Abstract
Β-glucans (BGs) are dietary fibers with human health benefits. Due to their emulsifying, thickening, and water-holding properties, they are frequently utilized in food formulations. Hull-less barley is one of the important sources of BGs. This research was performed to extract BGs from hull-less barley using microwave-assisted pressurized CO2/H2O (MW-PCO2) extraction, a combination that had never been employed before. The MW-PCO2 extraction conditions (temperature, time and water: barley flour ratio) were optimized using response surface methodology (RSM) with a Box-Behnken design for the maximum BG yield (%). Temperature of 47.74 °C, time of 19.92 min, and water: barley flour ratio of 10.10:1 (g/g) were found to be the optimum conditions for extraction with a BG yield of 62.43%. Additionally, MW-PCO2 extraction was compared with conventional water extraction (CE) and BG extracts obtained by both methods were characterized. Chemical composition, molecular weight, thermal properties, water solubility, water holding capacity, surface morphology, and Fourier Transform Infrared Spectrum (FTIR) of the BG extracts were determined. MW-PCO2 extraction gave higher BG yield and purity, molecular weight, and water holding capacity using less solvent in a shorter time. Furthermore, both extracts have similar morphological images, FTIR spectrum, and thermal properties. The results of this work demonstrate the potential of the MW-PCO2 approach for extracting BGs from hull-less barley with improved selectivity and recovery, which can then be added to a variety of food and drug formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-Glucans (BGs) are water-soluble dietary fibers found in various cereals such as oats, barley, and rye. They consist of glucose units linked by β-(1-4)- and β-(1-3)-glycosidic linkages. BGs belong to the dietary fibers (DF) like cellulose and hemicellulose. DF is believed to play an important role in lowering serum lipid concentrations, cholesterol levels, and blood pressure (Cajzek et al., 2020). Daily intake of DF (25–35 g/day) is recommended to reduce the risk of coronary heart disease, stroke, and diabetes (Kendall et al., 2010). It is also known that BGs have good properties in food processing because of their emulsifying, thickening, and water-holding capacities (Dimopoulos et al., 2020; Sharma & Gujral, 2013).
Oats, barley, and rye are known to be good sources of BGs (Ahmad et al., 2009; Wood, 2010). Hull-less barley has also been shown to be an important source of BGs at 4–8% (Izydorczyk et al., 2003). Nie et al. (2021) studied the BG structure of hull-less barley and its in vitro fermentation properties. They found that hull-less barley BG has a molecular weight of 3.45 × 104 Da and serves as a prebiotic. Due to the increasing interest in BGs, researchers and producers are focusing on their better separation techniques with higher efficiency and purity from various sources. Various techniques have been used for the extraction/isolation of BGs, such as hot water and pressurized hot water extraction (Ahmad et al., 2009; Benito-Román et al., 2013a, b; Rodríguez-Seoane et al., 2018; Sakdasri et al., 2022), alkaline, acidic, and enzymatic extraction (Ahmad et al., 2009), ultrasound-assisted extraction (Benito-Román et al., 2013c; Liu et al., 2021), and microwave-assisted extraction (Gangopadhyay et al., 2015; Liu et al., 2021).
The methods mentioned here have some advantages and disadvantages. Hot water, caustic, and acid methods are comparatively old techniques whose main disadvantage is that they are time consuming and not very efficient. The non-conventional techniques such as ultrasound-assisted extraction and microwave-assisted extraction have several advantages such as providing fast and homogenous heating, reducing extraction time and formation of by-products, and saving energy compared to the conventional techniques. Microwave irradiation uses an indirect way of heating and accelerates energy transfer. Microwave is used in the food industry for various purposes such as sterilization, drying, thawing, and blanching (Guzik et al., 2022). Microwave technology has also been used for the extraction of various compounds such as colorings, essential oils, and bioactive compounds (Bansod et al., 2023; Guzik et al., 2022). Lower solvent, energy, and time consumption, as well as high purity, are among the advantages of microwave-assisted extraction. It is also an environmentally friendly technology that uses water as a solvent in most applications (Liu et al., 2021). Carbon dioxide is also a cheap, non-flammable, and non-toxic green solvent. It dissolves in water as carbonic acid and lowers the pH up to 2.9, which is an acidic medium suitable for the degradation of lignocellulose structure with the dissolution of cellulose and hemicellulose (Sasaki & Ohsawa, 2021).
Pressurized carbon dioxide/water mixture could be used as a solvent for lignocellulosic biomass degradation and BG extraction in the presence of microwave heating. The technique used here has not been applied in any BG extraction. A great future strength of the technique is that it may help to eliminate the use of alkalis/acid in the traditional extraction methods. This study aims to the following: (1) explore the effect of parameters (temperature, time, and water: barley flour ratio) in the microwave-assisted pressurized CO2/H2O extraction (MW-PCO2) of BG from hull-less barley and optimize processing parameters for maximum yield of BG using response surface methodology (RSM); (2) compare the yield, physical and chemical properties of BG extracted by MW-PCO2 system with those of BG extracted by CE method.
Materials and Methods
Materials and Reagents
Yalın variety hull-less barley was obtained from the Field Crops Central Research Institute affiliated to the Ministry of Agriculture and Forestry of the Republic of Turkey. A new generation whole grain milling machine (Erdal Özkan Makine A.Ş., Crabcut, CR0, 2021, Turkey) was used to grind barley to a size below 500 µm. Up to its use in the extraction process, barley flour was kept in plastic containers at +4 °C. Acetic acid, ethanol, sulfuric acid, xylose and arabinose standards, sodium dihydrogen phosphate dihydrate, and sodium hydroxide used in the experiments were obtained from Sigma-Aldrich (St. Louis, MO, USA). BG analysis kit and total starch kit were purchased from Megazyme (Megazyme, Bray, Ireland).
