Abstract
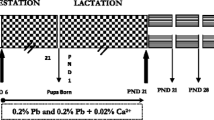
Docosahexaenoic acid (DHA, C22:6, ω-3), an ω-3 polyunsaturated fatty acid (PUFA), is critical for brain growth, development, and cognitive ability. It is consumed by offspring via milk during lactation. However, the toxic heavy metal lead (Pb) readily passes into the mammary glands of mother animals and then to offspring through milk. Here, we investigated whether DHA composition of milk and mammary gland tissues is affected by Pb exposure. Mother rats were exposed to Pb via drinking water (0.1%). The fatty acid profile and levels of reduced glutathione (GSH), lipid peroxide (LPO), and pro-inflammatory TNF-α in milk and mammary tissues were measured. Levels of DHA and antioxidant GSH decreased (P < 0.05), while LPO and TNF-α levels increased (P < 0.05) both in milk and mammary tissues. Our results suggest that toxic Pb exposure can upset the level of milk DHA, which may affect brain growth and development, and hence cognitive ability in adulthood and later life.




Similar content being viewed by others
References
Kawakita E, Hashimoto M, Shido O (2006) Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 139:991–997. https://doi.org/10.1016/j.neuroscience.2006.01.021
Oster T, Pillot T (2010) Docosahexaenoic acid and synaptic protection in Alzheimer’s disease mice. Biochim Biophys Acta 1801(8):791–798. https://doi.org/10.1016/j.bbalip.2010.02.011
Tanaka K, Farooqui AA, Siddiqi NJ et al (2012) Effects of docosahexaenoic acid on neurotransmission. Biomol Ther (Seoul) 20:152–157. https://doi.org/10.4062/biomolther.2012.20.2.152
Bradbury J (2011) Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain. Nutrients 3:529–554. https://doi.org/10.3390/nu3050529
Hashimoto M, Hossain S, Al Mamun A et al (2017) Docosahexaenoic acid: one molecule diverse functions. Crit Rev Biotechnol 37:579–597. https://doi.org/10.1080/07388551.2016.1207153
Hashimoto M, Hossain MS, Yamasaki H et al (1999a) Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids 34:1297–1304. https://doi.org/10.1007/s11745-999-0481-6
Hashimoto M, Hossain MS, Shimada T et al (2001) Effects of docosahexaenoic acid on annular lipid fluidity of the rat bile canalicular plasma membrane. J Lipid Res 42(7):1160–1168
Hashimoto M, Hossain S, Shimada T, Shido O (2006) Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol 33:934–939. https://doi.org/10.1111/j.1440-1681.2006.04467.x
Hashimoto M, Hossain S, Shido O (2006) Docosahexaenoic acid but not eicosapentaenoic acid withstands dietary cholesterol-induced decreases in platelet membrane fluidity. Mol Cell Biochem 293:1–8. https://doi.org/10.1007/s11010-006-0164-x
Onuki Y, Morishita M, Chiba Y, Tokiwa S, Takayama K (2006) Docosahexaenoic acid and eicosapentaenoic acid induce changes in the physical properties of a lipid bilayer model membrane. Chem Pharm Bull (Tokyo) 54(1):68–71. https://doi.org/10.1248/cpb.54.68
Brenner RR (1984) Effect of unsaturated acids on membrane structure and enzyme kinetics. Prog Lipid Res 23:69–96. https://doi.org/10.1016/0163-7827(84)90008-0
Kim H-Y, Spector AA, Xiong Z-M (2011) A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins Other Lipid Mediat 96:114–120. https://doi.org/10.1016/j.prostaglandins.2011.07.002
Suzuki H, Manabe S, Wada O, Crawford MA (1997) Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int J Vitam Nutr Res 67:272–278
Salem NJ, Litman B, Kim HY, Gawrisch K (2001) Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 36:945–959. https://doi.org/10.1007/s11745-001-0805-6
Bazan NG (2009) Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J Lipid Res 50(Suppl):S400–S405. https://doi.org/10.1194/jlr.R800068-JLR200
Molloy C, Doyle LW, Makrides M, Anderson PJ (2012) Docosahexaenoic acid and visual functioning in preterm infants: a review. Neuropsychol Rev 22:425–437. https://doi.org/10.1007/s11065-012-9216-z
