Cr > Ag and in the El-Qanatir station as Mn > Li > Cu > Ni > Cr.
The concentrations of copper and manganese in sediment of studied stations were lower than the standard acceptable values, according to [31]. However, the Cr level in the El-Rahawi drain sediment was above the acceptable limit [31]. Levels of Cr in the current study were within those recorded [32, 33]. The levels of Cu in the current study were within range of those detected [32]. However, it was higher than those recorded [28, 33]. Levels of Ni in the current study were higher than those reported [28, 32, 33]. Levels of Mn in the current study were higher than those determined [28]. Moreover, it was lower than those recorded [32, 33].
Elements Levels in Crayfish Tissues
Elements are accumulated by freshwater P. clarkii, crayfish in their tissues from the water, and sediments in which they live. Environmental factors affect metal bioaccumulation in aquatic species, but so do internal factors, including life cycle, habitat, and feeding habits [8]. The levels of heavy metals and lithium in the tissues of crayfish, P. clarkii collected from El-Qanatir and El-Rahawi stations, were represented in Table 3. The highest value of elements levels in the muscles was recorded in El-Rahawi drain for manganese (5.41 ± 0.08 µg/g dw), and the lowest value (0.24 ± 0.05 µg/g dw) was determined in El-Qanatir station for silver. However, the maximum values of elements levels in the crayfish exoskeleton collected from El-Rahawi drain were recorded for manganese (51.77 ± 2.08 µg/g dw), and the minimum values were determined in El-Qanatir station for chromium (1.20 ± 0.10 µg/g dw). Moreover, the maximal value of elements levels in the crayfish gills was recorded in El-Rahawi drain for manganese (63.40 ± 1.94 µg/g dw), while the minimal value (0.44 ± 0.02 µg/g dw) was determined in El-Qanatir station for silver. Furthermore, the highest value of elements levels in the hepatopancreas was recorded in El-Rahawi drain for copper (100.12 ± 3.21 µg/g dw), while the lowest value (3.29 ± 0.91 µg/g dw) was determined in the El-Qanatir station for lithium. There were significant differences (p < 0.05) in the one-way analysis of variance between the different stations and organs, for all metals. Table 4 shows the Pearson correlation based on values of heavy metals and lithium in water, sediment, and tissues of crayfish collected from El-Qanatir and El-Rahawi drain stations. El-Rahawi drain had significantly higher levels of elements in water, sediment, and crayfish tissues than El-Qanatir station (t test, p < 0.05), which may be due to the increased influence of sewage, industrial, and agricultural discharge, as well as deposition of these metals from the atmosphere. This finding agrees with [23, 34]
The presented data reports that one-way analysis of variance (ANOVA) based on heavy metals and lithium in the different tissues of crayfish revealed significant differences between crayfish tissues in both studied locations (ANOVA, p < 0.05). Additionally, heavy metals and lithium in the different tissues of crayfish were ordered in descending order according to; hepatopancreas > gills > exoskeleton > muscles for Cu and Cr; hepatopancreas > exoskeleton > gills > muscles for Ni and Ag; exoskeleton > gills > hepatopancreas > muscles for Li and Mn. This finding agrees with [7], who mentioned that the bio-accumulation of Cu in the tissues of Procambarus clarkii was hepatopancreas > gills > exoskeleton > muscles. However, Mn was ordered in the following order: exoskeleton > gills > hepatopancreas > muscles of crayfish. Manganese is in direct contact with the crayfish shell, and its level in the shell was higher than in water, due to its assimilation into the calcium carbonate structure [7].
The highest values of Bio-AF and Bio-SF of heavy metals and lithium in the tissues of P. clarkii crayfish taken from El-Qanatir and El-Rahawi stations were recorded in hepatopancreas and the lowest observed in the muscles (Figs. 2–3).The high metal accumulation in the crayfish hepatopancreas may explain the synthesis of low-molecular-weight metal-binding (metallothionein-like) proteins, which have been widely recorded in different crustacean species [35]. On the other hand, the metal concentrations were accumulated in the muscles of crayfish at the lowest values [22]. Many authors have noted a similar accumulation pattern for various HMs, indicating that higher accumulations were observed in the exoskeleton, gills, and hepatopancreas, whereas lower accumulations were observed in the muscle tissues [6, 7]. Each metal exhibits a different accumulation pattern based on its interaction with crayfish metabolism. Non-essential metals have no metabolic function in crustacean species, and the tissue contents of these metals are unregulated [35].
The levels of Li and Ag in crayfish tissues can thus be used to estimate metal levels in the environment. Thus, the levels of Li and Ag in P. clarkii reflect the levels of these metals in the water and sediment. Cu and Ni are also widely found in the hepatopancreas of other crayfish species [6]. Regardless of their environmental levels, Cu is found in high concentrations in crayfish tissues. Cu bioaccumulation in crayfish tissues increases significantly whenever their environmental content is increased [36].
