Abstract
Purpose of Review
To summarize pathophysiology, key conflicts, and therapeutic approaches in managing concomitant severe acute brain injury (SABI) and acute respiratory distress syndrome (ARDS).
Recent Findings
ARDS is common in SABI and independently associated with worse outcomes in all SABI subtypes. Most landmark ARDS trials excluded patients with SABI, and evidence to guide decisions is limited in this population. Potential areas of conflict in the management of patients with both SABI and ARDS are (1) risk of intracranial pressure (ICP) elevation with high levels of positive end-expiratory pressure (PEEP), permissive hypercapnia due to lung protective ventilation (LPV), or prone ventilation; (2) balancing a conservative fluid management strategy with ensuring adequate cerebral perfusion, particularly in patients with symptomatic vasospasm or impaired cerebrovascular blood flow; and (3) uncertainty about the benefit and harm of corticosteroids in this population, with a mortality benefit in ARDS, increased mortality shown in TBI, and conflicting data in other SABI subtypes. Also, the widely adapted partial pressure of oxygen (PaO2) target of > 55 mmHg for ARDS may exacerbate secondary brain injury, and recent guidelines recommend higher goals of 80–120 mmHg in SABI. Distinct pathophysiology and trajectories among different SABI subtypes need to be considered.
Summary
The management of SABI with ARDS is highly complex, and conventional ARDS management strategies may result in increased ICP and decreased cerebral perfusion. A crucial aspect of concurrent management is to recognize the risk of secondary brain injury in the individual patient, monitor with vigilance, and adjust management during critical time windows. The care of these patients requires meticulous attention to oxygenation and ventilation, hemodynamics, temperature management, and the neurological exam. LPV and prone ventilation should be utilized, and supplemented with invasive ICP monitoring if there is concern for cerebral edema and increased ICP. PEEP titration should be deliberate, involving measures of hemodynamic, pulmonary, and brain physiology. Serial volume status assessments should be performed in SABI and ARDS, and fluid management should be individualized based on measures of brain perfusion, the neurological exam, and cardiopulmonary status. More research is needed to define risks and benefits in corticosteroids in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute respiratory distress syndrome (ARDS) is commonly encountered in severe acute brain injury (SABI). Among subtypes of SABI, ARDS has been reported in up to 30% of patients with traumatic brain injury (TBI) [1–5], 38% of patients with non-traumatic subarachnoid hemorrhage (SAH) [6–9], 28% of patients with spontaneous intracranial hemorrhage (sICH) [10], 4% of patients with acute ischemic stroke (AIS) [11, 12], and 48% of patients after cardiac arrest [13, 14]. Given the high prevalence of ARDS and a wide range of reported neurological manifestations with COVID-19 [15–18], the concurrent occurrence of SABI and ARDS will likely rise further. ARDS is independently associated with increased mortality and poor neurological outcome in all SABI subtypes [2, 3, 5, 6, 8, 13, 14, 19].
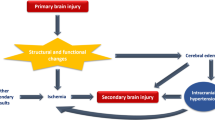
In critically ill patients with SABI, pathophysiological interactions between the brain and lungs are complex (Fig. 1). SABI can induce and worsen ARDS via multiple pathways [20–22]; conversely, hypoxemia and systemic inflammatory responses encountered in ARDS can further precipitate secondary brain injury. Cognitive deficits and mood disorders are frequently encountered as long-term sequelae of ARDS [23–25], even in the absence of known SABI.
Strategies for managing SABI and ARDS may conflict. Ventilatory and hemodynamic targets considered standard ARDS care may insufficiently support or even harm the acutely injured brain. Most major randomized controlled trials (RCTs) supporting ARDS treatment strategies excluded patients with neurological injury or elevated intracranial pressure (ICP) [26•, 27•, 28–31], and the results of these studies are not generalizable to this population.
In this article, we review key principles and evidence in the treatment of SABI and ARDS, and provide [32] guidance on how to approach conflicts in managing concomitant SABI and ARDS based on the available literature and practical considerations.
Mechanical ventilation targets in SABI
Mechanical ventilation (MV) in SABI is typically indicated due to impaired consciousness, resulting in loss of airway protective reflexes and decreased respiratory drive or in the context of secondary respiratory events, such as aspiration pneumonia, pulmonary contusions, pulmonary edema, pulmonary embolism, or ARDS. On occasions, MV is deemed necessary when deep sedation is required to treat status epilepticus, elevated ICP, extreme agitation, or to facilitate emergent neuroimaging studies. However, the optimal PaO2 and PaCO2 targets are not yet established.
Hypoxemia has shown to be detrimental in SABI [33–36], due to secondary ischemic injury and reflexive cerebral vasodilation resulting in increased ICP. Hyperoxemia (PaO2 > 300 mmHg) may also be potentially harmful due to the creation of reactive oxygen species and exacerbation of inflammation and cellular injury [34, 35, 37, 38]. Several large trials in a general ICU population demonstrated no difference between conservative and liberal oxygen therapy [39–41], though questions remain about the subgroup of patients with brain injury in these trials. A recent ESICM consensus statement recommended higher PaO2 targets of 80–120 mmHg in SABI and strict avoidance of hypoxemia [42•]. Ideally, targets would be individualized based on the type and extent of brain injury. Also, the optimal hemoglobin goal to optimize oxygen-carrying capacity and cerebral oxygen delivery remains under investigation. Both anemia and blood transfusions have been associated with worse neurological outcomes in SABI [43–46]. Based on evidence in the general ICU population [47–49], hemoglobin goals around 7 gm/dl and conservative transfusion strategies have been widely adopted. However, patients with SABI were underrepresented in these studies. A RCT in TBI showed higher adverse events with hemoglobin goals > 10 gm/dl with no improvement in neurological outcome [50]. SAH guidelines recommend transfusing to a hemoglobin goal of 8–10 gm/dl in patients at risk for delayed cerebral ischemia (DCI) [51], but the impact on neurological outcomes remains to be established, and a large RCT comparing transfusion strategies is ongoing [52]. The value of adjusting hemodynamic and ventilator parameters based on brain tissue oxygen (PbtO2) is being investigated. Observational studies in TBI and SAH have shown higher mortality in association with decreased PbtO2 levels and suggested outcome benefits with PbtO2-directed therapy [53–58]. The Brain Oxygen Optimization in Severe TBI (BOOST-3) trial, a phase 3 RCT, is assessing the potential to improve neurological outcomes in TBI by comparing ICU care guided by ICP monitoring only against an ICP plus PbtO2-guided management strategy [59].
Partial pressure of carbon dioxide (PaCO2) acts as a fundamental regulator of cerebral blood flow (CBF) [60]. Hypercapnia causes dilatation of the cerebral vasculature and can result in ICP elevations. Lowering PaCO2 via therapeutic hyperventilation is a rapid, effective measure to treat elevated ICP, but the effect diminishes over 6–24 h, and hypocapnia can cause cerebral vasoconstriction and cerebral ischemia [61–63]. Also, normocapnia following hypocapnia can result in rebound ICP spikes. Due to these concerns, the use of therapeutic hyperventilation remains controversial and should only be considered as a short-term rescue strategy. Both hypo- and hypercapnia are associated with higher mortality and poor outcomes in TBI, AIS, and PCABI [64•, 65, 66]. Mild hypocapnia (30–35 mmHg) can be considered in patients with elevated ICPs. Potential benefits of mild hypercarbia in restoring cerebral perfusion after cardiac arrest are being investigated [67–69]. In most patients with SABI, vigilant monitoring and avoidance of extreme PaCO2 fluctuations are recommended [42•].
