Abstract
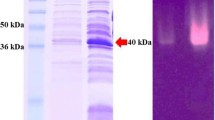
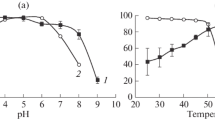
The 1014 nucleotides long gene-encoding β-mannanase from Bacillus subtilis strain MA139 was cloned using PCR. To obtain high expression levels in Pichia pastoris, the β-mannanase gene was optimized according to the codon usage bias of P. pastoris and fused downstream of GAP promoter. The reconstituted plasmid pGAP-mann was transformed into P. pastoris X-33 strain to constitutively express β-mannanase. When cultured at 28 °C for 3 days protein yields up to 2.7 mg/mL was obtained with the enzyme activity of up to 230 U/mL. In comparison, wild-type gene product yielded 1.9 mg/mL and 170 U/mL, respectively indicating that the protein yield and enzyme activity were significantly improved by codon modification. After purification, the enzyme properties were characterized. The optimal activity was at pH 6.0 and 50 °C. In the pH range of 3.0 to 9.0, β-mannanase showed above 60% of its peak activity. Among the numerous ions tested copper significantly inhibited the enzyme activity. These results suggested that codon-optimized β-mannanase expressed in P. pastoris could potentially be used as an additive in the feed for monogastric animals.





Similar content being viewed by others
References
Matheson, N. K., & McCleary, B. V. (1985). In G. O. Aspinall (Ed.), The Polysachharides, vol. 3 (p. 1). New York: Academic.
Singh, S., Madlala, A. M., & Prior, B. A. (2003). FEMS Microbiology Reviews, 27, 3–16.
Rättö, M., & Poutanen, K. (1988). Biotechnology Letters, 10, 661–664.
Abe, J., Hossain, Z. M., & Hizukuri, S. (1994). Journal of Fermentation and Bioengineering, 3, 259–261.
Araujo, A., & Ward, O. P. (1990). Journal of Applied Bacteriology, 68, 253–261.
Araujo, A., & Ward, O. P. (1990). Applied and Environmental Microbiology, 56, 1954–1956.
Khanongnuch, C., Ooi, T., & Kinoshita, S. (1999). World Journal of Microbiology and Biotechnology, 15, 249–258.
Oda, Y., Komaki, T., & Tonomura, K. (1993). Journal of Fermentation and Bioengineering, 76, 14–18.
Takahashi, R., Kusakabe, I., Kobayashi, H., Murakami, K., Maekawa, A., & Suzuki, T. (1984). Agricultural and Biological Chemistry, 48, 2189–2195.
Titapoka, S., Keawsompong, S., Haltrich, D., & Nitisinprasert, S. (2008). World Journal of Microbiology and Biotechnology, 24, 1425–1433.
Guo, X. H., Li, D. F., Lu, W. Q., Piao, X Sh, & Chen, X. L. (2006). Antonie Van Leeuwenhoek, 90, 139–146.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning: A laboratory manual (3rd ed.). New York: Cold Spring Harbor Laboratory Press.
Zhao, X., Huo, K. K., & Li, Y. Y. (2000). Chinese Journal of Biotechnology, 16, 308–311.
Sreekrishna, K. (1993). Strategies for optimizing protein expression and secretion in the methylotrophic yeast Pichia pastoris. In R. H. Baltz, G. D. Hegeman & P. L. Skatrud (Eds.), Industrial microorganism: basic and applied molecular genetics (pp. 119–126). Washington, DC: American Society of Microbiology.
Sinclair, G., & Choy, F. Y. M. (2002). Protein Expression and Purification, 26, 96–105.
Chen, X. L., Cao, Y. H., Ding, Y. H., Lu, W. Q., & Li, D. F. (2006). Journal of Biotechnology, 128, 452–461.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428.
Kim, T. R., Goto, Y., Hirota, N., Dawata, K., Denton, H., Wu, S. Y., et al. (1997). Protein Engineering, 10, 1339–1345.
Reverter, D., Ventura, S., Villegas, V., Vendrell, J., & Avilés, F. X. (1998). Journal of Biological Chemistry, 273, 3535–3541.
Sharp, P. M., & Li, W. H. (1986). Journal of Molecular Evolution, 24, 28–38.
Hu, S. Y., Li, L. W., Qiao, J. J., Guo, Y. J., Cheng, L. S., & Liu, J. (2006). Protein Expression and Purification, 47, 249–257.
Chen, X. L., Qiao, J. Y., Yu, H. F., & Cao, Y. H. (2009). Biologia, 64, 235–238.
Waterham, H. R., Digan, M. E., Koutz, P. J., Lair, S. V., & Cregg, J. M. (1997). Gene, 186, 37–44.
Moreira, L. R. S., & Filho, E. X. F. (2008). Applied Microbiology and Biotechnology, 79, 165–178.
Jiang, Z. Q., Wei, Y., Li, D. Y., Li, L. T., Chai, P. P., & Kusakabe, I. (2006). Carbohydrate Polymers, 66, 88–96.
He, X. P., Liu, N., Li, W. W., Zhang, Zh Y, Zhang, B. R., & Ma, Y. H. (2008). Enzyme and Microbial Technology, 43, 13–18.
Zakaria, M. M., Yamamoto, S., & Yagi, T. (2006). FEMS Microbiology Letters, 158, 25–31.
Mendoza, N. S., Arai, M., Kawaguchi, T., Yoshida, T., & Joson, L. M. (1994). World Journal of Microbiology and Biotechnology, 10, 551–555.
Acknowledgements
This work was supported by the National High Technology Research and Development Program (2007AA100601), the Program for New Century Excellent Talents in University (NCET-07-0807), and the Project of State Key Laboratory of Animal Nutrition (2004DA125184 (team) 0806).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qiao, J., Rao, Z., Dong, B. et al. Expression of Bacillus subtilis MA139 β-mannanase in Pichia pastoris and the Enzyme Characterization. Appl Biochem Biotechnol 160, 1362–1370 (2010). https://doi.org/10.1007/s12010-009-8688-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8688-7