The BIO-SF is the ratio of metal levels in the liver (hepatopancreas) to sediment levels [37]. Organisms are divided into three groups based on their BIO-SF values: (1) If the BIO-SF is greater than 2, the organism is a macro-concentrator. (2) If 1 < BIO-SF < 2, it indicates that the organism is a micro-concentrator. (3) If BIO-SF is lower than 1, the organism is de-concentrating the metal and releasing it into the sediment [38]. Based on the above, the crayfish tissues from El-Qanatir station showed that BIO-SF of muscles is de-concentrators for all metals and releases the metals in sediment. BIO-SF of the exoskeleton is de-concentrators for all metals except Ag. BIO-SF of gills is de-concentrators for all metals except Cu. BIO-SF of exoskeleton is de-concentrators for all metals except Ag.
Human Health Risk Assessment
The EDIs of the metal ions under investigation are lower than RfDs (oral reference dosages) [21, 22]. As a result, normal crayfish ingestion should not pose a serious health risk to consumers, while caution should be exercised to avoid excessive consumption. The estimated daily intake (EDI, mg/kg/day) and the target hazard quotients (THQ) for metals in crayfish muscles was shown in Table 5. The sequence of EDI values in crayfish from El-Qanatir stations, it was ranged from 3.61E − 05 mg/kg/day for children consumers to 1.25E − 03 mg/kg/day for adults. However, in El-Rahawi drain, it fluctuated between 1.05E − 05 mg/kg/day for children consumers and 2.08E − 03 mg/kg/day for adult consumers. The 95th percentile of the THQ and HI was used to rank the non-carcinogenic risk of each metal among species and tissues [39]. The values of THQ in the studied stations were varied from 2.5E − 03 for children to 4.02E − 02 for adults. Additionally, the hazard index (HI) in the muscles of crayfish was 4.86E − 02 for the children’s consumer and 1.08E − 01 for the adult consumers (Fig. 4). THQs and HI calculated for heavy metals and lithium in crayfish are less than 1, which indicates that crayfish consumption does not pose a non-carcinogenic hazard for children and adults who consume crayfish in sufficient quantities. The CR values for Ni and Cr in crayfish muscles from the El-Rahawi drain and El-Qanatir stations were calculated for both children and adult consumers, and the findings are shown in Fig. 5. In both study locations, the CR values of Cr varied between 1.9E − 06 and 4.9E − 06. However, CR values of Ni in both study locations ranged from 4.2E − 08 to 1.2E − 07. Using the acceptable limit of E − 4, the carcinogenic risk (CR) values for Ni and Cr do not pose a carcinogenic risk to children and adult consumers of crayfish muscles in both studied stations as the CR values are less than 1E − 6 for both children and adult consumers [21, 22, 39].
Conclusion
Elements in water, sediment, and crayfish tissues were significantly higher in El-Rahawi drain than in El-Qanatir station. This indicates that El-Rahawi drain has more elements pollution than El-Qanatir station. The hepatopancreas of crayfish accumulated higher levels of elements than the other tissues, which is consistent with previous research. However, crayfish muscle accumulated elements at the lowest levels. Finally, the studied heavy metals and lithium will not cause any considerable adverse health effects to humans based on estimated daily intake, carcinogenic hazards, and non-carcinogenic hazards. Therefore, crayfish muscles are suitable for human consumption.
Data Availability
The authors declare that data are available from the corresponding author at readers’ request.
Code Availability
Not applicable.