The optimal tidal volume (Vt), respiratory rate (RR), positive end-expiratory pressure (PEEP), and preferred mode of ventilation in SABI remain unknown. Many patients who require MV due to the loss of airway protective reflexes often retain their ventilatory drive. If safely tolerated, a spontaneous mode may diminish the need for sedation. An assisted mode will ensure tighter control of ventilation and PaCO2. Abnormal breathing patterns are commonly encountered in SABI [69], and changes in respiratory drive may further exacerbate ventilator dyssynchrony, requiring nuanced management of sedation to minimize barotrauma and avoid PaCO2 derangements.
Overview of ARDS
ARDS is defined by four components: (1) acute onset within 7 days of a clinical insult, (2) hypoxemia (PaO2:FiO2 ≤ 300), (3) radiographic bilateral pulmonary opacities, and (4) findings not fully explained by fluid overload or heart failure [70]. The Berlin definition stratifies ARDS into three categories based on hypoxemia severity (mild: P:F ratio 201–300 mmHg, moderate: 101–200 mmHg, severe: ≤ 100 mmHg with PEEP ≥ 5 cm H2O) [71].
The most common contributing risk factors, accounting for approximately 85% of ARDS cases, are pneumonia, non-pulmonary sepsis, and aspiration of gastric contents [72]. A wide range of other pulmonary and non-pulmonary etiologies, including major trauma, intracranial hypertension, pulmonary contusions, pancreatitis, inhalation and drowning injuries, severe burns, non-cardiogenic shock, blood transfusions, pulmonary vasculitis, and drug overdoses, have been associated with ARDS [72, 73].
Despite advances in understanding the pathophysiology and development of therapeutic interventions, ARDS remains a common and lethal condition. In a study of nearly 30,000 patients from 50 countries, 23% of mechanically ventilated patients and 10% of patients admitted to an ICU had ARDS [74]. Mortality ranged from 35% in patients with mild ARDS to 46% in the subgroup with severe ARDS. Since this study, the prevalence of ARDS has dramatically increased due to the COVID-19 pandemic. Mortality from COVID-19-related ARDS ranges widely (12–78%), and limited comparative data suggests similar outcomes between COVID-19-related ARDS and non-COVID-ARDS [75, 76].
The main therapeutic pillars of ARDS consist of treatment of the underlying cause, lung protective ventilation (LPV), PEEP titration, neuromuscular blockade (NMB), prone positioning (PP), conservative fluid management, corticosteroids, and extracorporeal membrane oxygenation (ECMO). A detailed overview of physiological benefits and evidence for these treatments is provided in Table 1. Within the broad consensus definition of ARDS, there is substantial heterogeneity among the population, and further investigation of ARDS subphenotypes may allow for more nuanced, tailored treatment strategies in the future [77].
Management of brain-lung conflicts in concurrent SABI and ARDS
An overview of potential conflicts arising in SABI with various ARDS management principles and strategies to balance brain and lung pathology is provided in Fig. 2. Figure 3 delineates an algorithm with specific considerations for ARDS with SABI. Key considerations in the management of ARDS and SABI based on the available evidence are highlighted below.
Conflicts of ARDS management principles in SABI. Red boxes: potential conflicts, green boxes: management strategies. AC anticoagulation, ARDS acute respiratory distress syndrome, CBF cerebral blood flow, CPP cerebral perfusion pressure, ICP intracranial pressure, ITP intrathoracic pressure, MAP mean arterial pressure, NMB neuromuscular blockade, PaO2 partial pressure of oxygen, PaCO2 partial pressure of carbon dioxide, PbtO2 brain tissue oxygen, pECLA pumpless extracorporeal lung assist, PEEP positive end-expiratory pressure, RAP right atrial pressure, TBI traumatic brain injury.
Treatment algorithm for concomitant ARDS and SABI. ARDS acute respiratory distress syndrome, AED antiepileptic drug, cEEG continuous electroencephalogram, CPP cerebral perfusion pressure, CT computed tomography, CVC central venous catheter, ECMO extracorporeal membrane oxygenation, GCS Glasgow Coma Scale, HD hemodialysis, ICP intracranial pressure, MAP mean arterial pressure, NCSE non-convulsive status epilepticus, NCSz non-convulsive seizures, PaO2 partial pressure of oxygen, PaCO2 partial pressure of carbon dioxide, PbtO2 brain tissue oxygen, PEEP positive end-expiratory pressure, RM recruitment maneuver, SABI severe acute brain injury, TBI traumatic brain injury, TCD transcranial Doppler, SE status epilepticus.
Lung protective ventilation
LPV, defined as low tidal volume ventilation coupled with PEEP optimization and minimization of barotrauma, is the standard of care for patients with ARDS. The landmark ARMA trial was stopped early after demonstrating a 9% decrease in mortality and fewer days of MV with Vt of 4–6 cc/kg of predicted body weight (PBW) compared to 12 cc/kg PBW [26•]. Further evidence to support the use of LPV in ARDS has emerged since, and studies have suggested that LPV may reduce progression to acute lung injury (ALI) or ARDS in ventilated patients without ARDS [78–80]. Moreover, studies have identified high Vt as a predictor of ARDS in various subtypes of SABI [10, 81–83].
While permissive hypercapnia with LPV is commonly tolerated in patients with ARDS and no known brain injury, a rising PaCO2 can result in elevated ICPs, and poor neurological outcomes in SABI [64•, 65]. Also, the widely adapted PaO2 goal target of > 55 mmHg in ARDS based on the ARMA study protocol may be insufficient in SABI and exacerbate secondary brain injury.
Overall, LPV should be utilized in concurrent SABI and ARDS, with vigilant monitoring of PaCO2 and higher PaO2 goals of 80–120 mmHg. The balance between lung protection and PaCO2 control should be determined based on ARDS severity, lung compliance, and concern for worsening brain edema and herniation. If hypercarbia is unavoidable in patients at high risk for neurological decompensation due to elevated ICP, direct measures of brain physiology should be considered. Invasive ICP and PbtO2 monitoring, and cerebral autoregulation with CO2 reactivity studies can be valuable in determining if higher PaCO2 values are tolerated from a cerebral pressure and localized perfusion standpoint.
High positive end-expiratory pressure
PEEP is often utilized in patients with severe ARDS to optimize lung recruitment, thereby improving oxygenation and maximizing compliance. PEEP is adjusted based on various measures, including empirical titration tables, pressure–volume loops, esophageal manometry to estimate transpulmonary pressure, and optimization of driving pressure [84–86]. Several RCTs have compared high versus low PEEP strategies in ARDS. No individual trial demonstrated improved mortality with a higher PEEP strategy, but some did note improved oxygenation [87–89]. A subsequent meta-analysis demonstrated possible benefit in moderate-severe ARDS with higher PEEP [90]. Lower driving pressure, defined as the difference between the static pressure and PEEP in a volume-controlled ventilator mode, is associated with reduced mortality [91].
The use of high PEEP is controversial in SABI. PEEP increases intrathoracic and right atrial pressures, and may subsequently cause elevated ICPs by impeding cerebral venous drainage. Higher PEEP may also decrease cerebral perfusion pressure (CPP) in patients with impaired cerebral autoregulation.
Studies in SABI show mixed results regarding the effect of PEEP on ICP [92, 93, 94•, 95–97]. Small studies in TBI, SAH, ICH, and AIS did not observe a significant effect on CPP when increasing PEEP up to 15 cmH2O [93, 95, 98]. Other studies suggested that a principal mechanism resulting in ICP elevations and CPP reductions appeared to be a PEEP-dependent decrease in mean arterial pressure (MAP) [96, 97], with ICP and CPP improving once MAP was restored. High PEEP was more likely to affect CPP when cerebral autoregulation was impaired [92]. One study suggested that intrathoracic pressure augmentation would only impact ICP when PEEP values exceeded ICP [98]. A small prospective study of patients with SABI and ALI found substantial differences in the effect of PEEP on ICP depending on whether increasing PEEP resulted in alveolar recruitment or hyperinflation based on static volume-pressure curves, with only the latter group showing a rise in PaCO2 and ICP [99]. Recent studies have also shown an association between decreased respiratory compliance and PEEP-mediated ICP elevations [94•, 100].
