Abstract
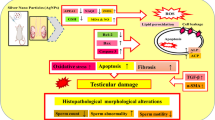
Reproductive toxicity is a major challenge associated with aluminum (Al) exposure. Therefore, this study aimed to investigate the effects of zinc oxide nanoparticle (ZnONP) treatment on Al-induced reproductive toxicity in rats. Thirty-two adult male albino rats were allocated into four equal groups as follows: control, AlCl3 orally administered group (100 mg/kg bwt), ZnONPs injected intraperitoneally (i.p.) group (4 mg/kg bwt), and ZnONPs + AlCl3–treated group. The treatment was daily extended for 42 consecutive days. Oral administration of AlCl3 showed an oxidative damage confirmed by an increase in malondialdehyde and nitric oxide levels and superoxide dismutase activity and accompanied by a decrease in glutathione content and catalase activity. Also, AlCl3 administration increased the pro-inflammatory mediator tumor necrosis factor-alpha. Furthermore, significant declines in the levels of serum male reproductive hormones testosterone, luteinizing hormone, and follicle-stimulating hormone in AlCl3-intoxicated rats were noticed. In parallel, severe histopathological alterations were observed in testis tissues. Additionally, the immunohistochemical analysis showed that AlCl3 administration potentiates cell death in the testicular tissue by elevating the immunostaining intensity signal for the pro-apoptotic protein, cysteinyl aspartate specific protease-3 (caspase-3) and a marked depletion in the cell proliferation expression marker, Ki-67, in germinal cells of AlCl3-treated group. On the other hand, the daily i.p. injection to rats with ZnONPs before AlCl3 was found to ameliorate the reproductive toxicity induced by Al administration through reducing the testicular oxidative stress and improving the inflammatory, apoptotic, and reproductive markers as well as histopathological alterations in the testis. These results suggest that ZnONPs could be used as an alternative agent to minimize the reproductive toxicity associated with Al exposure through its antioxidant, anti-inflammatory, anti-apoptotic, and reproductive modulatory activities.






Similar content being viewed by others
Data Availability
All relevant data are within the paper.
References
Liu J, Wang Q, Sun X, Yang X, Zhuang C, Xu F, Cao Z, Li Y (2016) The toxicity of aluminum chloride on kidney of rats. Biol Trace Elem Res 173(2):339–344
Sun X, Sun H, Yu K, Wang Z, Liu Y, Liu K, Zhu Y, Li Y (2018) Aluminum chloride causes the dysfunction of testes through inhibiting the ATPase enzyme activities and gonadotropin receptor expression in rats. Biol Trace Elem Res 183(2):296–304
Kinawy AA (2019) Potential toxicity of aluminum and fluoride on some biochemical aspects of male rat’s offspring. J Basic Appl Zool 80(1):18
Mouro VG, Menezes TP, Lima GD, Domingues RR, Souza ACF, Oliveira JA, Matta SL, Machado-Neves M (2018) How bad is aluminum exposure to reproductive parameters in rats? Biol Trace Elem Res 183(2):314–324
Mohamed DA, Abdelrahman SA (2019) The possible protective role of zinc oxide nanoparticles (ZnONPs) on testicular and epididymal structure and sperm parameters in nicotine-treated adult rats (a histological and biochemical study). Cell Tissue Res 375(2):543–558
Semercioz A, Baltaci AK, Mogulkoc R, Avunduk MC (2017) Effect of zinc and melatonin on oxidative stress and serum inhibin-B levels in a rat testicular torsion–detorsion model. Biochem Genet 55(5–6):395–409
Sacan O, Turkyilmaz IB, Bayrak BB, Mutlu O, Akev N, Yanardag R (2016) Zinc supplementation ameliorates glycoprotein components and oxidative stress changes in the lung of streptozotocin diabetic rats. Biometals 29(2):239–248
Babaknejad N, Bahrami S, Moshtaghie AA, Nayeri H, Rajabi P, Iranpour FG (2018) Cadmium testicular toxicity in male Wistar rats: protective roles of zinc and magnesium. Biol Trace Elem Res 185(1):106–115
Mahmoud ARH, Shalaby NMM (2019) Ameliorative effect of zinc oxide nanoparticles on nicotine induced testicular dysfunction; biochemical and histological study. Toxicol Environ Heal Sci 11(2):104–113
