Abstract
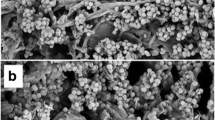
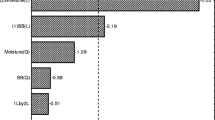
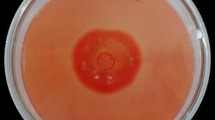
A study was conducted to appraise the potential of using kinnow pulp for production of cellulases by Trichoderma reesei Rut C-30. Out of the different combinations tried out, dried kinnow pulp supplemented with wheat bran in the ratio of 4:1 resulted in the highest filter paper cellulase (FPase) activity of 13.4 IU/gds whereas endo-1,4-β-glucanase (CMCase) activity was found to be best when kinnow pulp was supplemented with wheat bran in the ratio 3:2 using Mandel Weber (MW) medium. β-glucosidase activity of 18 IU/gds was again found to be maximum in treatment involving 3:2 ratio of kinnow pulp to wheat bran in MW medium. However, supplementing kinnow pulp with wheat bran in 3:2 using water as medium resulted in an FPase:β-glucosidase ratio of nearly 1:1 which is considered to be most appropriate for achieving ideal saccharification efficiency in case of pretreated lignocellulosic material. Thus, this study involved the utilisation of kinnow pulp for production of cellulases and demonstrated that a substrate which does not find any commercial significance and causes environmental pollution due to its poor disposal holds promise as a substrate for production of cellulases.

Similar content being viewed by others
References
Adsul, M. G., Bastawade, K. B., Varma, A. J., & Gokhale, D. V. (2007). Strain improvement of Penicillium janthinellum NCIM 1171 for increased cellulase production. Bioresource Technology, 98, 1467–1473.
Ananda Muniswaran, P. K., Selvakumar, P., & Narasimha Charyulu, N. C. L. (1994). Production of cellulases from coconut coir pith in solid state fermentation. Journal of Chemical Technology and Biotechnology, 60, 147–151.
AOAC (2000). Official methods of analysis of AOAC international vol. 2 (17th ed., p. 14). Gaithersburg, MD: Association of Official Analytical Chemists.
Aravantinos-Zafiris, G., Oreopouou, V., Tzia, C., & Thomopoulos, C. D. (1992). Utilization of orange by-products-orange peel carotenoids. Journal of the Science of Food and Agriculture, 59, 77–79.
Awafo, V. A., Chahal, D. S., Simpson, B. K., & Le, G. B. B. (1996). Production of cellulase systems by selected mutants of Trichoderma reesei in solid-state fermentation and their hydrolytic potentials. Applied Biochemistry and Biotechnology, 57–58, 461–470.
Bhat, M. K. (2000). Cellulases and related enzymes in biotechnology. Biotechnology Advances, 18, 355–383.
Butt, M. S., Ihsanulla Qamar, M., Anjum, F. M., Aziz, A., & Atif Randhawa, M. (2004). Development of minerals enriched brown flour by utilizing wheat milling by-products. Internet Journal of Food Safety, 3, 15–20.
Cen, P., & Xia, L. (1999). Production of cellulases by solid state fermentation. Advances in Biochemical Engineering/Biotechnology, 65(6), 69–92.
Chahal, D. S., Mc Guire, S., Pikor, H., & Noble, G. (1982). Production of cellulase complex by Trichoderma reesei Rut-C30 on lignocellulose and its hydrolytic potential. Biomass, 2, 127–138.
Chen, S., & Wyman, M. (1991). Cellulase production induced by carbon sources derived from waste newspaper. Process Biochemistry, 26, 93–100.
Crampton, E. W., & Maynard, L. A. (1938). The relation of cellulose and lignin content to the nutritive value of animal feeds. Journal of Nutrition, 16, 383–395.
Depaula, E. H., Ramos, L. P., & Azevedo, M. D. (1999). The potential of Humicola grisea var. thermoidea for bioconversion of sugarcane bagasse. Bioresource Technology, 68, 35–41.
Devi, S. P., & Singh, H. D. (1995). Bioconversion of water hyacinth hemicellulose and xylose rich sugar mixtures to ethanol by Neurospora crassa. Enzyme Microbial Technology, 8, 149–152.