Su H-M (2010) Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J Nutr Biochem 21:364–373. https://doi.org/10.1016/j.jnutbio.2009.11.003
Gamoh S, Hashimoto M, Hossain S, Masumura S (2001) Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol 28:266–270. https://doi.org/10.1046/j.1440-1681.2001.03437.x
Gamoh S, Hashimoto M, Sugioka K et al (1999) Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience 93:237–241
Jensen CL, Lapillonne A (2009) Docosahexaenoic acid and lactation. Prostaglandins Leukot Essent Fat Acids 81:175–178. https://doi.org/10.1016/j.plefa.2009.05.006
Hashimoto M, Hossain S, Shimada T et al (2002) Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem 81:1084–1091. https://doi.org/10.1046/j.1471-4159.2002.00905.x
Söderberg M, Edlund C, Kristensson K, Dallner G (1991) Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 26:421–425. https://doi.org/10.1007/BF02536067
Ip MM, Shoemaker SF, Darcy KM (1992) Regulation of rat mammary epithelial cell proliferation and differentiation by tumor necrosis factor-alpha. Endocrinology 130:2833–2844. https://doi.org/10.1210/endo.130.5.1572296
Goldman AS, Chheda S, Garofalo R, Schmalstieg FC (1996) Cytokines in human milk: properties and potential effects upon the mammary gland and the neonate. J Mammary Gland Biol Neoplasia 1:251–258
Holman RT (1986) Control of polyunsaturated acids in tissue lipids. J Am Coll Nutr 5:183–211
Agostoni C, Marangoni F, Stival G et al (2008) Whole blood fatty acid composition differs in term versus mildly preterm infants: small versus matched appropriate for gestational age. Pediatr Res 64:298–302. https://doi.org/10.1203/PDR.0b013e31817d9c23
Yang X, Sheng W, Sun GY, Lee JC-M (2011) Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem Int 58:321–329. https://doi.org/10.1016/j.neuint.2010.12.004
Bakulski KM, Rozek LS, Dolinoy DC et al (2012) Alzheimer’s disease and environmental exposure to lead: the epidemiologic evidence and potential role of epigenetics. Curr Alzheimer Res 9:563–573. https://doi.org/10.2174/156720512800617991
Singh PK, Singh MK, Yadav RS et al (2017) Omega-3 fatty acid attenuates oxidative stress in cerebral cortex, cerebellum, and hippocampus tissue and improves neurobehavioral activity in chronic lead-induced neurotoxicity. Nutr Neurosci 22(2):83–97. 1–15. https://doi.org/10.1080/1028415X.2017.1354542
Hossain MS, Hashimoto M, Gamoh S, Masumura S (1999) Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J Neurochem 72:1133–1138. https://doi.org/10.1046/j.1471-4159.1999.0721133.x
Jarrar BM, Taib NT (2012) Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J Biol Sci 19:203–210. https://doi.org/10.1016/j.sjbs.2011.12.005
Sharma S, Singh B (2014) Effects of acute and chronic lead exposure on kidney lipid peroxidation and antioxidant enzyme activities in BALB-C mice (Mus musculus). Int J Sci Res 3:1564–1566. www.ijsr.net: Paper ID: SEP1442
Aldahmash BA, El-Nagar DM (2016) Antioxidant effects of captopril against lead acetate-induced hepatic and splenic tissue toxicity in Swiss albino mice. Saudi J Biol Sci 23:667–673. https://doi.org/10.1016/j.sjbs.2016.05.005
Kilikdar D, Mukherjee D, Mitra E et al (2011) Protective effect of aqueous garlic extract against lead-induced hepatic injury in rats. Indian J Exp Biol 49:498–510
Gurer H, Ozgunes H, Neal R et al (1998) Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology 128:181–189. https://doi.org/10.1016/S0300-483X(98)00074-2
Apostoli P, Kiss P, Porru S et al (1998) Male reproductive toxicity of lead in animals and humans. ASCLEPIOS Study Group. Occup Environ Med 55:364–374. https://doi.org/10.1136/oem.55.6.364
Cecil KM, Brubaker CJ, Adler CM et al (2008) Decreased brain volume in adults with childhood lead exposure. PLoS Med 5:e112. https://doi.org/10.1371/journal.pmed.0050112