Determination of BG, Moisture, Ash, Protein, Starch, and Fat Content
The amount of BG in barley flour and BG extracts was determined according to the procedure of Megazyme β-Glucan Assay kit (Mixed Linkage). Moisture, ash, and protein contents of barley flour and BG extracts were determined according to AOAC 925.10 (AOAC, 2014), AOAC 923.03 (AOAC, 2015), and AOAC 960.52 (AOAC, 2010) methods, respectively. Starch content of the samples was determined using the Megazyme total starch assay kit. Fat content of the barley flour was determined by the Soxhlet method.
Beta-Glucan Extraction
Conventional Water Extraction (CE)
Conventional water extraction was performed using the procedure described by Benito-Román et al. (2011) with slight modifications. Hull-less barley flour was mixed with distilled water at a solid/liquid ratio of 1:15 (g/g). The mixture was then incubated in a shaker incubator (Heidolph Inkubator 1000, Germany) for 3 h at 55 °C and 150 rpm. At the end of the incubation, the liquid and solid parts were separated by centrifugation (Eppendorf, Centrifuge 5810 R, Hamburg, Germany) at 6000 rpm for 15 min. The supernatant was mixed with ethanol (1:1 v/v) and incubated at 4 °C for 24 h to precipitate the BGs. The precipitate was filtered, rinsed with ethanol, and then freeze-dried using a freeze dryer (FreeZone 6, Labconco, USA) at − 55 °C for 48 h. Once the samples had dried, they were ground using an electrical grinder (Isolab laboratory mill 602, Interlab, İstanbul, Turkey), sieved to particles smaller than 0.5 mm, and stored at 4 °C until additional analysis was performed. The BG yield (%) was calculated using the following equation:
Microwave-Assisted Pressurized CO2/H2O Extraction (MW-PCO2)
A closed vessel microwave system (Milestone SynthWave, Sorisole, Italy) outfitted with a gas input system and water cycling chiller adjusted to 10 °C was used to MW-PCO2 extraction of BG from barley flour (Fig. 1). A 1 L polytetrafluoroethylene vessel (inner dimensions:17.7 cm of height and 8 cm of diameter) covered with a stainless steel reaction chamber which serves as a microwave cavity and a reaction vessel was filled with barley flour and distilled water. After pre-pressurization with CO2, the chamber was sealed. An initial CO2 pressure of 30 bar was used for all experimental trials. A propeller type stirrer (215 rpm) was used during the whole process. A built-in cooling mechanism quickly reduced the temperature after the microwave cycle. This microwave reactor allows the system to operate at constant temperature. Microwave power was set to 750 W for quick heating and throughout the procedure, power was applied intermittently between 0 and 750 W to maintain the temperature at set value. Furthermore, a mass loading of 120 g (the total of barley flour and water) was employed in every experiment. Extraction experiments were conducted with varying time, temperature, and water/barley flour ratio. Following extraction, the liquid and solid parts were separated by centrifugation (Eppendorf, Centrifuge 5810 R, Hamburg, Germany) at 6000 rpm for 15 min. After that, BG was obtained by applying the same procedures as in the CE method.
Experimental Design and Optimization of MW-PCO2 Extraction
For the experimental design, data analysis and optimization of MW-PCO2 extraction conditions, Design Expert 7.1.6 (StatEase, Minneapolis, MN, USA) was used. The three-factor-three-level Box–Behnken design with response surface methodology (RSM) was applied to study the independent variables influencing the extraction process, their interactions, and the optimization of the process parameters. The independent variables were chosen as temperature, time, and water/barley flour ratio with the ranges of 40–60 °C, 5–20 min, and 10:1–20:1 (g/g), respectively. MW-PCO2 extraction experiments were conducted at 17 points, five of which were central points, as the experimental design indicated. Table 1 shows the experimental and predicted values obtained for each experiment. The extraction process was optimized to obtain maximum BG yield (%).
Characterization of BG Extracts
Color Analysis
The color of BG samples was measured using a HunterLab ColorFlex, A60-1010-615 model colormeter (HunterLab, Reston, VA, USA). L*, a*, and b* color parameters were measured.
Arabinoxylan and Lignin Analysis
Acid-soluble and insoluble lignin contents of the samples were determined according to NREL Laboratory Procedure NREL/TP-510-42618 (Sluiter et al., 2008). According to this method, 0.3 g of extract was weighed into test tube and 3 mL of 72% H2SO4 was added. The tube was placed in a water bath at 30° C and incubated for 1 h. The sample was stirred every 5 to 10 min using a stir rod without removing the sample from the bath. Then, the acid concentration was diluted to 4% by adding 84 mL of deionized water and the mixture was autoclaved at 121° C for 1 h. At the end of the period, the remaining mixture was cooled and filtered through sinter glass filter crucible. The crucible and the residue were dried at 105° C until a constant weight and then placed in a muffle furnace at 575 °C for 24 h. The change in the mass before and after the combustion process was calculated as the amount of acid-insoluble lignin. In order to calculate the amount of acid-soluble lignin, the absorbance of the hydrolysis liquor was adjusted into the range of 0.7–1.0 by diluting the solution with distilled water and the dilution factor was recorded. The absorbance of the sample was measured at 320 nm using a UV-Vis spectrophotometer (Optima SP 3000 Nano, Tokyo, Japan). The amount of acid-soluble lignin was calculated using the following equation:
where UVabs represents UV-Vis absorbance of the sample at 320 nm wavelength, Vfiltrate is volume of filtrate (mL), dilution factor is (volume of the sample+volume of the distilled water) / volume of the sample, ε is biomass absorptivity at definite wavelength (L/g.cm), and pathlength represents UV-Vis path length of the cell (cm).
