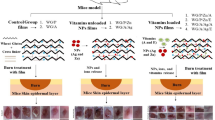
Abstract
Treatment of burn wounds has many requirements to ensure wound closure with healthy tissue, increased vascularization, guarantee edema resolution, and control bacterial infection. We propose that titanium oxide (TiO2) nanoparticles (NPs) will be more efficient than silver dioxide (Ag2O) in the treatment of burn wounds. Herein, gelatin loaded NPs (GLT-NPs) were evaluated for their efficacy to regenerate second-degree burn wound in rabbit skin. TEM results revealed that the average particle sizes were ⁓ 7.5 and 17 nm for Ag2O and TiO2 NPs, respectively. The results of the in vivo application of GLT-NPs on burn wound in the rabbit revealed that both Ag2O and TiO2 NPs were efficient than the control none treated (CTRL) and GLT group. In terms of the healing rate, the GLT-TiO2 did not show any significant difference than GLT-Ag2O (99.57% vs. 99.85%, p = 0.2). Meanwhile, the healing rate was significantly higher in both NPs’ treated groups than CTRL (94.16%, p < 0.01) and GLT group (95.07%, p < 0.05). Also, the histological analysis using H&E staining showed re-epithelization, less edema, and enhanced vascularization in both GLT-NPs than CTRL and GLT groups. Furthermore, immunohistochemical analysis of TGF-β1 and α-SMA revealed significantly a higher expression in both GLT-NPs groups than CTRL and GLT groups at weeks 1 and 2 (p < 0.05). Interestingly, TGF-β1 and α-SMA were substantially higher in GLT- TiO2 than GLT-Ag2O at weeks 1 and 2 (p < 0.05), but the expression was not significant at week 3. In conclusion, GLT-NPs showed higher regenerative capacity and enhanced the healing quality after burn wound compared to CTRL and GLT.

Graphical abstract






Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included within the article.
References
Abadi AD, Vaheb M, Rakhshani MH, Tofighian T (2018) Comparison of the effect of nanosilver spray and 1% silver sulfadiazine cream on the healing of second-degree burn wound. Transl Biomed 09(01). https://doi.org/10.21767/2172-0479.100141
Çakir B, Yeğen BC (2004) Systemic responses to burn injury. Turk J Med Sci 34(4):215–226
Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK (2011) Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol 9(1):30
Avalos A, Haza AI, Mateo D, Morales P (2016) Interactions of manufactured silver nanoparticles of different sizes with normal human dermal fibroblasts. Int Wound J 13(1):101–109
Fong J (2005) The use of silver products in the management of burn wounds: change in practice for the burn unit at Royal Perth Hospital. Primary Intention: The Australian Journal of Wound Management 13(4):S16
Mallmann EJJ, Cunha FA, Castro BNMF, Maciel AM, Menezes EA, Fechine PBA (2015) Antifungal activity of silver nanoparticles obtained by green synthesis. Rev Inst Med Trop Sao Paulo 57(2):165–167. https://doi.org/10.1590/S0036-46652015000200011
Qing Y, Cheng L, Li R, Liu G, Zhang Y, Tang X, Wang J, Liu H, Qin Y (2018) Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomedicine 13:3311–3327. https://doi.org/10.2147/IJN.S165125
El-Rafie HM, Hamed MA-A (2014) Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Adv Nat Sci Nanosci Nanotechnol 5(3):035008
Shokoofeh N, Moradi-Shoeili Z, Naeemi AS, Jalali A, Hedayati M, Salehzadeh A (2019) Biosynthesis of Fe3O4@Ag nanocomposite and evaluation of its performance on expression of norA and norB efflux pump genes in ciprofloxacin-resistant Staphylococcus aureus. Biol Trace Elem Res 191(2):522–530. https://doi.org/10.1007/s12011-019-1632-y
Shandiz SAS, Montazeri A, Abdolhosseini M, Shahrestani SH, Hedayati M, Moradi-Shoeili Z, Salehzadeh A (2018) Functionalization of Ag nanoparticles by glutamic acid and conjugation of Ag@ Glu by thiosemicarbazide enhances the apoptosis of human breast cancer MCF-7 cells. J Clust Sci 29(6):1107–1114