Verina T, Rohde CA, Guilarte TR (2007) Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience 145:1037–1047. https://doi.org/10.1016/j.neuroscience.2006.12.040
Hossain S, Bhowmick S, Jahan S et al (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158. https://doi.org/10.1016/j.neuro.2016.07.013
Hashimoto M, Shinozuka K, Gamoh S, Tanabe Y, Hossain SMS, Kwon Y, Hata N, Misawa Y, Kunimoto M, Masumura S (1999) The hypotensive effect of docosahexaenoic acid is associated with the enhanced release of ATP from the caudal artery of aged rats. J Nutr 129:70–76. https://doi.org/10.1093/jn129.1.70
Hashimoto M, Hossain S, Katakura M et al (2015) The binding of Abeta1-42 to lipid rafts of RBC is enhanced by dietary docosahexaenoic acid in rats: Implicates to Alzheimer’s disease. Biochim Biophys Acta 1848:1402–1409. https://doi.org/10.1016/j.bbamem.2015.03.008
Etem-Piskin I, Nur Karavar H, Arasli M, Ermis B (2012) Effect of maternal smoking on colostrum and breast milk cytokines. Eur Cytokine Netw 23:187–190. https://doi.org/10.1684/ecn.2013.0324
Lehtolainen T, Rontved C, Pyorala S (2004) Serum amyloid A and TNF alpha in serum and milk during experimental endotoxin mastitis. Vet Res 35:651–659. https://doi.org/10.1051/vetres:2004043
Hossain S, Bhowmick S, Islam S et al (2015) Oral administration of Ganoderma lucidum to lead-exposed rats protects erythrocytes against hemolysis: implicates to anti-anemia. Evidence-based Complement Altern Med 2015:8. https://doi.org/10.1155/2015/463703
Halliwell B, Gutteridge JMC (1989) Protection against oxidants in biological systems: the superoxide theory of oxygen toxicity. In: Halliwell B, Gutteridge JMC (eds) Free Radical in Biology and Medicine. Clarendon Press, Oxford, pp 86–123
Yiin SJ, Lin TH (1995) Lead-catalyzed peroxidation of essential unsaturated fatty acid. Biol Trace Elem Res 50:167–172. https://doi.org/10.1007/BF02789419
Hossain MS, Hashimoto M, Masumura S (1998) Influence of docosahexaenoic acid on cerebral lipid peroxide level in aged rats with and without hypercholesterolemia. Neurosci Lett 244:157–160. https://doi.org/10.1016/S0304-3940(98)00147-5
Egan RW, Paxton J, Kuehl FAJ (1976) Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem 251:7329–7335
Zimmermann L, Pages N, Antebi H et al (1993) Lead effect on the oxidation resistance of erythrocyte membrane in rat triton-induced hyperlipidemia. Biol Trace Elem Res 38:311–318. https://doi.org/10.1007/BF02785314
Knowles SO, Donaldson WE, Andrews JE (1998) Changes in fatty acid composition of lipids from birds, rodents, and preschool children exposed to lead. Biol Trace Elem Res 61:113–125. https://doi.org/10.1007/BF02784024
Osterode W, Ulberth F (2000) Increased concentration of arachidonic acid in erythrocyte membranes in chronically lead-exposed men. J Toxicol Environ Health Part A 59(2):87–95. https://doi.org/10.1080/009841000156998
Adegbesan BO, Adenuga GA (2007) Effect of lead exposure on liver lipid peroxidative and antioxidant defense systems of protein-undernourished rats. Biol Trace Elem Res 116:219–225. https://doi.org/10.1007/BF02685932
Wang J, Wu J, Zhang Z (2006) Oxidative stress in mouse brain exposed to lead. Ann Occup Hyg 50:405–409. https://doi.org/10.1093/annhyg/mei079
Kasperczyk S, Slowinska-Lozynska L, Kasperczyk A et al (2015) The effect of occupational lead exposure on lipid peroxidation, protein carbonylation, and plasma viscosity. Toxicol Ind Health 31:1165–1171. https://doi.org/10.1177/0748233713491804
Patra RC, Rautray AK, Swarup D (2011) Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 2011:457327. https://doi.org/10.4061/2011/457327