Ellouz Chaabouni, S., Belguith, H., Hassairi, I., Rad, K. M., & Ellouz, R. (1994). Optimization of cellulase production by Penicillium occitanis. Applied Microbiology and Biotechnology, 43, 267–269.
Garcia-Kirchner, O., Segura-Granados, M., & Rodriguez-Pascual, P. (2005). Effect of media composition and growth conditions on production of beta-glucosidase by Aspergillus niger C-6. Applied Biochemistry and Biotechnology, 121, 347–459.
Goering, H. K.,& Vansoest, P. J. (1970). Forage fibre analysis. Agricultural Research Services, United States Department of Agriculture, Agricultural Handbook, No. 379.
Grohmann, K., Cameron, R. G., & Buslig, B. S. (1995). Fractionation and pretreatment of orange peel by dilute acid hydrolysis. Bioresource Technology, 54, 129–141.
Holker, U., Hofer, M., & Lenz, J. (2004). Biotechnological advantages of lab scale solid state fermentation with fungi. Applied Microbiology and Biotechnology, 64, 175–189.
Immanuel, G., Akila Bhagavath, C. M., Iyappa Raj, P., Esakkiraj, P., & Palavesam, A. (2007). Production and partial purification of cellulase by Aspergillus niger and A. fumigatus fermented in Coir waste and sawdust. The Internet Journal of Microbiology, 3(1).
Juhász, T., Kozma, K., Szengyel, Z., & Réczey, K. (2003). Production of β-glucosidase in mixed culture of Aspergillus niger BKMF 1305 and Trichoderma reesei RUT C30. Food Technology Biotechnology, 41, 49–53.
Kadam, K. L. (1996). Cellulase production. In C. E. Wyman (Ed.), Handbook on bioethanol: Production and utilization Chapter 11 (pp. 213–252). Washington, DC: Taylor and Francis.
Kalra, K. L., Grewal, H. S., & Kahlon, S. S. (1989). Bioconversion of kinnow mandarin waste into single cell protein. MIRCEN J, 5, 32–39.
Kang, S. W., Park, Y. S., Lee, J. S., Hong, S. I., & Kim, S. W. (2004). Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresource Technology, 91, 153–156.
Kansoh, A. L., Essam, S. A., & Zeinat, A. N. (1999). Biodegradation and utilization of bagasse with Trichoderma reesei. Polymer Degradation Stability, 62, 173–178.
Khandelwal, P., Vijay, K., Das, N., & Tyagi, S. M. (2006). Development of process for preparation of pure and blended kinnow wine without debittering kinnow mandarin juice. Internet Journal of Food Safety, 6, 24–29.
Krishna, C. (1999). Production of bacterial cellulases by solid state bioprocessing of banana wastes. Bioresource Technology, 69, 231–239.
Latifian, M., Hamidi-Esfahini, Z., & Barzegar, M. (2007). Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresource Technology, 98, 3634–3637.
Levin, L., & Forchiassin, F. (1997). Effect of culture conditions on the production of cellulases by Trametes trogii. Revista Argentina de Microbiologia, 29, 16–23.
Miller, G. (1959). Dinitro salicylic acid reagent for determination of reducing sugars. Analytical Chemistry, 31, 426–428.
Mohagheghi, A., Tuker, M., Grohmann, K., & Wyman, C. (1992). High solids simultaneous saccharification and fermentation of pretreated wheat straw to ethanol. Applied Biochemistry and Biotechnology, 33, 67–81.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., et al. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 671–686.
Nakari-Stela, T., & Pentilla, M. (1995). Production of Trichoderma reesei cellulases on glucose containing medium. Applied and Environmental Microbiology, 61, 3650–3655.
Oberoi, H. S., Kalra, K. L., Uppal, D. S., & Tyagi, S. K. (2007). Effect of different drying methods of cauliflower waste on drying time, colour retention and glucoamylase production by Aspergillus niger NCIM 1054. International Journal of Food Science and Technology, 42, 228–234.
Olsson, L., Christensen Tove, M. I. E., Hansen Kim, P., & Palmquist Eva, A. (2003). Influence of the carbon source on production of cellulases, hemicellulases and pectinases by Trichoderma reesei Rut C-30. Enzyme and Microbial Technology, 33, 612–619.
Pandey, A. (2003). Solid state fermentation. Biochemical Engineering Journal, 13, 81–84.