Abdel Moneim AE, Othman MS, Mohmoud SM, El-Deib KM (2013) Pomegranate peel attenuates aluminum-induced hepatorenal toxicity. Toxicol Mech Methods. https://doi.org/10.3109/15376516.2013.823634
Othman MS, Hafez MM, Abdel Moneim AE (2019) The potential role of zinc oxide nanoparticles in microRNAs dysregulation in STZ-induced type 2 diabetes in rats. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-02012-x
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Niskikimi M, Rao N, Yog K (1972) Colormetric determination of superoxide dismutase activity. Biochem Biophys Res Commun 46:849–851
Johansson LH, Borg LH (1988) A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem 174(1):331–336
Pedrycz A, Czerny K (2008) Immunohistochemical study of proteins linked to apoptosis in rat fetal kidney cells following prepregnancy adriamycin administration in the mother. Acta Histochem 110(6):519–523
Al-Olayan EM, El-Khadragy MF, Abdel Moneim AE (2015) The protective properties of melatonin against aluminium-induced neuronal injury. Int J Exp Pathol 96(3):196–202. https://doi.org/10.1111/iep.12122
Cheraghi E (2019) Roshanaei K (2019) The protective effect of curcumin against aluminum chloride-induced oxidative stress and hepatotoxicity in rats. Pharm Biomed Res 5(1):11–18
Tasdemir M, Celikezen FC, Oto G, Ozbey F (2020) The effects of pretreatment with lithium metaborate dihydrate on lipid peroxidation and Ca, Fe, Mg, and K levels in serum of Wistar albino male rats exposed to Cd. Environ Sci Pollut Res Int 27(7):7702–7711. https://doi.org/10.1007/s11356-019-07516-6
Afolabi OK, Wusu AD, Ugbaja R, Fatoki JO (2018) Aluminium phosphide-induced testicular toxicity through oxidative stress in Wistar rats: ameliorative role of hesperidin. Toxicol Res Appl 2:2397847318812794. https://doi.org/10.1177/2397847318812794
Kalaiselvi A, Suganthy OM, Govindassamy P, Vasantharaja D, Gowri B, Ramalingam V (2014) Influence of aluminium chloride on antioxidant system in the testis and epididymis of rats. Iran J Toxicol 8(24):991–997
Bhalla P, Singla N, Dhawan DK (2010) Potential of lithium to reduce aluminium-induced cytotoxic effects in rat brain. Biometals 23(2):197–206. https://doi.org/10.1007/s10534-009-9278-4
Peter O, Ignatius M, Nnaemeka U, O O, (2017) Antioxidant status in aluminum intoxified male Wistar albino rats. The Pharmaceutical and Chemical Journal 4:102–108
Zakaria MMH, Hajipour B, Estakhri R, Saleh BM (2017) Anti-oxidative effect of resveratrol on aluminum induced toxicity in rat cerebral tissue. Bratisl Lek Listy 118(5):269–272. https://doi.org/10.4149/BLL_2017_053
Al Dera HS (2016) Protective effect of resveratrol against aluminum chloride induced nephrotoxicity in rats. Saudi Med J 37(4):369–378. https://doi.org/10.15537/smj.2016.4.13611
Wang ZL, Yang LY, Chen HH, Lin HH, Tsai YT, Huang WJ (2017) Effects of TNF-alpha on penile structure alteration in rats with hyperprolactinemia. PLoS ONE 12(8):e0181952. https://doi.org/10.1371/journal.pone.0181952
Campbell A, Becaria A, Lahiri DK, Sharman K, Bondy SC (2004) Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J Neurosci Res 75(4):565–572. https://doi.org/10.1002/jnr.10877
Abdel Moneim AE (2012) Evaluating the potential role of pomegranate peel in aluminum-induced oxidative stress and histopathological alterations in brain of female rats. Biol Trace Elem Res 150(1–3):328–336. https://doi.org/10.1007/s12011-012-9498-2
Rani V, Verma Y, Rana K, Rana SVS (2018) Zinc oxide nanoparticles inhibit dimethylnitrosamine induced liver injury in rat. Chem Biol Interact 295:84–92
Kim MH, Jeong HJ (2015) Zinc oxide nanoparticles suppress LPS-induced NF-kappaB activation by inducing A20, a negative regulator of NF-kappaB, in RAW 264.7 macrophages. J Nanosci Nanotechnol 15(9):6509–6515. https://doi.org/10.1166/jnn.2015.10319
Omu AE, Al-Azemi MK, Al-Maghrebi M, Mathew CT, Omu FE, Kehinde EO, Anim JT, Oriowo MA, Memon A (2015) Molecular basis for the effects of zinc deficiency on spermatogenesis: an experimental study in the Sprague-Dawley rat model. Indian J Urol 31(1):57–64. https://doi.org/10.4103/0970-1591.139570