For the analysis of xylose and arabinose, the hydrolyzate obtained from the two-stage hydrolysis process in lignin determination was analyzed using a Shimadzu SCL-10A HPLC system (Shimadzu, Kyoto, Japan). A refractive index detector (RID-10A, Shimadzu) was used as the detector and Aminex HPX-87H (Bio-Rad, Hercules, CA, USA) was used as the column. 5 mM H2SO4 was used as the mobile phase and eluted from the column at 65 °C and a flow rate of 0.6 mL/min. Calibration curves of xylose and arabinose standards were used to determine the amounts of xylose and arabinose (g/L) in the BG hydrolyzate. The xylan and arabinan content of BG extracts were determined using the amounts of monomeric sugars (Browning, 1967).
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR analysis of β-glucan extracts was performed according to Liu et al. (2021). BG samples were analyzed on a Perkin Elmer Spectrum 100 (PerkinElmer Ltd, Beaconsfield, UK) with 32 scans at 4 cm−1 resolution and frequency range of 4000–600 cm−1.
Differential Scanning Calorimetry (DSC) Analysis
A Perkin Elmer TGA 4000 (PerkinElmer Ltd, Waltham, USA) was used to determine the thermal properties of extracted BG using the method described by Ahmad et al. (2016) with slight modifications. Nearly 4 mg of sample was weighed into the aluminum pan. The container was then hermetically sealed and the samples were heated dynamically between 50 and 170 °C with a heating rate of 10 °C/min. The reference was the empty aluminum pan.
Molecular Weight Analysis
Gel permeation chromatography (GPC) system equipped with an RI detector (Agilent 1100 series, Agilent, Agilent Technologies, Germany) was used to determine the weight average molecular weight (Mw), number average molecular weight (Mn), molecular weight of the highest peak (Mp) and polydispersity index (Mw/Mn: PDI) of the samples. Analysis was performed at a flow rate of 1.0 mL/min at 45 °C. Polyethyleneoxide/glycol standards (Agilent, Santa Clara, CA, USA) with Mp in the range of 106 to 1 511 000 g/mol were used to prepare calibration curve.
Water Solubility Index and Water Holding Capacity
Water solubility index of samples was determined as described by Liu et al. (2021) with slight modifications. One gram of BG extract was mixed with 100 mL of distilled water and the mixture was stirred at 55 °C for 2 h. Then, the mixture was centrifuged in a Hettich-EBA 20 (Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany) centrifuge at 6000 rpm for 20 min. The supernatant was separated and dried at 105 °C until constant weight. The weight of the dried phase was determined and the solubility index was calculated as follows:
Water holding capacity of samples was determined according to the method described by Kurek et al. (2018).
Scanning Electron Microscope (SEM)
The SEM analysis of the samples was performed according to Ahmad et al. (2016) with slight modifications. A scanning electron microscope (SEM) Zeiss EVO LS10 (Carl Zeiss, Germany) was used to evaluate the surface morphologies of BG samples. The samples were coated with gold using a Cressington 108 auto (Cressington Scientific Instruments, UK) sputter coater after being mounted on aluminum SEM stubs. The SEM images were obtained at a magnification of ×10000 and at 3 kV.
Statistical Analysis
All experiments were performed in triplicate and the results are given as mean±standard deviation. SPSS 22 (SPSS Inc. Chicago, IL, USA) was used to statistical analysis of data and one-way ANOVA was applied to compare experimental results.
Results and Discussion
Composition of Barley Flour
According to the results of the composition analysis, barley flour contains 8.97 ± 0.22% moisture on wet basis. The protein, starch, ash, BG, fat, acid soluble, and insoluble lignin content of the barley flour were 17.26 ± 0.38%, 65.58 ± 0.18%, 1.87 ± 0.04%, 5.65 ± 0.21%, 2.13 ± 0.24%, 0.14 ± 0.01, and 0.35 ± 0.01 on a dry basis, respectively. Acar (2020) has reported that the protein, starch, ash, and BG content of hull-less barley were in the range of 12.3–14.5%, 62.1–63.6%, 1.65–1.83%, and 4.45–5.01%, respectively. In addition, Aslan (2022) stated that the hull-less barley contain 11.05% moisture, 2.35% ash, 4.18% BG, 48.97% starch, 11.55% protein, and 2.16% fat. Öner (2019) and Erbaş Köse et al. (2021) have reported similar results. The differences in the composition of barley could be related to differences in climatic and geographical conditions.
Effect of MW-PCO2 Extraction Variables on BG Yield and Optimization of Process
The effects of the independent variables (temperature, time, and water:barley flour ratio) on MW-PCO2 extraction of BG were analyzed using the Box-Behnken design (BBD). The generated BBD and the experimental and predicted response values are shown in Table 1. Statistical modeling of MW-PCO2 process was performed using RSM with multiple regression analysis. The best fitting model with a non-significant lack of fit value for BG yield was selected as the quadratic model. Non-significant factors and interaction terms were excluded from the model using the backward elimination. Model equation created with independent variables for BG yield is given in following equation in terms of coded factors:
where A, B, and C are temperature, time, and water:barley flour ratio, respectively.