Based on these findings, the use of increased PEEP to treat ARDS is reasonable and likely safe in most patients with SABI [42•]. PEEP titration should be deliberate based on lung compliance, and MAP should be strictly maintained. Non-invasive methods such as transcranial Doppler (TCD), pupillometry, and optic nerve sheath diameter (ONSD) may be helpful while titrating PEEP or during recruitment maneuvers, and should be considered in patients at risk for elevated ICP or impaired autoregulation. Simultaneous use of lung and brain ultrasound and a lung ultrasound score to guide PEEP titration in SABI have been described [94•, 101].
Prone positioning
Since the PROSEVA trial demonstrated a 17% absolute mortality reduction with ≥ 16 h/day of PP in patients with P/F ratio < 150 [27•], this therapy has become standard of care in moderate and severe ARDS and has been widely utilized during the COVID-19 pandemic [102].
While considered one of the most effective interventions in the management of moderate-severe ARDS, the impact of PP on ICP, CPP, and neurological outcomes in SABI is not fully understood, and PROSEVA excluded patients with ICP > 30 mmHg. PP in SABI also raises numerous logistical concerns (Table 2): worsening of concomitant cervical spine instability in TBI, positioning in patients with cranial bone flaps, accidental displacement of invasive brain monitors, and inadequate cerebral spinal fluid (CSF) drainage from external ventricular drains (EVD).
PP can result in ICP elevations due to decreased head elevation, increased abdominal pressure, and compression of neck veins affecting cerebral venous drainage. Most studies investigating the effect of PP on ICPs are small with fewer than 30 patients, included mixed types of brain injuries, did not assess long-term neurological outcome, and used varying degrees of head elevation [103–105]. Also, duration of PP in these studies was shorter (1–8 h), a criticism of negative PP trials before PROSEVA. Many of these studies demonstrated statistically significant transient elevations in ICP by 5–15 mmHg, with mixed effects on CPP, and overall substantial improvement of PaO2 and PbtO2 [106, 107]. The clinical relevance of these findings is not clear. The largest study including 111 patients showed a significant increase in ICP, and decrease in CPP when ICP exceeded 20 mmHg, the mean PaO2/FiO2 ratio improved from 135 to 340 [107]. The only prospective RCT included 51 patients but excluded those with ICP > 20 and PaO2/FiO2 ratio < 150, limiting the applicability to patients with elevated ICPs and severe ARDS. PP for 4 h daily resulted in significantly improved hypoxemia and initially elevated ICPs that gradually down-trended over 4 h [108•]. Overall, in most studies, the benefit on oxygenation and hemodynamics appeared to outweigh transient rises in ICP.
Given the substantial mortality benefit and potential impact on cerebral oxygen delivery, PP should be utilized in patients with SABI and moderate-severe ARDS. In those with concern for elevated ICP, invasive neuromonitoring is particularly important in the absence of the ability to follow a neurological examination. Elevating the head, positioning to minimize neck compression, aggressive bowel regimens and padding to decrease abdominal pressure, prophylactic and therapeutic ICP treatment with hyperosmolar therapy (HT) or CSF diversion, and MAP elevations with vasopressors are strategies to optimize CPP. The prone angle can also be modified to facilitate serial neurological assessments.
Sedation and neuromuscular blockade
Patients with ARDS often require deep sedation and NMB to facilitate ventilator synchrony, especially in patients requiring LPV. Both sedation and NMB are also thought to reduce global oxygen consumption. The ACURASYS trial demonstrated a mortality benefit for continuous infusion of NMB in moderate-severe ARDS [28], but the subsequent ROSE trial found no such benefit [29]. Importantly, no significant harm was demonstrated in either trial.
In patients with SABI, sedatives are used as a strategy to treat increased ICP by decreasing cerebral metabolism and oxygen consumption. NMB can also be used to optimize ICP and CPP, typically in cases when ventilator dyssynchrony is felt to worsen ICPs. However, the ability to perform serial neurological assessments becomes impaired. In addition, side effects of sedative medications, such as hypotension and reduced cardiac output, may affect CPP. Deep prolonged sedation may also compound neurocognitive sequelae and result in protracted recovery in critically ill patients.
In general, short-sacting agents such as propofol are preferred to allow for intermittent neurological assessments and reduce the risk of delirium [109, 110]. The need to utilize sedation for ICP control has to be considered and reassessed over time. When the neurological exam is limited by deep sedation, additional neuromonitoring such as pupillometry, quantitative electroencephalography (EEG), invasive and non-invasive ICP monitoring, and serial imaging should be considered in patients at risk for acute neurological deterioration.
Inhaled pulmonary vasodilators
Inhaled pulmonary vasodilators (IPV), typically inhaled prostacyclins or nitric oxide (NO), have been shown to improve oxygenation and reduce pulmonary arterial pressures, but have not demonstrated a survival benefit in ARDS [111–113]. As a result, they are not routinely recommended in ARDS, but rather used selectively as a bridge to other treatments in truly refractory hypoxemia, or in specific populations. Potential benefits include improvement of right ventricular dysfunction, acute or chronic pulmonary hypertension, and right-to-left shunting, and adverse effects include worsening renal failure or inhibition of platelet function. Pre-clinical studies have implicated impaired NO metabolism in the pathogenesis of various SABI subtypes and suggested both deleterious and neuroprotective effects of inhaled NO [114]. While thought to potentially improve cerebral blood flow and oxygenation, a better understanding of NO pathways in the brain is needed to establish benefits or harms in different SABI subtypes [115–118]. In patients with ICH at increased risk of bleeding, close monitoring for hematoma expansion could be considered due to the theoretical risk of platelet inhibition.
Extracorporeal membrane oxygenation
ECMO provides circulatory support and gas exchange for patients experiencing profound cardiopulmonary failure. Venovenous (VV)-ECMO can be an effective rescue therapy in patients with severe ARDS refractory to conventional therapies. The CESAR trial demonstrated lower mortality and improved outcomes in patients with ARDS who were transferred to an ECMO Center [30]; the subsequent EOLIA trial showed a trend towards improved outcome with VV-ECMO that was not statistically significant [31], though a high probability for benefit was indicated in subsequent Bayesian analysis [119]. The utilization of ECMO has increased substantially over the past two decades, and has increased even further due to the COVID-19 pandemic [120].
Historically, SABI has been considered a relative contraindication for ECMO. Concerns include the risk of hematoma expansion or hemorrhagic conversion with therapeutic anticoagulation, decreased cerebral venous return with large venous cannulas placed in the internal jugular vein, and extreme fluctuations in PaCO2 which can result in ICH [121]. However, major recent technological advances have allowed for increased ECMO utilization in SABI. Heparin-bonded circuits and polymethylpentene oxygenators have allowed for extended VV-ECMO support with low-dose or no systemic anticoagulation without premature oxygenator failure or excessive thrombotic complications [122, 123]. Femoral access for cannulation avoids concerns regarding impaired cerebral venous drainage. Initial low sweep gas rates and titration can avoid overly rapid PaCO2 correction. Overall, ECMO is feasible in highly selected patients with SABI, and may be considered on a case-by-case basis, with anticipated neurological prognosis weighing heavily in patient selection.