Pandey, A., Selvakumar, P., Soccol, C. R., & Nigam, P. (1999). Solid state fermentation for production of industrial enzymes. Current Science, 77, 149–162.
Pothiraj, C., Balaji, P., & Eyini, M. (2006). Enhanced production of cellulases by various fungal cultures in solid state fermentation of cassava waste. African Journal of Biotechnology, 5, 1882–1885.
Ramos, L. P., Nazhad, M. M., & Saddler, J. N. (1993). Effect of enzymatic hydrolysis on the morphology and fine structure of pretreated cellulosic residues. Enzyme Microbial Technology, 15, 821–831.
Ryu, D. D. Y., & Mandels, M. (1980). Cellulases: Biosynthesis and applications. Enzyme Microbial Technology, 2, 91–102.
Sami, A. J., Akhtar, M. W., Malik, N. N., & Naz, B. A. (1988). Production of free and substrate-bound cellulases of Cellulomonas flavigena. Enzyme Microbial Technology, 10, 626–631.
Shah, N. (2007). Optimization of an enzyme assisted process for juice extraction and clarification from Litchis (Litchi Chinensis Sonn.). International Journal of Food Engineering, 3(3), 1–17 Article 8.
Shiang, M., Linden, J. C., Mohagheghi, A., Rivard, C. J., Grohmann, K., & Himmel, M. E. (1990). Cellulase production by Acidothermus cellulolyticus. Applied Biochemistry and Biotechnology, 24/25, 223–235.
Shin, C. S., Joon, L. P., Lee, J. S., & Park, S. C. (2000). Enzyme production of Trichoderma reesei Rut C-30 on various lignocellulosic substrates. Applied Biochemistry and Biotechnology, 84–86, 237–245.
Singhania, R. R., Sukumaran, R. K., & Pandey, A. (2007). Improved cellulase production by Trichoderma reesei RUT C30 under SSF through process optimization. Applied Biochemistry and Biotechnology, 142, 60–70.
Steiner, G., Socha, C., & Eyzaguirre, J. (1994). Culture conditions for enhanced cellulase production by a native strain of Penicillium purpurogenum. World Journal of Microbiology and Biotechnology, 10, 280–284.
Sun, X., Liu, Z., Qu, Y., & Li, X. (2008). The effects of wheat bran composition on the production of biomass-hydrolyzing enzymes by Penicillium decumbens. Applied Biochemistry and Biotechnology, 146, 119–128.
Teeri, T. T. (1997). Crystalline cellulose degradation: New insight into the function of cellobiohydrolases. Focus, 15, 160–167.
Tengerdy, R. P. (1998). In A. Pandey (Ed.), Advances in biotechnology (pp. 13–16). New Delhi: Educational Publishers and Distributors.
Tolan, J. S., & Foody, B. (1999). Cellulases from submerged fermentation. Advances in Biochemical Engineering/Biotechnology, 65, 41–67.
Wang, J. S., Wang, J., & Gulfraz, M. (2005). Efficient cellulase production from corn straw by Trichoderma Reesei LW1 through solid state fermentation process. Ethnobot. Leaf. www.siu.edu.
Wilkins, M. R., Widmer, W. W., & Grohmann, K. (2007). Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochemistry, 42, 1614–1661.
Wooley, R., Ruth, M., Glassner, D., & Sheehan, J. (1999). Process design and costing of bioethanol technology: A tool for determining the status and direction of research and development. Biotechnology Progress, 15, 794–803.
Xu, G. H., Chen, J. C., Liu, D. H., Zhang, Y. H., Jiang, P., & Ye, X. Q. (2008). Minerals, phenolic compounds, and antioxidant capacity of citrus peel extract by hot water. Journal of Food Science, 73, 11–18.
Yu, X. B., Yun, H. S. K., & Mo, Y. (1998). Production of cellulase by Trichoderma reesei Rut C30 in wheat bran-containing media. Journal of Microbiology and Biotechnology, 8, 208–213.
Acknowledgement
The authors thankfully acknowledge the financial assistance received from NBAIM/ICAR under AMAAS for carrying out this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oberoi, H.S., Chavan, Y., Bansal, S. et al. Production of Cellulases through Solid State Fermentation Using Kinnow Pulp as a Major Substrate. Food Bioprocess Technol 3, 528–536 (2010). https://doi.org/10.1007/s11947-008-0092-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0092-8