Muselin F, Cristina RT, Igna V, Dumitrescu E, Brezovan D, Trif A (2016) The consequences of aluminium intake on reproductive function in male rats: a three-generation study. Turk J Med Sci 46(4):1240–1248. https://doi.org/10.3906/sag-1501-101
Cheraghi E, Golkar A, Roshanaei K, Alani B (2017) Aluminium-induced oxidative stress, apoptosis and alterations in testicular tissue and sperm quality in Wistar rats: ameliorative effects of curcumin. Int J Fertil Steril 11(3):166
Guo CH, Lin CY, Yeh MS, Hsu GS (2005) Aluminum-induced suppression of testosterone through nitric oxide production in male mice. Environ Toxicol Pharmacol 19(1):33–40
Fallah A, Mohammad-Hasani A, Colagar AH (2018) Zinc is an essential element for male fertility: a review of Zn roles in men’s health, germination, sperm quality, and fertilization. J Reprod Infertil 19(2):69–81
Afifi M, Almaghrabi OA, Kadasa NM (2015) Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res Int 2015:153573. https://doi.org/10.1155/2015/153573
Komatsu T, Tabata M, Kubo-Irie M, Shimizu T, Suzuki K, Nihei Y, Takeda K (2008) The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol In Vitro 22(8):1825–1831
Stocco DM (2000) Intramitochondrial cholesterol transfer. Biochim Biophys Acta 1486(1):184–197
Pinho AR, Rebelo S, Pereira ML (2020) The impact of zinc oxide nanoparticles on male (in)fertility. Materials (Basel) 13 (4)
Mesole SB, Alfred OO, Yusuf UA, Lukubi L, Ndhlovu D (2020) Apoptotic inducement of neuronal cells by aluminium chloride and the neuroprotective effect of eugenol in Wistar rats. Oxid Med Cell Longev 2020:8425643. https://doi.org/10.1155/2020/8425643
Anan HH, Zidan RA, Abd El-Baset SA, Ali MM (2018) Ameliorative effect of zinc oxide nanoparticles on cyclophosphamide induced testicular injury in adult rat. Tissue Cell 54:80–93
Eron SJ, MacPherson DJ, Dagbay KB, Hardy JA (2018) Multiple mechanisms of zinc-mediated inhibition for the apoptotic caspases-3,-6,-7, and-8. ACS Chem Biol 13(5):1279–1290
Kabel AM (2018) Zinc/alogliptin combination attenuates testicular toxicity induced by doxorubicin in rats: role of oxidative stress, apoptosis and TGF-beta1/NF-kappaB signaling. Biomed Pharmacother 97:439–449
Huber KL, Hardy JA (2012) Mechanism of zinc-mediated inhibition of caspase-9. Protein Sci 21(7):1056–1065. https://doi.org/10.1002/pro.2090
Bulan ÖK, Bayrak BB, Sarikaya-Unal G, Yanardag R (2019) The influence of melatonin supplementation against aluminum-induced toxicity in brains of male rats. J Res Pharm 23(2):275–283
Wang C, Zhang L, Ying Z, He J, Zhou L, Zhang L, Zhong X, Wang T (2018) Effects of dietary zinc oxide nanoparticles on growth, diarrhea, mineral deposition, intestinal morphology, and barrier of weaned piglets. Biol Trace Elem Res 185(2):364–374
Miska-Schramm A, Kapusta J, Kruczek M (2017) The effect of aluminum exposure on reproductive ability in the bank vole (Myodes glareolus). Biol Trace Elem Res 177(1):97–106. https://doi.org/10.1007/s12011-016-0848-3
Al-Ani N, Al-Kawaz U, Saeed T (2015) Protective influence of zinc on reproductive parameters in male rat treated with cadmium. Am J Med Sci 5(2):73–81. https://doi.org/10.5923/j.ajmms.20150502.03
Author information
Authors and Affiliations
Contributions
N.A. El-Yamany designed the project. E. Ashraf performed the experiments and analyzed the data. M. Lokman, R.B. Kassab, and A.E. Abdel Moneim interpreted the data. All the authors drafted and edited the manuscript. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved, and all experimental procedures were performed by and according to the guidelines of the Committee of Research Ethics for Laboratory Animal Care, Department of Zoology and Entomology, Faculty of Science, Helwan University (Cairo, Egypt; approval no, HU2017/Z/EAE0817-09).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lokman, M., Ashraf, E., Kassab, R.B. et al. Aluminum Chloride–Induced Reproductive Toxicity in Rats: the Protective Role of Zinc Oxide Nanoparticles. Biol Trace Elem Res 200, 4035–4044 (2022). https://doi.org/10.1007/s12011-021-03010-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03010-8