Fluid and hemodynamic management
The FACTT trial and a large meta-analysis demonstrated a decrease in MV duration, ICU length of stay, and improved gas exchange, but no change in mortality with a conservative fluid management strategy in ARDS [124, 125]. While initial volume resuscitation may be indicated in patients with hypovolemic or septic shock, hypervolemia is strictly avoided in ARDS to minimize alveolar capillary hydrostatic pressure and pulmonary edema.
However, hypotension and hypovolemia may result in decreased CPP and precipitate or exacerbate brain injury. Concerns about adequate cerebral perfusion are particularly high in patients with symptomatic vasospasm, acute cerebrovascular occlusions or high-grade stenoses, or impaired cerebral autoregulation.
Hypotension is associated with higher mortality and worse outcomes in all SABI subtypes [126–132]. While there is limited evidence for induced hypertension, higher BP targets and even BP augmentation may be considered in scenarios with high concern for cerebral perfusion. There is limited evidence to guide volume management in SABI. Low fluid balance in the first days is associated with worse neurological outcomes in TBI [133, 134]. Fluid restriction has shown to result in higher risk of cerebral infarction in SAH [135] and may cause watershed infarcts in patients with preexisting cervical vascular stenoses. However, fluid balance does not always reflect intravascular volume status. Hypervolemia can augment cerebral edema in patients with blood–brain barrier (BBB) disruption, exacerbate heart failure, cardiogenic shock, and pulmonary edema, and is also associated with high ICU mortality and worse outcomes in SABI [133, 136, 137]. Guidelines recommend targeting intravascular euvolemia and avoiding a restrictive or negative fluid balance in SABI [138].
The use of HT to treat elevated ICP may have differential effects on intravascular volume status depending on the type of HT used and the patient’s organ function. Mannitol causes an osmotic diuresis and may lead to decreased preload and cardiac output, and also precipitate or exacerbate acute kidney injury. Hypertonic saline may contribute to hypervolemia and pulmonary edema.
For patients with concurrent ARDS and SABI, a tailored fluid strategy utilizing serial multimodal volume status assessments is critical in guiding the optimal strategy for the individual patient. For most patients, targeting normotension and intravascular euvolemia is appropriate. Hypotension and intravascular hypovolemia should be strictly avoided in patients with impaired cerebral perfusion, and the use of diuretics may have to be limited during critical time windows. Measures of cerebral perfusion, such as TCD or CT-angiogram (CT-A) and CT-perfusion (CT-P), can help guide BP and volume targets.
Corticosteroids
Following decades of inconclusive and negative clinical trials [139–141], several recent RCTs have demonstrated benefits of corticosteroids in ARDS. The DEXA-ARDS study showed lower mortality and reduced duration of MV in patients with moderate-severe ARDS who received a 10-day course of corticosteroids after 24 h of disease onset [142]. Delayed corticosteroids within ≥ 7 days of onset have not shown a benefit, with higher mortality at ≥ 14 days [141]. Several studies from the COVID-19 era have suggested benefits of corticosteroids; the RECOVERY trial demonstrated a mortality reduction by one-third in ventilated patients with COVID-19 [143], and a meta-analysis of 7 RCTs also showed a significant mortality reduction [144•]. Treatment with corticosteroids has since become commonplace in ARDS. However, distinct ARDS subgroups may have varying responses to corticosteroids based on hypo- or hyperinflammatory phenotypes or the presence of infectious organisms, and future research is needed to establish benefits and harmful effects and effects of dosing in different sub-populations.
Corticosteroids can reduce cerebral vasogenic edema by decreasing BBB permeability [145], with benefits noted in the treatment of brain tumors [146] and subtypes of meningitis [147]. However, corticosteroids have not proven to be effective in SABI and might be harmful. Specifically, the Brain Trauma Foundation (BTF) guidelines state that corticosteroids are not recommended in TBI for ICP control, and high-dose steroids are contraindicated (level 1 recommendation) [148]. After several inconclusive RCTs [149–152], a large multicenter RCT (MRC CRASH) stopped early after showing higher mortality at 2 weeks [153] and 6 months [154•] with a 48-h high-dose methylprednisolone infusion. Disability at 6 months did not differ, and reasons for the increased mortality remain unclear with no substantial difference in infections or gastrointestinal bleeding. Studies in AIS [155], SAH [156, 157], and ICH [158] have not demonstrated a significant improvement in survival or neurological outcome but have suggested adverse systemic effects. In cardiac arrest, some studies have suggested an association with increased return of spontaneous circulation when used in combination with vasopressin during cardiopulmonary resuscitation, with no benefit on long-term neurological outcomes [159, 160]. Two systematic reviews have not found a benefit with corticosteroids alone [161, 162].
With insufficient evidence to guide management in concurrent ARDS and SABI, decisions may be guided by ARDS severity, ARDS and SABI etiologies, underlying infectious etiologies, potential for iatrogenic harm (e.g., risk for hyperglycemia, infection, and myopathy), and time from ARDS onset. Steroids should generally be avoided when TBI is the dominant clinical problem. Given the mortality benefit in ARDS, they should be considered in other SABI subtypes and may be beneficial in some SAH and post-cardiac arrest populations. More data is needed to establish the overall benefit or harm in various ARDS and SABI subtypes.
Specific considerations in SABI subtypes
The different subtypes of SABI have very distinct underlying pathophysiology and clinical trajectories. Specific concerns related to increased ICP, impaired cerebral perfusion, and critical time windows are summarized in Table 1. Key neuroprotective strategies to minimize secondary brain injury in all SABI subtypes encompass maintaining physiological homeostasis; avoiding derangements in temperature, oxygenation, and ventilation; optimizing cerebral perfusions; averting detrimental ICP elevations and decreased CPP; and early recognition and treatment of seizures and status epilepticus.
In patients with TBI, the BTF has developed evidence-based guidelines, and a multitiered algorithm has been adopted to minimize secondary injury [148]. While there is large heterogeneity within TBI, these principles should be applied to patients with concomitant ARDS. In patients with clinical concerns for elevated ICP, invasive multimodal monitoring should be strongly considered, and target ICP < 20 mmHg, CPP 60–70 mmHg, PaCO2 35–40 mmHg, and PbtO2 > 20 mmHg.
The main challenge in concurrent SAH and ARDS is the prevention of DCI while minimizing volume overload. While DCI is thought to be multifactorial, the only known potentially reversible etiology is vasospasm. Radiographic vasospasm occurs in 70% of patients with SAH, and 30% of patients with radiographic vasospasm develop cerebral ischemia [163, 164]. Also, a subset of patients with SAH develop cerebral salt wasting syndrome and natriuresis, which can lead to acute intravascular hypovolemia. The currently recommended management approach during the vasospasm period (3–21 days, peak day 7–10, < 5% after day 14) is to maintain intravascular euvolemia and a combination of permissive and induced hypertension. Hypovolemia and fluid restriction have been associated with increased risk of cerebral infarction and poor outcomes [165]. However, induced hypervolemia and hemodilution have not demonstrated an outcome benefit and are associated with worsening pulmonary edema [44, 166–168]. In addition, up to 30% of patients with SAH can develop neurogenic heart failure [163]. Daily multimodal volume status assessments are recommended; while there is clinical concern for DCI, strict intravascular euvolemia should be targeted and diuresis should be minimized. TCD, CT-A, and CT-P can help stratify and reassess the risk for vasospasm.
A crucial part of sICH management is serial monitoring of the neurological exam and hemodynamic status to ensure early recognition and treatment of ICH expansion, hydrocephalus, and herniation [169]. If deep sedation and NMB are required for ARDS management during a critical time period, serial imaging or invasive ICP monitoring should be considered in patients with high clinical concerns for neurological deterioration. With regard to ventilatory and hemodynamic targets, a large retrospective cohort in mechanically ventilated patients with ICH identified high Vt as the strongest risk factor for ARDS development and in-hospital mortality, other modifiable risk factors included high fluid balance, hypoxemia, and transfusions [10].