MW-PCO2 extraction variables and their interactions were evaluated using ANOVA (Table 2). The significant p value (< 0.0001) and the non-significant lack of fit test indicated that the produced quadratic model was well adapted to the response with the coefficient of determination values of 0.9868. Temperature, time, and water:barley flour ratio were found to be statistically significant (p < 0.05) factors on BG yield. In addition, the interactions of temperature×water:barley flour ratio (AC), time×water: barley flour ratio (BC) and the quadratic effect of variables (A2, B2, C2) were statistically significant (p < 0.05) model terms. The most significant factor was water:barley flour ratio for the BG extraction from barley flour considering its higher F value (89.85) and low p-value (< 0.0001).
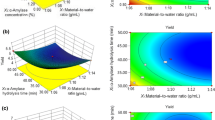
The BG yield obtained from barley flour by MW-PCO2 varies between 35.65 ± 0.83 and 63.09 ± 2.87% (Table 1). The highest BG yield was obtained at run 10, where temperature, time, and water: barley flour ratio were 50 °C, 20 min, and 10:1 (g/g), respectively. Figure 2 shows the three-dimensional response surface graphs which represent the change in BG yield as a function of independent variables. BG yield increased with the increasing temperature; however, when the temperature exceeded nearly 55 °C, the BG yield began to decrease (Fig. 2a and b). This can be explained by the increase in the amount of starch in the extract with increasing temperature. Starch in the extraction medium gelatinizes, makes the extraction liquid thicker, which also slows down mass transfer and makes extraction difficult. Similar results were reported in the literature by other researchers who performed BG extraction from barley (Benito-Román et al., 2013a; Gangopadhyay et al., 2015). As the water:barley flour ratio increased, BG yield decreased at low temperatures. At high temperatures from 55 to 60 °C, increasing the water:barley flour ratio from 10:1 (g/g) to nearly 13:1 (g/g) increased the yield, but above this value BG yield was decreased (Fig. 2b). The increase in the yield could be explained with a better extraction with increasing extraction medium. However, further increase in solvent results in rapid extraction into a dilute medium and extracted BG in a dilute system is more open to acidity and thermal and non-thermal effects of microwave to be degraded into simple sugars. This result shows that it is possible to work at low water:barley flour ratios. This is an important result because wet extraction of BG consumes large amounts of water, which must be removed in subsequent purification steps. Working at low water:barley flour ratios will simplify the subsequent purification process. Similar results were also reported by Benito-Román et al. (2013a), who performed BG extraction from different barley varieties. Extraction time is another factor that has a significant impact on BG yield. Increasing the extraction time at lower water:barley flour ratios enhanced the yield. However, when the water:barley flour ratio was high, increasing time decreased the yield (Fig. 2c). In line with this result, it was observed that, BG could be extracted in a short time when the water:barley flour ratio was high. With increasing extraction time, the BG yield decreased due to the degradation of BG because of both the non-thermal effect of microwaves and the acidic conditions created by carbonic acid formed when carbon dioxide dissolves in water.
MW-PCO2 extraction conditions were optimized to obtain the maximum BG yield (%). The optimum conditions were selected as temperature of 47.74 °C, the time of 19.92 min, and the water: barley flour ratio of 10.10:1 (g/g) with the desirability value of 1. The estimated BG yield under these conditions was 63.57%. Three parallel experiments were conducted under the recommended conditions to verify the results predicted by the model. The average BG yield of these experiments was found to be 62.43 ± 1.39%. The obtained experimental data were analyzed with one-sample t-test and the p-value for BG yield was found to be 0.291. These results showed that there was no statistically significant difference between the value recommended by the program and the values obtained experimentally (p > 0.05) which was also indicated the reliability of the extraction conditions obtained by RSM.
Comparisons of CE and MW-PCO2 Extraction Methods
Table 3 summarizes the conditions applied for CE and MW-PCO2 extraction methods and their effects on BG yield and purity. The extraction time of MW-PCO2 method (19.92 min) performed under optimum conditions was considerably shorter than that of CE method (180 min). The temperature applied and the solvent used in extraction was lower in MW-PCO2 method compared to CE method. BG yield and purity were found statistically significant and higher in MW-PCO2 method than in the CE method.
Although shorter time, lower temperature, and less solvent were used in the MW-PCO2 extraction method, obtaining higher yield and purity shows that this method is more advantageous. This could be explained by differences in the heating mechanisms of microwave and conventional methods. The direct interaction between the heated material and the electromagnetic field provides rapid heating in microwave (Özbek et al., 2021). In addition, CO2 pressure (30 bar) and the acidic environment (pH 2.9) provided by the CO2 could be effective on breakdown of barley endosperm and lignocellulosic structure to extract the BG more easily (Gangopadhyay et al., 2015; Lee et al., 2015).
Compositional Analysis of BG Extracts
The composition of the BG extracts obtained by CE and MW-PCO2 methods are given in Table 4. There is no statistical difference in moisture content. The extract obtained by the MW-PCO2 method had more BG, protein, starch, ash, arabinoxylan, and lignin. In a study on BG extraction from oat and barley fractions, the BG content obtained from barley varied between 22 and 63%, and the arabinoxylan ratio varied between 4.9 and 13.6% (Aktas-Akyildiz et al., 2018). The BG and starch content of BG extracts obtained by CE were reported as 48.83% and 15.79%, respectively (Aslan, 2022). Liu et al. (2021) purified starch and protein using enzymes after extraction, and thus they could reach 84.91% and 88.17% of BG purity. Performing a series of purification procedures, Ahmad et al. (2009) reported the BG, starch, protein, and ash content of BG extracts in the range of 78.1–83.1%, 3.19–3.25%, 6.86–7.72%, and 1.15–1.42%, respectively.