References
Abdo MH (2013) Physico-chemical studies on the pollutants effect in the aquatic environment of Rosetta Branch River Nile. Egypt Life Sci J 10:493–501
Abou El-Anwar EA, Samy YM, Salman SA (2018) Heavy metals hazard in Rosetta Branch sediments. Egypt J Mater Environ Sci 9:2142–2152
- Griboff J, Wunderlin DA, Monferran MV (2017) Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health?. Microchem J 130:236–244. 10.1016%2Fj.microc.2016.09.013
- Nagarajan R, Anandkumar A, Hussain SM (2019) Chapter 12-geochemical characterization of Beach sediments of miri, NW borneo, SE Asia: implications on provenance, weathering intensity, and assessment of coastal environmental Status. In: Ramkumar, Mu, Arthur James, R., Menier, David, Kumaraswamy, K. (Eds.), Coastal Zone Management. London UK Elsevier 279–330. https://doi.org/10.1016/B978-0-12-814350-6.00012-4
Anandkumar A, Nagarajan R, Prabakaran K et al (2018) Human health risk assessment and bioaccumulation of trace metals in fish species collected from the Miri coast, Sarawak, Borneo. Mar Pollut Bull 133:655–663. https://doi.org/10.1016/j.marpolbul.2018.06.033
Kuklina I, Kouba A, Buřič M et al (2014) Accumulation of heavy metals in crayfish and fish from selected Czech reservoirs. Biomed Res Int 306103.https://doi.org/10.1155/2014/306103
El Assal FM, Abdel-Meguid ZA (2017) Impact of HM pollution on Procambarus clarkii (Crustacea: Decapoda), from Egypt. Int J Waste Resour 7:270. https://doi.org/10.4172/2252-5211.1000270
Anandkumara A, Lia J, Prabakarana K et al (2020) Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans. J F Compo Ana 88:103449. https://doi.org/10.1016/j.jfca.2020.103449
Liu HB, Yang J, Gan JL (2010) Trace element accumulation in bivalve mussels Anodonta woodiana from Taihu Lake, China. Arch Environ Contam Toxicol 59:593–601. https://doi.org/10.1007/s00244-010-9521-6
Zhao L, Yang F, Yan X et al (2012) Heavy metal concentrations in surface sediments and manila clams (Ruditapes philippinarum) from the Dalian Coast, China after the Dalian Port Oil Spill. Biol Trace Elem Res 149:241–247. https://doi.org/10.1007/s12011-012-9412-y
- Abd El-Aziz, ME, Hassan AM, El-Naggar HA et al (2022) Potential carcinogenic and non-carcinogenic health risks of heavy metals ingestion from consumption of the crayfish, Procambarus clarkii in El-Rahawy Drain and ElKanater in the River Nile, Egypt. Egypt J Aquat Biol Fish 26: 667– 686. https://doi.org/10.21608/ejabf.2022.244364
Jia Y, Wang L, Qu Z et al (2017) Effects on heavy metals accumulation in freshwater fishes: species, tissues, and sizes. Environ Sci Pollut Rse 24:9379–9386. https://doi.org/10.1007/s11356-017-8606-4
- Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2:112–118. http://www.academicjournals.org/IJPS
- AOAC, Association of Official Analytical Chemists (2012) Official methods of analysis. (15th ed.) Assoc Off Anal Chem Inc Washington, DC USA 478p
Wang X, Wu J, Yu B et al (2020) Heavy metals in aquatic products and the health risk assessment to population in China. Environ Sci Pollut Res 27:22708–22719. https://doi.org/10.1007/s11356-020-08685-5
Adolfsson-Erici M, Åkerman G, Mclachlan MS (2012) Measuring bioconcentration factors in fish using exposure to multiple chemicals and internal benchmarking to correct for growth dilution. Environ Toxicol Chem 31:1853–1860. https://doi.org/10.1002/etc.1897
Usero J, Marilla J, Graccia I (2005) Heavy metals concentrations in mollusc from the Atlantic Coast of Sothern Spain. Chemosphere 59:1175–1181. https://doi.org/10.1016/j.chemosphere.2004.11.089
Mwakalapa EB, Simukoko CK, Mmochi AJ et al (2019) Heavy metals in farmed and wild milkfish (Chanos chanos) and wild mullet (Mugil cephalus) along the coasts of Tanzania and associated health risk for humans and fish. Chemosphere 224:176–186. https://doi.org/10.1016/j.chemosphere.2019.02.063
- USEPA, United States Environmental Protection Agency (2008) Child-specific exposure factors handbook. EPA/600/R-06/096F, National Center for Environmental Assessment Office of Research and Development, Washington. District of Columbia, pp. 1–687. http://www.epa.gov/ncea
- USEPA, United States Environmental Protection Agency (2011) Risk assessment guidance for superfund. Volume I: (Part A: Human health evaluation manual; part e, supplemental guidance for dermal risk assessment; Part F, Supplemental guidance for inhalation risk assessment). US Environ Prot Agency Washington, DC
Varol M, Kaya GK, Alp A (2017) Heavy metals and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the firat (euphrates) river: risk-based consumption advisories. Sci Total Environ 599:1288–1296. https://doi.org/10.1016/j.scitotenv.2017.05.052
Xiong B, Xu T, Li R et al (2020) Heavy metal accumulation and health risk assessment of crayfish collected from cultivated and uncultivated ponds in the middle reach of Yangtze river. Sci Total Environ 739:139963. https://doi.org/10.1016/j.scitotenv.2020.139963