The management of AIS revolves around ensuring adequate cerebral perfusion and salvaging the ischemic penumbra. The degree of concern is based on the presence and chronicity of occlusions and stenoses, revascularization and collateral status, and concern for reperfusion injury and hemorrhagic conversion [13]. BP and volume targets need to be determined based on these factors and weighed against ARDS severity as well as presence of cardiopulmonary disease as a common stroke risk factor. Concerns about ICPs crises and herniation are high in patients with large middle cerebral artery infarctions, or cerebellar infarcts resulting in mass effect on the brainstem or obliteration of the fourth ventricle, and close neurological monitoring is warranted in these patients.
The post-cardiac arrest syndrome is characterized by a systemic inflammatory state, and there is substantial overlap in pathophysiological mechanisms encountered in ARDS. Interventions that have shown to impact outcomes include temperature management, hemodynamic optimization (MAP > 65 mmHg), adequate oxygenation and ventilation with PaO2 and PCO2 goals in a physiological range, and appropriate neuroprognostication [13, 170–172]. Studies have suggested decreased occurrence of ARDS, more ventilator-free days, and improved neurological outcomes with LPV after cardiac arrest [173]. Seizures are common after cardiac arrest [174], and may be another potentially modifiable target in minimizing secondary brain injury. Cerebral edema and elevated ICP have been described in up to 22% [175], and are associated with worse outcomes [176]. The use of invasive ICP monitoring is controversial and less established in this population and its utility remains under investigation.
Conclusions
ARDS is common and associated with higher mortality and worse neurological outcomes in SABI. ARDS and SABI management strategies may conflict. High PEEP, permissive hypercapnia due to LPV, and prone ventilation may result in increased ICP and decreased CPP. Measures to enhance cerebral perfusion, including volume resuscitation, BP augmentation, and HT, can interfere with the conservative fluid management strategy recommended in ARDS. More research is needed to determine risks and benefits in corticosteroids, especially in light of conflicting data about mortality in ARDS and TBI.
The care of patients with SABI and ARDS requires meticulous attention to oxygenation and ventilation, hemodynamics and volume status, temperature management, and the neurological exam. In general, LPV and PP should be utilized, and PEEP titration should be deliberate based on measures of lung and brain physiology. Intravascular euvolemia and normotension should be targeted in SABI, particularly for patients at risk for brain ischemia due to impaired cerebral perfusion. In patients with high concern for increased ICP and insufficient cerebral perfusion, multimodal monitoring and serial hemodynamic assessments can help determine individualized targets to support the acutely injured brain 177.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Aisiku IP, Yamal JM, Doshi P, et al. The incidence of ARDS and associated mortality in severe TBI using the Berlin definition. J Trauma Acute Care Surg. 2016;80(2):308–12. https://doi.org/10.1097/TA.0000000000000903.
Holland MC, Mackersie RC, Morabito D, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003;55(1):106–11. https://doi.org/10.1097/01.TA.0000071620.27375.BE.
Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. 2001;95(4):560–8. https://doi.org/10.3171/jns.2001.95.4.0560.
Bratton SL, Davis RL. Acute lung injury in isolated traumatic brain injury. Neurosurgery. 1997;40(4):707–712; discussion 712. https://doi.org/10.1097/00006123-199704000-00009.
Rincon F, Ghosh S, Dey S, et al. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery. 2012;71(4):795–803. https://doi.org/10.1227/NEU.0b013e3182672ae5.
Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006;34(1):196–202. https://doi.org/10.1097/01.ccm.0000194540.44020.8e.
Veeravagu A, Chen YR, Ludwig C, et al. Acute lung injury in patients with subarachnoid hemorrhage: a nationwide inpatient sample study. World Neurosurg. 2014;82(1–2):e235-241. https://doi.org/10.1016/j.wneu.2014.02.030.
Solenski NJ, Haley EC, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995;23(6):1007–1017. https://doi.org/10.1097/00003246-199506000-00004.
Gruber A, Reinprecht A, Illievich UM, et al. Extracerebral organ dysfunction and neurologic outcome after aneurysmal subarachnoid hemorrhage. Crit Care Med. 1999;27(3):505–14. https://doi.org/10.1097/00003246-199903000-00026.
Elmer J, Hou P, Wilcox SR, et al. Acute respiratory distress syndrome after spontaneous intracerebral hemorrhage*. Crit Care Med. 2013;41(8):1992–2001. https://doi.org/10.1097/CCM.0b013e31828a3f4d.
Rincon F, Maltenfort M, Dey S, et al. The prevalence and impact of mortality of the acute respiratory distress syndrome on admissions of patients with ischemic stroke in the United States. J Intensive Care Med. 2014;29(6):357–64. https://doi.org/10.1177/0885066613491919.
Zhao JN, Liu Y, Li HC. Aspiration-related acute respiratory distress syndrome in acute stroke patient. Plos One. 2015;10(3):e0118682. https://doi.org/10.1371/journal.pone.0118682.
Johnson NJ, Caldwell E, Carlbom DJ, et al. The acute respiratory distress syndrome after out-of-hospital cardiac arrest: incidence, risk factors, and outcomes. Resuscitation. 2019;135:37–44. https://doi.org/10.1016/j.resuscitation.2019.01.009.
Kim JS, Kim YJ, Kim M, et al. The impact of severity of acute respiratory distress syndrome following cardiac arrest on neurologic outcomes. Ther Hypothermia Temp Manag. 2021;11(2):96–102. https://doi.org/10.1089/ther.2019.0047.
Chou SHY, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. 2021;4(5): e2112131. https://doi.org/10.1001/jamanetworkopen.2021.12131.
Siegler JE, Cardona P, Arenillas JF, et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 Multinational Registry. Int J Stroke. 2021;16(4):437–47. https://doi.org/10.1177/1747493020959216.
Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19. Stroke. 2021;52(3):905–12. https://doi.org/10.1161/STROKEAHA.120.031786.
Leasure AC, Khan YM, Iyer R, et al. Intracerebral hemorrhage in patients with COVID-19. Stroke. 2021;52(7):e321–3. https://doi.org/10.1161/STROKEAHA.121.034215.
Tejerina E, Pelosi P, Muriel A, et al. Association between ventilatory settings and development of acute respiratory distress syndrome in mechanically ventilated patients due to brain injury. J Crit Care. 2017;38:341–5. https://doi.org/10.1016/j.jcrc.2016.11.010.
Mrozek S, Constantin JM, Geeraerts T. Brain-lung crosstalk: implications for neurocritical care patients. World J Crit Care Med. 2015;4(3):163–78. https://doi.org/10.5492/wjccm.v4.i3.163.
Ziaka M, Exadaktylos A. Brain–lung interactions and mechanical ventilation in patients with isolated brain injury. Crit Care. 2021;25(1):358. https://doi.org/10.1186/s13054-021-03778-0.
Huang M, Gedansky A, Hassett CE, et al. Pathophysiology of brain injury and neurological outcome in acute respiratory distress syndrome: a scoping review of preclinical to clinical studies. Neurocrit Care. 2021;35(2):518–27. https://doi.org/10.1007/s12028-021-01309-x.
Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327(6):559–65. https://doi.org/10.1001/jama.2022.0040.
Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352. https://doi.org/10.1186/s13054-019-2626-z.
Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42(5):725–38. https://doi.org/10.1007/s00134-016-4321-8.
• Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome | NEJM. https://doi.org/10.1056/nejm200005043421801. (Accessed 18 Feb 2022). Ventilation with lower tidal volumes has been shown to decrease mortality and duration if mechanical ventilation in ARDS.
• Prone positioning in severe acute respiratory distress syndrome | NEJM. https://doi.org/10.1056/nejmoa1214103. (Accessed 18 Feb 2022). Prone ventilation for 16 h/day has been shown to decrease mortality in moderate to severe ARDS.
Neuromuscular blockers in early acute respiratory distress syndrome | NEJM. https://doi.org/10.1056/nejmoa1005372. (Accessed 18 Feb 2022).
Early neuromuscular blockade in the acute respiratory distress syndrome | NEJM. https://doi.org/10.1056/nejmoa1901686. (Accessed 18 Feb 2022).
Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–63. https://doi.org/10.1016/S0140-6736(09)61069-2.
Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–75. https://doi.org/10.1056/NEJMoa1800385.
Rowat AM, Dennis MS, Wardlaw JM. Hypoxaemia in acute stroke is frequent and worsens outcome. Cerebrovasc Dis. 2006;21(3):166–72. https://doi.org/10.1159/000090528.
McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):287–93. https://doi.org/10.1089/neu.2006.0031.
Davis DP, Meade W, Sise MJ, et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26(12):2217–23. https://doi.org/10.1089/neu.2009.0940.
Alali AS, Temkin N, Vavilala MS, et al. Matching early arterial oxygenation to long-term outcome in severe traumatic brain injury: target values. J Neurosurg. 2019;132(2):537–44. https://doi.org/10.3171/2018.10.JNS18964.
Moroney JT, Bagiella E, Desmond DW, Paik MC, Stern Y, Tatemichi TK. Cerebral hypoxia and ischemia in the pathogenesis of dementia after stroke. Ann N Y Acad Sci. 1997;826:433–6. https://doi.org/10.1111/j.1749-6632.1997.tb48498.x.
Helmerhorst HJF, Schultz MJ, van der Voort PHJ, de Jonge E, van Westerloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19:284. https://doi.org/10.1186/s13054-015-0996-4.
Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–71. https://doi.org/10.1001/jama.2010.707.
Conservative oxygen therapy during mechanical ventilation in the ICU | NEJM. https://doi.org/10.1056/NEJMoa1903297. (Accessed 18 Feb 2022).
Lower or higher oxygenation targets for acute hypoxemic respiratory failure | NEJM. https://doi.org/10.1056/NEJMoa2032510. (Accessed 18 Feb 2022).
Liberal or conservative oxygen therapy for acute respiratory distress syndrome | NEJM. https://doi.org/10.1056/NEJMoa1916431. (Accessed 18 Feb 2022).
• Robba C, Poole D, McNett M, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med. 2020;46(12):2397–2410. https://doi.org/10.1007/s00134-020-06283-0. The optimal oxygenation and ventilation targets and ventilator parameters in patients with acute brain injury are unknown, with limited evidence to guide clinical decisions. An expert panel reviewed the relevant existing literature, identified the need for future research, and provided recommendations based on the available evidence.
Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13(3):R89. https://doi.org/10.1186/cc7916.
Kumar MA, Levine J, Faerber J, et al. The effects of red blood cell transfusion on functional outcome after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2017;108:807–16. https://doi.org/10.1016/j.wneu.2017.09.038.
Leal-Noval SR, Múñoz-Gómez M, Murillo-Cabezas F. Optimal hemoglobin concentration in patients with subarachnoid hemorrhage, acute ischemic stroke and traumatic brain injury. Curr Opin Crit Care. 2008;14(2):156–62. https://doi.org/10.1097/MCC.0b013e3282f57577.
Ayling OGS, Ibrahim GM, Alotaibi NM, Gooderham PA, Macdonald RL. Anemia after aneurysmal subarachnoid hemorrhage is associated with poor outcome and death. Stroke. 2018;49(8):1859–65. https://doi.org/10.1161/STROKEAHA.117.020260.
Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. https://doi.org/10.1056/NEJM199902113400601.
Carless PA, Henry DA, Carson JL, Hebert PP, McClelland B, Ker K. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2010;(10):CD002042. https://doi.org/10.1002/14651858.CD002042.pub2.
Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36(9):2667–74. https://doi.org/10.1097/CCM.0b013e3181844677.
Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312(1):36–47. https://doi.org/10.1001/jama.2014.6490.
Diringer MN, Bleck TP, Claude Hemphill J, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211–40. https://doi.org/10.1007/s12028-011-9605-9.
English SW, Fergusson D, Chassé M, et al. Aneurysmal subarachnoid hemorrhage—red blood cell transfusion and outcome (SAHaRA): a pilot randomised controlled trial protocol. BMJ Open. 2016;6(12): e012623. https://doi.org/10.1136/bmjopen-2016-012623.
Maloney-Wilensky E, Gracias V, Itkin A, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med. 2009;37(6):2057–63. https://doi.org/10.1097/CCM.0b013e3181a009f8.
Xie Q, Wu HB, Yan YF, Liu M, Wang ES. Mortality and outcome comparison between brain tissue oxygen combined with intracranial pressure/cerebral perfusion pressure-guided therapy and intracranial pressure/cerebral perfusion pressure-guided therapy in traumatic brain injury: a meta-analysis. World Neurosurg. 2017;100:118–27. https://doi.org/10.1016/j.wneu.2016.12.097.
Narotam PK, Morrison JF, Nathoo N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg. 2009;111(4):672–82. https://doi.org/10.3171/2009.4.JNS081150.
Gouvea Bogossian E, Diaferia D, Ndieugnou Djangang N, et al. Brain tissue oxygenation guided therapy and outcome in non-traumatic subarachnoid hemorrhage. Sci Rep. 2021;11(1):16235. https://doi.org/10.1038/s41598-021-95602-6.
Lubillo ST, Parrilla DM, Blanco J, et al. Prognostic value of changes in brain tissue oxygen pressure before and after decompressive craniectomy following severe traumatic brain injury. J Neurosurg. 2018;128(5):1538–46. https://doi.org/10.3171/2017.1.JNS161840.
Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg. 2010;113(3):571–80. https://doi.org/10.3171/2010.1.JNS09506.
BOOST-3 | SIREN. https://siren.network/clinical-trials/boost-3. (Accessed 18 Feb 2022).
Frontiers | pCO2 and pH regulation of cerebral blood flow | Physiology. https://doi.org/10.3389/fphys.2012.00365/full. (Accessed 18 Feb 2022).
Diringer MN, Videen TO, Yundt K, et al. Regional cerebrovascular and metabolic effects of hyperventilation after severe traumatic brain injury. J Neurosurg. 2002;96(1):103–8. https://doi.org/10.3171/jns.2002.96.1.0103.
Coles JP, Fryer TD, Coleman MR, et al. Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35(2):568–78. https://doi.org/10.1097/01.CCM.0000254066.37187.88.
Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75(5):731–9. https://doi.org/10.3171/jns.1991.75.5.0731.
• Roberts BW, Karagiannis P, Coletta M, Kilgannon JH, Chansky ME, Trzeciak S. Effects of PaCO2 derangements on clinical outcomes after cerebral injury: a systematic review. Resuscitation. 2015;91:32–41. https://doi.org/10.1016/j.resuscitation.2015.03.015. Both hyper- and hypocarbia have been associated with poor neurological outcomes and increased mortality in brain injured patients.
Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127(21):2107–13. https://doi.org/10.1161/CIRCULATIONAHA.112.000168.
Davis DP, Idris AH, Sise MJ, et al. Early ventilation and outcome in patients with moderate to severe traumatic brain injury. Crit Care Med. 2006;34(4):1202–8. https://doi.org/10.1097/01.CCM.0000208359.74623.1C.