Smith JN, Thomas DG, Jolley H, Kodali VK, Littke MH, Munusamy P, Baer DR, Gaffrey MJ, Thrall BD, Teeguarden JG (2018) All that is silver is not toxic: silver ion and particle kinetics reveals the role of silver ion aging and dosimetry on the toxicity of silver nanoparticles. Part Fibre Toxicol 15(1):47. https://doi.org/10.1186/s12989-018-0283-z
Wang X, Ji Z, Chang CH, Zhang H, Wang M, Liao YP, Lin S, Meng H, Li R, Sun B (2014) Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small 10(2):385–398
Sankar R, Dhivya R, Shivashangari KS, Ravikumar V (2014) Wound healing activity of Origanum vulgare engineered titanium dioxide nanoparticles in Wistar Albino rats. J Mater Sci Mater Med 25(7):1701–1708. https://doi.org/10.1007/s10856-014-5193-5
Tang C, Chen N, Zhang Q, Wang K, Fu Q, Zhang X (2009) Preparation and properties of chitosan nanocomposites with nanofillers of different dimensions. Polym Degrad Stab 94(1):124–131
Vasilichin VA, Tsymbal SA, Fakhardo AF, Anastasova EI, Marchenko AS, Shtil AA, Vinogradov VV, Koshel EI (2020) Effects of metal oxide nanoparticles on toll-like receptor mRNAs in human monocytes. Nanomaterials 10(1):127
Khader SZA, Ahmed SSZ, Ganesan GM, Mahboob MR, Vetrivel M, Sankarappan M, Manickam P (2020) Rhynchosia rufescens AgNPs enhance cytotoxicity by ROS-mediated apoptosis in MCF-7 cell lines. Environ Sci Pollut Res 27(2):2155–2164
Carriere M, Arnal M-E, Douki T (2020) TiO2 genotoxicity: an update of the results published over the last six years. Mutat Res Genet Toxicol Environ Mutagen 854-855:503198
Fan X, Chen K, He X, Li N, Huang J, Tang K, Li Y, Wang F (2016) Nano-TiO2/collagen-chitosan porous scaffold for wound repairing. Int J Biol Macromol 91:15–22
Niska K, Pyszka K, Tukaj C, Wozniak M, Radomski MW, Inkielewicz-Stepniak I (2015) Titanium dioxide nanoparticles enhance production of superoxide anion and alter the antioxidant system in human osteoblast cells. Int J Nanomedicine 10:1095
Klevens R (2007) Active bacterial core surveillance (ABCs) MRSA investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama 298:1763–1771
Javanmardi S, Ghojoghi A, Divband B, Ashrafi J (2018) Titanium dioxide nanoparticle/gelatin: a potential burn wound healing biomaterial. Wounds 30(12):372–379
Nikpasand A, Parvizi MR (2019) Evaluation of the effect of titatnium dioxide nanoparticles/gelatin composite on infected skin wound healing; an animal model study. Bull Emerg Trauma 7(4):366–372
Abd Elkodous M, Hassaan A, Ghoneim A, Abdeen Z (2018) C-dots dispersed macro-mesoporous TiO2 phtocatalyst for effective waste water treatment. Characterization and Application of Nanomaterials 1(2). https://doi.org/10.24294/can.v2i1.585
Suresh AK, Pelletier DA, Wang W, Broich ML, Moon J-W, Gu B, Allison DP, Joy DC, Phelps TJ, Doktycz MJ (2011) Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater 7(5):2148–2152
Khafaga AF, Abu-Ahmed HM, El-Khamary AN, Elmehasseb IM, Shaheen HM (2018) Enhancement of equid distal limb wounds healing by topical application of silver nanoparticles. JEVS 61:76–87
Wang XQ, Kravchuk O, Winterford C, Kimble RM (2011) The correlation of in vivo burn scar contraction with the level of α-smooth muscle actin expression. Burns 37(8):1367–1377. https://doi.org/10.1016/j.burns.2011.07.018
Chen L, Schrementi ME, Ranzer MJ, Wilgus TA, DiPietro LA (2014) Blockade of mast cell activation reduces cutaneous scar formation. PLoS One 9(1):e85226. https://doi.org/10.1371/journal.pone.0085226
Abd Elkodous M, El-Sayyad GS, Youssry SM, Nada HG, Gobara M, Elsayed MA, El-Khawaga AM, Kawamura G, Tan WK, El-Batal AI, Matsuda A (2020) Carbon-dot-loaded CoxNi1 − xFe2O4; x = 0.9/SiO2/TiO2 nanocomposite with enhanced photocatalytic and antimicrobial potential: an engineered nanocomposite for wastewater treatment. Sci Rep 10(1):11534. https://doi.org/10.1038/s41598-020-68173-1