Molecular Weight Distribution
Molecular weight distribution of BG extracts obtained by CE and MW-PCO2 methods are given in Table 5. The results showed that the molecular weights of the extracts obtained by both methods were close to each other. Although there is no statistically significant difference between Mw results, there are significant differences between Mn, Mp, and PDI values (p < 0.05). The PDI (Mw/Mn) of BG extracts obtained by MW-PCO2 is slightly smaller than that of extracts obtained by the CE method. This indicates that the chain lengths of the extracts obtained with MW-PCO2 are more homogeneous. It has been reported by Lante and Canazza (2023) that the molecular weight of BGs of barley is highly variable, between 50 and 2000 kDa. This variability might be because of environmental factors, physical state of BG in plant tissue, β-glucanase activity, process parameters (extraction, purification, depolymerization steps), storage conditions, and molecular weight calculation methods (EFSA, 2011). The molecular weight of the extracts obtained by the UE method (2.682 × 106 Da) was reported be significantly lower than that of the extracts obtained by the CE method (4.989 × 106 Da) (Aslan, 2022). It is seen that the results of this study are comparable with the literature. In addition, the molecular weight of BG obtained by the MW-PCO2 method is good enough for dietary purpose (El Khoury et al., 2012).
Solubility and Water Holding Capacity (WHC)
The water solubility index and WHC of BG extracts obtained by CE and MW-PCO2 methods are shown in Table 5. The results obtained for both methods were found as significantly different (p < 0.05). Lower water solubility index of BG extract obtained by MW-PCO2 method is consistent with its higher molecular weight and WHC compared to that obtained by CE. Liu et al. (2021) reported the water solubility index of BG extracts obtained from hull-less barley as 69.01%, 75.62%, 77.63%, 78.08% in the extracts obtained by the microwave-assisted ultrasonic extraction (MUE), hot water extraction (HWE), microwave extraction (ME), and ultrasonic extraction (UE) methods, respectively. WHC of BG extracts was 5.59 g/g and 8.05 g/g for CE and MW-PCO2 methods, respectively. The higher WHC value for BG in MW-PCO2 process indicated that this material might be effectively used as a functional ingredient to prevent syneresis issues in a variety of food products, including cheese, sauces, jams, and jellies. WHC of BG extracts obtained from barley and oat by enzymatic method has been reported as 4.56 and 7.42 g/g, respectively (Kurek et al., 2018). The results found in this study for water solubility index and WHC are compatible with the literature. High WHC of BG extract makes it suitable to be used as a thickener for industrial use.
Thermal Properties of BG Extracts
DSC is an important tool used to study transitions that occur in polymers during heating in an inert atmosphere. Thermal scans in Fig. 3 show the differentiation of BG extracts obtained by CE and MW-PCO2 methods against heat. Both BG extracts showed two peaks in the range of 50–170 °C. The first peak is the glass transition peak which is the temperature at which molecular mobility decreases to a minimum and viscosity becomes very high. If the polymer is heated after this temperature, it loses its viscous properties and becomes brittle and hard like glass (Karaoğlu et al., 2009). The glass transition temperatures were 127.41 °C and 127.37 °C for BG extracts obtained by CE and MW-PCO2 methods, respectively. The second peak shows the melting point and this value was 148.05 °C and 146.83 °C for the BG extracts obtained by CE and MW-PCO2 methods, respectively. These peaks indicate melting of the polymer and dehydration of water due to weight loss. DSC analysis results showed that BG extracts are suitable for thermal processes, can be added to foods to be heated, and can withstand high temperatures in processes such as cooking and sterilization. In a study examining the thermal properties of BGs, the glass transition temperatures of standard BG, commercial BG and extracted BG samples were reported as 123.11 °C, 129.52 °C, and 122.34 °C, respectively, while the melting points of the samples were 144.23 °C, 153.08 °C, and 145.28 °C, respectively (Hasbay, 2018). Our results are quite consistent with this research. In another study conducted on barley BG concentrate and its hydrolysates, DSC analysis showed that a single endothermic peak at around 82 °C were observed in BG concentrate and BG hydrolysate with shorter hydrolysis time, while two endothermic peaks at arounds 150 °C and 183 °C were obtained in the hydrolysate with longer hydrolysis time (Dangi & Yadav, 2021). Thermal properties of BGs may be affected by their sources and extraction methods.
Fourier Transform Infrared (FTIR) Spectroscopy Analysis
FTIR spectra for BG extracted by the CE and the MW-PCO2 methods are presented in Fig. 4. The FTIR spectra of BG extracted by different methods revealed that both BGs had same characteristic peaks and no difference was observed between them. The absorption peak at around 3300 cm−1 was caused by stretching of hydroxyl groups (O–H) because polysaccharides have a large number of OH groups and the peak at around 2900 cm−1 was attributed to the methylene opposed stretching vibration (Fusté et al., 2019; Zhang et al., 2018). The absorption peak at approximately 1645 cm−1 could be assigned to the O–H bending of hydration water and NH groups of residual proteins (Liu et al., 2021; Nie et al., 2021). The band at 1370 cm−1 represents the deviational vibration of C–H moiety and the sharp peak at approximately 1025 cm−1 was attributed to the C–O bonds of the alcohol groups. Peak shoulder at 1070 cm−1 and peak at 1155 cm−1 represent the linear structure of BG linked through 1–3 linkages (Fusté et al., 2019). The characteristic absorbtion peak at 896.4 cm−1 refer to the presence of β-linked glycosidic bond and confirms the presence of β-d-glucan molecule (Fusté et al., 2019; Zhang et al., 2018). These results indicated that the MW-PCO2 and CE methods did not effect the primary functional groups of BG. The BG samples extracted by MW-PCO2 and CE methods had characteristic absorption peaks of BGs and their FTIR spectrum are consistent with those of previously reported cereal BGs (Nie et al., 2021; Zhang et al., 2018).