Eastwood GM, Schneider AG, Suzuki S, et al. Targeted therapeutic mild hypercapnia after cardiac arrest: a phase II multi-centre randomised controlled trial (the CCC trial). Resuscitation. 2016;104:83–90. https://doi.org/10.1016/j.resuscitation.2016.03.023.
Jakkula P, Reinikainen M, Hästbacka J, et al. Targeting two different levels of both arterial carbon dioxide and arterial oxygen after cardiac arrest and resuscitation: a randomised pilot trial. Intensive Care Med. 2018;44(12):2112–21. https://doi.org/10.1007/s00134-018-5453-9.
Abnormal breathing patterns associated with acute brain damage | JAMA Neurology | JAMA Network. https://jamanetwork.com/journals/jamaneurology/article-abstract/573367. (Accessed 18 Feb 2022).
Pham T, Rubenfeld GD. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Care Med. 2017;195(7):860–870. https://doi.org/10.1164/rccm.201609-1773CP.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. https://doi.org/10.1001/jama.2012.5669.
Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–72. https://doi.org/10.1056/NEJMra1608077.
Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–82. https://doi.org/10.1007/s00134-012-2682-1.
Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. https://doi.org/10.1001/jama.2016.0291.
Sjoding MW, Admon AJ, Saha AK, et al. Comparing clinical features and outcomes in mechanically ventilated patients with COVID-19 and acute respiratory distress syndrome. Ann Am Thorac Soc. 2021;18(11):1876–85. https://doi.org/10.1513/AnnalsATS.202008-1076OC.
Dmytriw AA, Chibbar R, Chen PPY, et al. Outcomes of acute respiratory distress syndrome in COVID-19 patients compared to the general population: a systematic review and meta-analysis. Expert Rev Respir Med. 2021;15(10):1347–54. https://doi.org/10.1080/17476348.2021.1920927.
Sinha P, Delucchi KL, McAuley DF, O’Kane CM, Matthay MA, Calfee CS. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir Med. 2020;8(3):247–57. https://doi.org/10.1016/S2213-2600(19)30369-8.
Neto AS, Simonis FD, Barbas CSV, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med. 2015;43(10):2155–63. https://doi.org/10.1097/CCM.0000000000001189.
Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817–24. https://doi.org/10.1097/01.ccm.0000133019.52531.30.
Fuller BM, Mohr NM, Drewry AM, Carpenter CR. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome: a systematic review. Crit Care. 2013;17(1):R11. https://doi.org/10.1186/cc11936.
Mascia L, Zavala E, Bosma K, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35(8):1815–20. https://doi.org/10.1097/01.CCM.0000275269.77467.DF.
Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest - PubMed. https://pubmed.ncbi.nlm.nih.gov/28267376/. (Accessed 18 Feb 2022).
Asehnoune K, Mrozek S, Perrigault PF, et al. A multi-faceted strategy to reduce ventilation-associated mortality in brain-injured patients. The BI-VILI project: a nationwide quality improvement project. Intensive Care Med. 2017;43(7):957–970. https://doi.org/10.1007/s00134-017-4764-6.
Sahetya SK, Goligher EC, Brower RG. Fifty years of research in ARDS. Setting positive end-expiratory pressure in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(11):1429–1438. https://doi.org/10.1164/rccm.201610-2035CI.
Mechanical ventilation guided by esophageal pressure in acute lung injury | NEJM. https://doi.org/10.1056/nejmoa0708638. (Accessed 18 Feb 2022).
Schiller HJ, Steinberg J, Halter J, et al. Alveolar inflation during generation of a quasi-static pressure/volume curve in the acutely injured lung. Crit Care Med. 2003;31(4):1126–33. https://doi.org/10.1097/01.CCM.0000059997.90832.29.
Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–45. https://doi.org/10.1001/jama.299.6.637.
Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome | NEJM. https://doi.org/10.1056/nejmoa032193. (Accessed 18 Feb 2022).
Mercat A, Richard JCM, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–55. https://doi.org/10.1001/jama.299.6.646.
Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–73. https://doi.org/10.1001/jama.2010.218.
Amato MBP, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55. https://doi.org/10.1056/NEJMsa1410639.
Videtta W, Villarejo F, Cohen M, et al. Effects of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Acta Neurochir Suppl. 2002;81:93–7. https://doi.org/10.1007/978-3-7091-6738-0_25.
Caricato A, Conti G, Della Corte F, et al. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma. 2005;58(3):571–6. https://doi.org/10.1097/01.ta.0000152806.19198.db.
• Robba C, Ball L, Battaglini D, et al. Effects of positive end-expiratory pressure on lung ultrasound patterns and their correlation with intracranial pressure in mechanically ventilated brain injured patients. Crit Care. 2022;26:1 31. https://doi.org/10.1186/s13054-022-03903-7. PEEP tiitration up to 15 appears feasible and safe in patients with acute brain injury. Specific findings on lung ultrasound, the effect of PEEP on MAP, and respiratory compliance appear to impact the effect of PEEP on ICP.
Huynh T, Messer M, Sing RF, Miles W, Jacobs DG, Thomason MH. Positive end-expiratory pressure alters intracranial and cerebral perfusion pressure in severe traumatic brain injury. J Trauma. 2002;53(3):488–492; discussion 492–493. https://doi.org/10.1097/00005373-200209000-00016.
Georgiadis D, Schwarz S, Baumgartner RW, Veltkamp R, Schwab S. Influence of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke. 2001;32(9):2088–2092. https://doi.org/10.1161/hs0901.095406.
Muench E, Bauhuf C, Roth H, et al. Effects of positive end-expiratory pressure on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation. Crit Care Med. 2005;33(10):2367–2372. https://doi.org/10.1097/01.CCM.0000181732.37319.DF.
McGuire G, Crossley D, Richards J, Wong D. Effects of varying levels of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Crit Care Med. 1997;25(6):1059–62. https://doi.org/10.1097/00003246-199706000-00025.
Mascia L, Grasso S, Fiore T, Bruno F, Berardino M, Ducati A. Cerebro-pulmonary interactions during the application of low levels of positive end-expiratory pressure. Intensive Care Med. 2005;31(3):373–9. https://doi.org/10.1007/s00134-004-2491-2.
Robba C, Ball L, Nogas S, et al. Effects of positive end-expiratory pressure on lung recruitment, respiratory mechanics, and intracranial pressure in mechanically ventilated brain-injured patients. Front Physiol. 2021;12: 711273. https://doi.org/10.3389/fphys.2021.711273.
Corradi F, Robba C, Tavazzi G, Via G. Combined lung and brain ultrasonography for an individualized “brain-protective ventilation strategy” in neurocritical care patients with challenging ventilation needs. Crit Ultrasound J. 2018;10(1):24. https://doi.org/10.1186/s13089-018-0105-4.
Mathews KS, Soh H, Shaefi S, et al. Prone positioning and survival in mechanically ventilated patients with coronavirus disease 2019-related respiratory failure. Crit Care Med. 2021;49(7):1026–37. https://doi.org/10.1097/CCM.0000000000004938.
Reinprecht A, Greher M, Wolfsberger S, Dietrich W, Illievich UM, Gruber A. Prone position in subarachnoid hemorrhage patients with acute respiratory distress syndrome: effects on cerebral tissue oxygenation and intracranial pressure. Crit Care Med. 2003;31(6):1831–8. https://doi.org/10.1097/01.CCM.0000063453.93855.0A.
Nekludov M, Bellander BM, Mure M. Oxygenation and cerebral perfusion pressure improved in the prone position. Acta Anaesthesiol Scand. 2006;50(8):932–6. https://doi.org/10.1111/j.1399-6576.2006.01099.x.