Malhat FM, Nasr I (2012) Metals in water from the River Nile tributaries in Egypt. Bull Environ Contam Toxicol 88:594–596. https://doi.org/10.1007/s00128-012-0562-6
- WHO, World Health Organization (2011) Guidelines for drinking water quality 4th edn, http://www.who.int/watersanitationhealth/publications/dwqguidelines/en/
El-Sheekh MM (2016) Impact of water quality on ecosystems of the Nile River. The Nile River 3:357–385
Gad M, Elsayed S, Moghanm S et al (2020) Combining water quality indices and multivariate modeling to assess surface water quality in the Northern Nile Delta. Egypt Water 12:2142. https://doi.org/10.3390/w12082142
Ibrahim M, Kamel A, Omar A, Madkour A (2017) Environmental impacts of metal pollution sources on Rosetta Branch Water Quality. Nature Sci 15:117–123. https://doi.org/10.7537/marsnsj150717.16
El Amier YA, Zahran MA, Al-Mamoori SO (2015) Environmental changes along Damietta branch of the River Nile. Egypt J Environ Sci Mansoura Univ 44:235–255
Taher ME, Ghoneium AM, Hopcroft RR, El Tohamy WS (2021) Temporal and spatial variations of surface water quality in the Nile River of Damietta Region. Egypt Environ Monit Assess 193:1–18. https://doi.org/10.1007/s10661-021-08919-0
Mucha AP, Vasconcelos MD, Bordalo AA (2003) Macro benthic community in the Douro Estuary: relations with heavy metals and natural sediment characteristics. Environ Pollut 121:169–180. https://doi.org/10.1016/S0269-7491(02)00229-4
- FAO/WHO (2011) Joint FAO/WHO food standards programme codex committee on contaminants in foods fifth session, http://www.fao.org/tempref/codex/Meetings/CCCF/CCCF5/cf05_INF.pdf
El Bouraie MM, Barbary AA, El YMM, Motawea EA (2010) Heavy metal concentrations in surface river water and bed sediments at Nile Delta in Egypt. Suo 61:1–12
Abou El-Anwar EA (2019) Assessment of heavy metal pollution in soil and bottom sediment of Upper Egypt: comparison study. Bull Natl Res Cent 43:1–11. https://doi.org/10.1186/s42269-019-0233-4
El Bouraie MM, El Barbary AA, Yehia MM (2011) Determination of organochlorine pesticide (OCPs) in shallow observation wells from El Rahawy contaminated area. Egypt Environ Res Eng Manag 3:28–38
- Cogun HY Firat O, Aytekin T et al (2017) Heavy metals in the Blue Crab (Callinectes sapidus) in Mersin Bay, Turkey. Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-017-2086-6
Mac Donald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31. https://doi.org/10.1007/s002440010075
Abdallah M, Abdallah A (2008) Biomonitoring study of heavy metals in biota and sediments in the South Eastern coast of Mediterranean sea. Egypt Environ Monit Assess 146:139–145. https://doi.org/10.1007/s10661-007-0066-8
Ziyaadini M, Yousefiyanpour Z, Ghasemzadeh J, Zahedi M (2017) Biota-sediment accumulation factor and concentration of heavy metals (Hg, Cd, As, Ni, Pb and Cu) in sediments and tissues of Chiton lamyi (Mollusca: Polyplacophora: Chitonidae) in Chabahar Bay Iran. Iran J Fish Sci 16:1123–1134
El-Said GF, El-Sadaawy MM, Shobier AH et al (2021) Human health implication of major and trace elements present in commercial crustaceans of a traditional seafood marketing region. Egypt Biol Trace Elem Res 199:315–328. https://doi.org/10.1007/s12011-020-02126-7
Acknowledgements
Thanks are indebted to ALLAH, always and foremost, for his mercy guiding and helping. The authors gratefully acknowledge the Department of Zoology, Faculty of Science, Al-Azhar University, Cairo, Egypt for providing the necessary support. Finally, we thank the anonymous reviewers of this article for their careful work and constructive suggestions.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MA: writing—original draft, statics and formal analysis, methodology, investigation. MAEA: methodology, investigation, visualization. AKH: writing, methodology, visualization, writing—review and editing. MR: writing, formal analysis, methodology, editing. MA: formal analysis, methodology, conceptualization, editing. MK: investigation, statics and formal analysis, review and editing. MET: statics and formal analysis, review and editing. HEN: statics and formal analysis, review and editing. M B: methodology, conceptualization, prepared figures and tables. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
The participants signed an informed consent form before the study started.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbas, M.M.M., Abd El-Aziz, M.A., Kaddah, M.M. et al. Bioaccumulation, Biosedimentation, and Health Hazards of Elements in Crayfish, Procambarus clarkii from El-Rahawi Drain and El-Qanatir in the River Nile, Egypt. Biol Trace Elem Res 201, 3050–3059 (2023). https://doi.org/10.1007/s12011-022-03380-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03380-7