Color
Color values of BG extracts obtained by CE and MW-PCO2 methods are presented in Table 5. While there is no statistical difference between the a* values of the extracts (p > 0.05), the L* and b* values are statistically different (p < 0.05). Lower L* value and higher b* value of the BG extract obtained by the CE method may be due to the better separation of coloring components in MP-PCO2 extraction. Ahmad et al. (2009) reported the color values of BG extracts as L* = 67.55, a* = 7.70, b* = 21.50 for alkaline extraction method, L* = 69.86, a* = 8.39, b* = 18.08 for acid extraction method, L* = 77.34, a* = 7.45, b* = 16.22 for CE method, and L* = 71.48, a* = 6.75, b* = 19.32 for enzymatic extraction method. Compared to the literature, both BG extracts obtained in this study have lighter color with lower redness and yellowness. This might be because of the difference in raw materials.
Scanning Electron Microscope Images of BG Extracts
The surface images of BG extracts are given in Fig. 5. Both images are similar in structure. It has a porous spongy appearance with no cell wall, appearing like a polymer structure. In the literature, images of BGs, which may vary depending on drying methods, are generally similar. Utama et al. (2021) analyzed the BG extracts extracted from four different microorganism sources and reported a common sponge-like appearance, that is, a porous and rough texture with an invisible cell wall structure although they had different particle sizes and shapes. Limberger-Bayer et al. (2014) reported that the morphological characteristics of barley BG and commercial BG are similar, that they have a porous and spongy appearance, and that there is no visible trace of the cell wall structure. In another study conducted with oat BG, the same porous spongy appearance is mentioned (Singh et al., 2018).
Conclusion
In this research, MW-PCO2 extraction of BG from hull-less barley was performed and the effect of extraction parameters was evaluated to obtain maximum BG yield. The temperature of 47.74 °C, the water:barley flour ratio of 10.10:1 (g/g), and the duration of 19.92 min yielded the maximum BG yield (62.43%). When this technique was compared with CE, the findings demonstrated that MW-PCO2 extraction produced greater BG yield and purity while requiring less solvent and less time. The BG extract obtained from MW-PCO2 extraction has a greater WHC, which qualifies it for use as an industrial thickener. The results of DSC analysis showed that BG extracts are suitable for thermal processes, can be added to foods to be heated, and can withstand high temperatures in processes such as cooking and sterilization. The molecular weight, morphological characteristics, and FTIR spectra of the BGs produced using the MW-PCO2 and CE procedures were comparable, suggesting that the polymer’s chemical structure was unaffected by the MW-PCO2 method. However, further studies should be performed on the potential use of hull-less barley in human nutrition and for the economic analysis to determine the applicability of MW-PCO2 to produce BG from different sources at industrial level.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Acar, O. (2020). An investigation on the effects of biofortification on minerals and functional constituents of barley, oats and milling fractions. Retrieved January 2, 2024, from https://openaccess.hacettepe.edu.tr/xmlui/handle/11655/22698
Ahmad, A., Anjum, F. M., Zahoor, T., Nawaz, H., & Din, A. (2009). Physicochemical and functional properties of barley b-glucan as affected by different extraction procedures. International Journal of Food Science and Technology, 44(1), 181–187. https://doi.org/10.1111/j.1365-2621.2008.01721.x
Ahmad, M., Gani, A., Shah, A., Gani, A., & Masoodi, F. A. (2016). Germination and microwave processing of barley (Hordeum vulgare L) changes the structural and physicochemical properties of β-D-glucan & enhances its antioxidant potential. Carbohydrate Polymers, 153, 696–702.
Aktas-Akyildiz, E., Sibakov, J., Nappa, M., Hytönen, E., Koksel, H., & Poutanen, K. (2018). Extraction of soluble β-glucan from oat and barley fractions: Process efficiency and dispersion stability. Journal of Cereal Science, 81, 60–68. https://doi.org/10.1016/j.jcs.2018.03.007
AOAC-Official Methods of Analysis. (2010). AOAC 960.52: Microchemical determination of nitrogen. Micro-kjeldahl method.
AOAC-Official Methods of Analysis. (2014). AOAC 925.10: Solids (total) and loss on drying (moisture) in flour.
AOAC 923.03:Ash of flour. (2015). AOAC-Official Methods of Analysis.
Aslan, Ö. (2022). Optimization of ultrasound assisted beta-glucan extraction from barley and its application in a model food. Retrieved December 15, 2023, from https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp
Bansod, S. P., Parikh, J. K., & Sarangi, P. K. (2023). Pineapple peel waste valorization for extraction of bio-active compounds and protein: Microwave assisted method and Box Behnken design optimization. Environmental Research, 221, 115237. https://doi.org/10.1016/j.envres.2023.115237
Benito-Román, Ó., Alonso, E., & Lucas, S. (2011). Optimization of the β-glucan extraction conditions from different waxy barley cultivars. Journal of Cereal Science, 53(3), 271–276. https://doi.org/10.1016/j.jcs.2011.01.003
Benito-Román, Ó., Alonso, E., & Cocero, M. J. (2013a). Pressurized hot water extraction of β-glucans from waxy barley. The Journal of Supercritical Fluids, 73, 120–125.