Cheng Y-J, Yang B-C, Liu M-Y (2006) Lead increases lipopolysaccharide-induced liver-injury through tumor necrosis factor-alpha overexpression by monocytes/macrophages: role of protein kinase C and P42/44 mitogen-activated protein kinase. Environ Health Perspect 114:507–513. https://doi.org/10.1289/ehp.8550
Liu B, Zupan B, Laird E et al (2014) Maternal hematopoietic TNF, via milk chemokines, programs hippocampal development and memory. Nat Neurosci 17:97–105. https://doi.org/10.1038/nn.3596
Hashimoto M, Hossain S, Katakura M et al (2018) Docosahexaenoic acid helps to lessen extinction memory in rats. Molecules 23:E451. https://doi.org/10.3390/molecules23020451
Sidhu VK, Huang BX, Desai A et al (2016) Role of DHA in aging-related changes in mouse brain synaptic plasma membrane proteome. Neurobiol Aging 41:73–85. https://doi.org/10.1016/j.neurobiolaging.2016.02.007
Jiang L-H, Yan S, Wang J, Liang Q (2013) Oral administration of docosahexaenoic acid activates the GDNF-MAPK-CERB pathway in hippocampus of natural aged rat. Pharm Biol 51:1188–1195. https://doi.org/10.3109/13880209.2013.784341
Ramirez-Ramirez V, Macias-Islas MA, Ortiz GG et al (2013) Efficacy of fish oil on serum of TNF alpha, IL-1 beta , and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxidative Med Cell Longev 2013:709493. https://doi.org/10.1155/2013/709493
Anderson DW, Mettil W, Schneider JS (2016) Effects of low level lead exposure on associative learning and memory in the rat: influences of sex and developmental timing of exposure. Toxicol Lett 246:57–64. https://doi.org/10.1016/j.toxlet.2016.01.011
de Oliveira FS, Viana MR, Antoniolli AR, Marchioro M (2001) Differential effects of lead and zinc on inhibitory avoidance learning in mice. Brazilian J Med Biol Res = Rev Bras Pesqui medicas e Biol 34:117–120
Krol E, Redman P, Thomson PJ et al (2005) Effect of photoperiod on body mass, food intake and body composition in the field vole, Microtus agrestis. J Exp Biol 208:571–584. https://doi.org/10.1242/jeb.01429
Valdearcos M, Robblee MM, Benjamin DI et al (2014) Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9:2124–2138. https://doi.org/10.1016/j.celrep.2014.11.018
Gibson RA, Muhlhausler B, Makrides M (2011) Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern Child Nutr 7(Suppl 2):17–26. https://doi.org/10.1111/j.1740-8709.2011.00299.x
Innis SM (2011) Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern Child Nutr 7(Suppl 2):112–123. https://doi.org/10.1111/j.1740-8709.2011.00318.x
Massiera F, Guesnet P, Ailhaud G (2006) The crucial role of dietary n-6 polyunsaturated fatty acids in excessive adipose tissue development: relationship to childhood obesity. Nestle Nutr Workshop Ser Pediatr Program 57:235. https://doi.org/10.1159/000091076
Hauner H, Brunner S, Amann-Gassner U (2013) The role of dietary fatty acids for early human adipose tissue growth. Am J Clin Nutr 98:549S–555S. https://doi.org/10.3945/ajcn.112.040733
Muhlhausler BS, Gibson RA, Makrides M (2011) The effect of maternal omega-3 long-chain polyunsaturated fatty acid (n-3 LCPUFA) supplementation during pregnancy and/or lactation on body fat mass in the offspring: a systematic review of animal studies. Prostaglandins Leukot Essent Fat Acids 85:83–88. https://doi.org/10.1016/j.plefa.2011.04.027
Acknowledgments
The authors gratefully acknowledge the contribution of the University Grant Commission-Higher Education Quality Enhancement Program (UGC-HEQEP) for the partial instrumental support (CP-358).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hossain, S., Hussain, J., Bhowmick, S. et al. Docosahexaenoic Acid (DHA, C22:6, ω-3) Composition of Milk and Mammary Gland Tissues of Lactating Mother Rats Is Severely Affected by Lead (Pb) Exposure. Biol Trace Elem Res 195, 525–534 (2020). https://doi.org/10.1007/s12011-019-01878-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01878-1