Manikandan V, Yi P-I, Velmurugan P, Jayanthi P, Hong S-C, Jang S-H, Suh J-M, Sivakumar S (2017) Production, optimization and characterization of silver oxide nanoparticles using Artocarpus heterophyllus rind extract and their antifungal activity. Afr J Biotechnol 16(36):1819–1825
El-batal A, El-Sayyad G (2014) Synthesis of silver nanoparticles and incorporation with certain antibiotic using gamma irradiation. Br J Pharm Res 4:1341–1363. https://doi.org/10.9734/BJPR/2014/9566
Abd Elkodous M, El-Sayyad GS, Abdel Maksoud MIA, Abdelrahman IY, Mosallam FM, Gobara M, El-Batal AI (2020) Fabrication of ultra-pure anisotropic zinc oxide nanoparticles via simple and cost-effective route: implications for UTI and EAC medications. Biol Trace Elem Res 196(1):297–317. https://doi.org/10.1007/s12011-019-01894-1
Elkhenany H, Abd Elkodous M, Ghoneim NI, Ahmed TA, Ahmed SM, Mohamed IK, El-Badri N (2020) Comparison of different uncoated and starch-coated superparamagnetic iron oxide nanoparticles: implications for stem cell tracking. Int J Biol Macromol 143:763–774. https://doi.org/10.1016/j.ijbiomac.2019.10.031
El-Sayyad GS, Abd Elkodous M, El-Khawaga AM, Elsayed MA, El-Batal AI, Gobara M (2020) Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1 − xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: implication for wastewater treatment. RSC Adv 10(9):5241–5259. https://doi.org/10.1039/C9RA10505K
León A, Reuquen P, Garín C, Segura R, Vargas P, Zapata P, Orihuela PA (2017) FTIR and Raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl Sci 7(1):49
Wang L, Qin W, Zhou Y, Chen B, Zhao X, Zhao H, Mi E, Mi E, Wang Q, Ning J (2017) Transforming growth factor β plays an important role in enhancing wound healing by topical application of Povidone-iodine. Sci Rep 7(1):991. https://doi.org/10.1038/s41598-017-01116-5
Chowdhury S, De M, Guha R, Batabyal S, Samanta I, Hazra SK, Ghosh TK, Konar A, Hazra S (2014) Influence of silver nanoparticles on post-surgical wound healing following topical application. Eur J Nanomed 6(4):237–247
Tian J, Wong KK, Ho CM, Lok CN, Yu WY, Che CM, Chiu JF, Tam PK (2007) Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2(1):129–136
Deepachitra R, Lakshmi RP, Sivaranjani K, Chandra JH, Sastry T (2015) Nanoparticles embedded biomaterials in wound treatment: a review. J Chem Pharm Sci 8:324–329
Kim J-Y, Kim S-E, Kim J-E, Lee J-C, Yoon J-Y (2005) The biocidal activity of nano-sized silver particles comparing with silver ion. J Korean Soc Environ Eng 27(7):771–776
Yacamán MJ, Ascencio J, Liu H, Gardea-Torresdey J (2001) Structure shape and stability of nanometric sized particles. JVSTB 19(4):1091–1103
Youn W, Ko EH, Kim MH, Park M, Hong D, Seisenbaeva GA, Kessler VG, Choi IS (2017) Cytoprotective encapsulation of individual Jurkat T cells within durable TiO2 shells for T-cell therapy. Angew Chem Int Ed 56(36):10702–10706
El-Batal A, Haroun BM, Farrag AA, Baraka A, El-Sayyad GS (2014) Synthesis of silver nanoparticles and incorporation with certain antibiotic using gamma irradiation. J Pharm Res Int 4(11)1341–1363. https://doi.org/10.9734/BJPR/2014/9566
Abd Elkodous M, El-Sayyad GS, Mohamed AE, Pal K, Asthana N, de Souza Junior FG, Mosallam FM, Gobara M, El-Batal AI (2019) Layer-by-layer preparation and characterization of recyclable nanocomposite (Co x Ni 1− x Fe 2 O 4; X = 0.9/SiO 2/TiO 2). J Mater Sci Mater Electron 30(9):8312–8328
Wan Y, Wang Y, Cheng G, Yao K (2000) Preparation and characterization of gelatin gel with a gradient structure. Polym Int 49(12):1600–1603
Li R, Chen Z, Ren N, Wang Y, Wang Y, Yu F (2019) Biosynthesis of silver oxide nanoparticles and their photocatalytic and antimicrobial activity evaluation for wound healing applications in nursing care. J Photochem Photobiol B Biol 199:111593