Benito-Román, Ó., Alonso, E., Gairola, K., & Cocero, M. J. (2013b). Fixed-bed extraction of β-glucan from cereals by means of pressurized hot water. The Journal of Supercritical Fluids, 82, 122–128. https://doi.org/10.1016/j.supflu.2013.07.003
Benito-Román, Ó., Alonso, E., & Cocero, M. J. (2013c). Ultrasound-assisted extraction of β-glucans from barley. LWT-Food Science and Technology, 50(1), 57–63. https://doi.org/10.1016/j.lwt.2012.07.006
Browning, B. L. (1967). Chemistry of wood. In K. V. Sarkanen & C. H. Ludwig (Eds.), Methods in wood chemistry (pp. 1943–1944). John, Wiley & Sons. https://doi.org/10.1002/pol.1968.160061112
Cajzek, F., Bertoncelj, J., Kreft, I., Poklar Ulrih, N., Polak, T., Požrl, T., Pravst, I., Polišenská, I., Vaculová, K., & Cigić, B. (2020). Preparation of β-glucan and antioxidant-rich fractions by stone milling of hull-less barley. International Journal of Food Science and Technology, 55(2), 681–689. https://doi.org/10.1111/ijfs.14322
Dangi, N., & Yadav, B. S. (2021). Characterization of acid hydrolysates from barley β-glucan concentrate for their physico-chemical and rheological properties. Journal of Food Science and Technology, 58(9), 3280–3292. https://doi.org/10.1007/s13197-020-04873-w
Dimopoulos, G., Tsantes, M., & Taoukis, P. (2020). Effect of high pressure homogenization on the production of yeast extract via autolysis and beta-glucan recovery. Innovative Food Science and Emerging Technologies, 62, 102340. https://doi.org/10.1016/j.ifset.2020.102340
EFSA. (2011). Scientific Opinion on the substantiation of a health claim related to barley beta-glucans and lowering of blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA Journal, 9(12), 2470. https://doi.org/10.2903/j.efsa.2011.2470
El Khoury, D., Cuda, C., Luhovyy, B. L., & Anderson, G. H. (2012). Beta glucan: Health benefits in obesity and metabolic syndrome. Journal of Nutrition and Metabolism, 2012, 851362. https://doi.org/10.1155/2012/851362
Erbaş Köse, Ö. D., Mut, Z., & Kardeş, Y. M. (2021). Determination of grain yield and some quality traits of hulless barley genotypes in Bilecik conditions. ISPEC Journal of Agricultural Sciences, 5(3), 608–615. http://ispecjournal.com/index.php/ispecjas/article/view/213/241
Fusté, N. P., Guasch, M., Guillen, P., Anerillas, C., Cemeli, T., Pedraza, N., Ferrezuelo, F., Encinas, M., Moralejo, M., & Garí, E. (2019). Barley β-glucan accelerates wound healing by favoring migration versus proliferation of human dermal fibroblasts. Carbohydrate Polymers, 210, 389–398. https://doi.org/10.1016/j.carbpol.2019.01.090
Gangopadhyay, N., Hossain, M. B., Rai, D. K., & Brunton, N. P. (2015). Optimisation of yield and molecular weight of β-glucan from barley flour using response surface methodology. Journal of Cereal Science, 62, 38–44. https://doi.org/10.1016/j.jcs.2014.10.007
Guzik, P., Kulawik, P., Zając, M., & Migdał, W. (2022). Microwave applications in the food industry: An overview of recent developments. Critical Reviews in Food Science and Nutrition, 62(29), 7989–8008. https://doi.org/10.1080/10408398.2021.1922871
Hasbay, İ. (2018). Arpadan β-glukan elde edilmesi, fonksiyonel gıda bileşeni ve doğal kıvam arttırıcı gıda katkı maddesi olarak kullanılması. Retrieved January 2, 2024, from https://search.trdizin.gov.tr/tr/yayin/detay/618983/arpadan-b-glukan-elde-edilmesi-fonksiyonel-gida-bileseni-ve-dogal-kivam-arttirici-gida-katki-maddesi-olarak-kullanilmasi
Izydorczyk, M. S., Jacobs, M., & Dexter, J. E. (2003). Distribution and structural variation of nonstarch polysaccharides in milling fractions of hull-less barley with variable amylose content. Cereal Chemistry, 80(6), 645–653. https://doi.org/10.1094/CCHEM.2003.80.6.645
Karaoğlu, M., Boz, H., Kotancılar, G. H., & Gerçekaslan, E. K. (2009). Glass transition temparature and it’s importance in cereal products. Atatürk University Journal of Agricultural Faculty, 40(2), 93–99.
Kendall, C. W. C., Esfahani, A., & Jenkins, D. J. A. (2010). The link between dietary fibre and human health. Food Hydrocolloids, 24(1), 42–48. https://doi.org/10.1016/j.foodhyd.2009.08.002
Kurek, M. A., Karp, S., Stelmasiak, A., Pieczykolan, E., Juszczyk, K., & Rieder, A. (2018). Effect of natural flocculants on purity and properties of β-glucan extracted from barley and oat. Carbohydrate Polymers, 188, 60–67. https://doi.org/10.1016/j.carbpol.2018.01.090
Lante, A., & Canazza, E. (2023). Insight on extraction and preservation of biological activity of cereal β-D-glucans. Applied Sciences, 13(19), 11080. https://doi.org/10.3390/app131911080
Lee, S. H., Jang, G. Y., Hwang, I. G., Kim, H. Y., Woo, K. S., Kim, K. J., Lee, M. J., Kim, T. J., Lee, J., & Jeong, H. S. (2015). Physicochemical properties of β-glucan from acid hydrolyzed barley. Preventive Nutrition and Food Science, 20(2), 110–118. https://doi.org/10.3746/pnf.2015.20.2.110
Limberger-Bayer, V. M., De Francisco, A., Chan, A., Oro, T., Ogliari, P. J., & Barreto, P. L. M. (2014). Barley β-glucans extraction and partial characterization. Food Chemistry, 154, 84–89. https://doi.org/10.1016/j.foodchem.2013.12.104
Liu, H., Li, Y., You, M., & Liu, X. (2021). Comparison of physicochemical properties of β-glucans extracted from hull-less barley bran by different methods. International Journal of Biological Macromolecules, 182, 1192–1199. https://doi.org/10.1016/j.ijbiomac.2021.05.043
Nie, C., Yan, X., Xie, X., Zhang, Z., Zhu, J., Wang, Y., Wang, X., Xu, N., Luo, Y., Sa, Z., Li, X., Liu, H., Tian, B., Ma, Q., Zhang, M., Chen, Z., & Li, J. (2021). Structure of β-glucan from Tibetan hull-less barley and its in vitro fermentation by human gut microbiota. Chemical and Biological Technologies in Agriculture, 8(1), 1–14. https://doi.org/10.1186/s40538-021-00212-z
Öner, A. (2019). Improvement of functional properties of hull-less barley and investigation of its possible usability in cookies. Retrieved April 03, 2024, from https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp
Özbek, H. N., Koçak Yanık, D., Fadıloğlu, S., & Göğüş, F. (2021). Effect of microwave-assisted alkali pre-treatment on fractionation of pistachio shell and enzymatic hydrolysis of cellulose-rich residues. Journal of Chemical Technology and Biotechnology, 96, 521–531. https://doi.org/10.1002/jctb.656
Rodríguez-Seoane, P., González-Muñoz, M. J., Falqué, E., & Domínguez, H. (2018). Pressurized hot water extraction of β-glucans from Cantharellus tubaeformis. Electrophoresis, 39(15), 1892–1898. https://doi.org/10.1002/elps.201700399
Sakdasri, W., Arnutpongchai, P., Phonsavat, S., Bumrungthaichaichan, E., & Sawangkeaw, R. (2022). Pressurized hot water extraction of crude polysaccharides, β-glucan, and phenolic compounds from dried gray oyster mushroom. LWT-Food Science and Technology, 168, 113895. https://doi.org/10.1016/j.lwt.2022.113895
Sasaki, M., & Ohsawa, K. (2021). Hydrolysis of lignocellulosic biomass in hot-compressed water with supercritical carbon dioxide. ACS Omega, 6(22), 14252–14259. https://doi.org/10.1021/acsomega.1c01026
Sharma, P., & Gujral, H. S. (2013). Extrusion of hulled barley affecting β-glucan and properties of extrudates. Food and Bioprocess Technology, 6(6), 1374–1389. https://doi.org/10.1007/s11947-011-0777-2
Singh, S., Kaur, M., & Sogi, D. S. (2018). β-glucan from different Indian oat (Avena sativa) cultivars: Chemical, functional, structural, and rheological properties. Journal of Food Processing and Preservation, 42(11), 1–10. https://doi.org/10.1111/jfpp.13788
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., & Templeton, D. (2008). Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Technical Report NREL/TP-510–42623. National Renewable Energy Laboratory, Laboratory, Colorado, USA, 1–14.
Utama, G. L., Dio, C., Sulistiyo, J., Yee Chye, F., Lembong, E., Cahyana, Y., Kumar Verma, D., Thakur, M., Patel, A. R., & Singh, S. (2021). Evaluating comparative β-glucan production aptitude of Saccharomyces cerevisiae, Aspergillus oryzae, Xanthomonas campestris, and Bacillus natto. Saudi Journal of Biological Sciences, 28(12), 6765–6773. https://doi.org/10.1016/j.sjbs.2021.07.051
Wood, P. J. (2010). Oat and rye β-glucan: Properties and function. Cereal Chemistry, 87(4), 305–314. https://doi.org/10.1094/CCHEM-87-4-0315
Zhang, H., Zhang, N., Xiong, Z., Wang, G., Xia, Y., Lai, P., & Ai, L. (2018). Structural characterization and rheological properties of β-D-glucan from hull-less barley (Hordeum vulgare L. var. nudum Hook. f.). Phytochemistry, 155, 155–163. https://doi.org/10.1016/j.phytochem.2018.08.004
Acknowledgements
The authors would like to thank Dr. Derya Koçak Yanık from Osmangazi University Food Engineering Department for her support in conducting the MW-PCO2 extraction experiments.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Saadet Özen: methodology, data curation, investigation, writing—original draft, visualization. Ayşe Ünlü: supervision, conceptualization, writing—review and editing. Hatice Neval Özbek: investigation, data curation, writing—original draft, conceptualization, methodology, writing—review and editing. Fahrettin Göğüş: conceptualization, methodology, investigation, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Özen, S., Ünlü, A., Özbek, H.N. et al. β-Glucan Extraction from Hull-Less Barley by a Novel Approach: Microwave-Assisted Pressurized CO2/H2O. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03420-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03420-1