Seisenbaeva GA, Fromell K, Vinogradov VV, Terekhov AN, Pakhomov AV, Nilsson B, Ekdahl KN, Vinogradov VV, Kessler VG (2017) Dispersion of TiO 2 nanoparticles improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins. Sci Rep 7(1):1–11
Wen T, Rothenberg ME (2017) The regulatory function of eosinophils. Microbiol Spectr 4(5). https://doi.org/10.1128/microbiolspec.MCHD-0020-2015
Gantwerker EA, Hom DB (2012) Skin: histology and physiology of wound healing. Clin Plast Surg 39(1):85–97. https://doi.org/10.1016/j.cps.2011.09.005
Beddy D, Watson R, Fitzpatrick J, O'Connell P (2004) Increased vascular endothelial growth factor production in fibroblasts isolated from strictures in patients with Crohn's disease. Br J Surg 91(1):72–77
Vong S, Kalluri R (2011) The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer 2(12):1139–1145
Spigoni V, Cito M, Alinovi R, Pinelli S, Passeri G, Zavaroni I, Goldoni M, Campanini M, Aliatis I, Mutti A, Bonadonna RC, Dei Cas A (2015) Effects of TiO2 and Co3O4 Nanoparticles on Circulating Angiogenic Cells. PLoS One 10(3):e0119310. https://doi.org/10.1371/journal.pone.0119310
Setyawati MI, Tay CY, Chia SL, Goh SL, Fang W, Neo MJ, Chong HC, Tan SM, Loo SCJ, Ng KW, Xie JP, Ong CN, Tan NS, Leong DT (2013) Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE–cadherin. Nat Commun 4(1):1673. https://doi.org/10.1038/ncomms2655
Bhagavathy S, Kancharla S (2016) Wound healing and angiogenesis of silver nanoparticle from Azadirachta indica in diabetes induced mice. Int J Herb Med 4:24–29
Baharara J, Namvar F, Mousavi M, Ramezani T, Mohamad R (2014) Anti-angiogenesis effect of biogenic silver nanoparticles synthesized using Saliva officinalis on chick chorioalantoic membrane (CAM). Molecules 19(9):13498–13508. https://doi.org/10.3390/molecules190913498
Kargozar S, Baino F, Hamzehlou S, Hamblin MR, Mozafari M (2020) Nanotechnology for angiogenesis: opportunities and challenges. Chem Soc Rev 49(14):5008–5057
Acknowledgments
H.E. would like to thank BioRender online software for their support.
Author information
Authors and Affiliations
Contributions
NE performed the experiments with assistance from HT, MA, HA, ME, HE, and RA. NE and HE performed data analysis, presented quantitative data, and co-wrote the manuscript. MA performed nanoparticle synthesis and characterization. HT performed the pathological examination. RA, ME, HA, and HE conceived and supervised the study. HE was responsible for the final review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
All animal experimental processes were approved by Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Alexandria University, and it was strictly designed under the consideration of animal welfares (AU0132019013015).
Conflict of Interest
The authors declare that they have no conflict of interest.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Fig. 1
Zeta potential of the prepared Ag2O (A) and TiO2 NPs (B) showing their stability index. (DOCX 95 kb).
Supplementary Fig. 2
Representative images of H&E staining of histological sections from different treatment groups at week 1 showing hemorrhage (H), neovascularization (V), fibroblastic cell proliferation (short black arrow), mononuclear cell (long black arrow), and pleomorphic nuclear eosinophilic cell (arrowhead). Scale bar:100 μm (c) and 200 μm (d). (DOCX 831 kb).
Rights and permissions
About this article
Cite this article
Eldebany, N., Abd Elkodous, M., Tohamy, H. et al. Gelatin Loaded Titanium Dioxide and Silver Oxide Nanoparticles: Implication for Skin Tissue Regeneration. Biol Trace Elem Res 199, 3688–3699 (2021). https://doi.org/10.1007/s12011-020-02489-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02489-x