Thelandersson A, Cider A, Nellgård B. Prone position in mechanically ventilated patients with reduced intracranial compliance. Acta Anaesthesiol Scand. 2006;50(8):937–41. https://doi.org/10.1111/j.1399-6576.2006.01037.x.
Wright JM, Gerges C, Shammassian B, et al. Prone position ventilation in neurologically ill patients: a systematic review and proposed protocol. Crit Care Med. 2021;49(3):e269–78. https://doi.org/10.1097/CCM.0000000000004820.
Roth C, Ferbert A, Deinsberger W, et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21(2):186–91. https://doi.org/10.1007/s12028-014-0004-x.
• Beuret P, Carton MJ, Nourdine K, Kaaki M, Tramoni G, Ducreux JC. Prone position as prevention of lung injury in comatose patients: a prospective, randomized, controlled study. Intensive Care Med. 2002;28(5):564 569. https://doi.org/10.1007/s00134-002-1266-x. Prone positioning in patients with acute brain injury can not only result in ICP elevations and decrease in CPP but also result in improved oxygenation. This suggests that prone positioning in acute brain injury is feasible and may be beneficial, but that close monitoring of ICP and CPP is warranted, especially when the ability to trend the neurological exam becomes limited.
Balas MC, Burke WJ, Gannon D, et al. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Crit Care Med. 2013;41(9 Suppl 1):S116-127. https://doi.org/10.1097/CCM.0b013e3182a17064.
Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47(1):3–14. https://doi.org/10.1097/CCM.0000000000003482.
Inhaled nitric oxide for the adult respiratory distress syndrome | NEJM. https://doi.org/10.1056/nejm199302113280605. (Accessed 18 Feb 2022).
Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291(13):1603–9. https://doi.org/10.1001/jama.291.13.1603.
Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26(1):15–23. https://doi.org/10.1097/00003246-199801000-00011.
Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KTS. The role of the nitric oxide pathway in brain injury and its treatment—from bench to bedside. Exp Neurol. 2015;263:235–43. https://doi.org/10.1016/j.expneurol.2014.10.017.
Goodman M. Respiratory mechanics following brain injury: the role of inhaled nitric oxide. 2022. https://clinicaltrials.gov/ct2/show/NCT03260569. (Accessed 17 Feb 2022).
Patel J. Improving outcomes in cardiac arrest with inhaled nitric oxide. 2019. https://clinicaltrials.gov/ct2/show/NCT04134078. (Accessed 17 Feb 2022).
Siuta M, Zuckerman SL, Mocco J. Nitric oxide in cerebral vasospasm: theories, measurement, and treatment. Neurol Res Int. 2013;2013: 972417. https://doi.org/10.1155/2013/972417.
Terpolilli NA, Moskowitz MA, Plesnila N. Nitric oxide: considerations for the treatment of ischemic stroke. J Cereb Blood Flow Metab. 2012;32(7):1332–46. https://doi.org/10.1038/jcbfm.2012.12.
Goligher EC, Tomlinson G, Hajage D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA. 2018;320(21):2251–9. https://doi.org/10.1001/jama.2018.14276.
Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–8. https://doi.org/10.1016/S0140-6736(20)32008-0.
Cavayas YA, Munshi L, Del Sorbo L, Fan E. The early change in PaCO2 after extracorporeal membrane oxygenation initiation is associated with neurological complications. Am J Respir Crit Care Med. 2020;201(12):1525–35. https://doi.org/10.1164/rccm.202001-0023OC.
Fina D, Matteucci M, Jiritano F, et al. Extracorporeal membrane oxygenation without therapeutic anticoagulation in adults: a systematic review of the current literature. Int J Artif Organs. 2020;43(9):570–8. https://doi.org/10.1177/0391398820904372.
Kurihara C, Walter JM, Karim A, et al. Feasibility of venovenous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg. 2020;110(4):1209–15. https://doi.org/10.1016/j.athoracsur.2020.02.011.
Comparison of two fluid-management strategies in acute lung injury | NEJM. https://doi.org/10.1056/nejmoa062200. (Accessed 18 Feb 2022).
Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–70. https://doi.org/10.1007/s00134-016-4573-3.
Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch Surg. 2001;136(10):1118–23. https://doi.org/10.1001/archsurg.136.10.1118.
Wohlfahrt P, Krajcoviechova A, Jozifova M, et al. Low blood pressure during the acute period of ischemic stroke is associated with decreased survival. J Hypertens. 2015;33(2):339–45. https://doi.org/10.1097/HJH.0000000000000414.
Vemmos KN, Tsivgoulis G, Spengos K, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med. 2004;255(2):257–65. https://doi.org/10.1046/j.1365-2796.2003.01291.x.
Stead LG, Gilmore RM, Decker WW, Weaver AL, Brown RD. Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology. 2005;65(8):1179–83. https://doi.org/10.1212/01.wnl.0000180939.24845.22.
Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome | Stroke. https://doi.org/10.1161/01.STR.0000109769.22917.B0. (Accessed 18 Feb 2022).
Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–24. https://doi.org/10.1016/j.resuscitation.2008.12.015.
Besmertis L, Bonovich DC, Hemphill JC. The role of hypotension in secondary brain injury after intracerebral hemorrhage. Stroke. 2000;32(suppl_1):358–358. https://doi.org/10.1161/str.32.suppl_1.358-d.
Zhao Z, Wang D, Jia Y, et al. Analysis of the association of fluid balance and short-term outcome in traumatic brain injury. J Neurol Sci. 2016;364:12–8. https://doi.org/10.1016/j.jns.2016.03.007.
Clifton GL, Miller ER, Choi SC, Levin HS. Fluid thresholds and outcome from severe brain injury. Crit Care Med. 2002;30(4):739–45. https://doi.org/10.1097/00003246-200204000-00003.
Wijdicks EF, Vermeulen M, ten Haaf JA, Hijdra A, Bakker WH, van Gijn J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann Neurol. 1985;18(2):211–6. https://doi.org/10.1002/ana.410180208.
Wiegers EJA, Lingsma HF, Huijben JA, et al. Fluid balance and outcome in critically ill patients with traumatic brain injury (CENTER-TBI and OzENTER-TBI): a prospective, multicentre, comparative effectiveness study. Lancet Neurol. 2021;20(8):627–38. https://doi.org/10.1016/S1474-4422(21)00162-9.
Fletcher JJ, Bergman K, Blostein PA, Kramer AH. Fluid balance, complications, and brain tissue oxygen tension monitoring following severe traumatic brain injury. Neurocrit Care. 2010;13(1):47–56. https://doi.org/10.1007/s12028-010-9345-2.
Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations - PubMed. https://pubmed.ncbi.nlm.nih.gov/29500701/. (Accessed 18 Feb 2022).
Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280(2):159–65. https://doi.org/10.1001/jama.280.2.159.
Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–71. https://doi.org/10.1001/jama.288.7.862.
Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. https://doi.org/10.1056/NEJMoa051693.
Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. https://www.thelancet.com/pdfs/journals/lanres/PIIS2213-2600(19)30417-5.pdf. (Accessed 18 Feb 2022).
Dexamethasone in hospitalized patients with COVID-19 | NEJM. https://doi.org/10.1056/nejmoa2021436. (Accessed 18 Feb 2022).
• Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. Crit Care Med. JAMA Network. https://jamanetwork.com/journals/jama/fullarticle/2770279. (Accessed 18 Feb 2022). Dexamethasone has shown to reduce mortality in COVID-related (143,144) and non-COVID related (142) ARDS.
Witt KA, Sandoval KE. Steroids and the blood-brain barrier: therapeutic implications. Adv Pharmacol. 2014;71:361–90. https://doi.org/10.1016/bs.apha.2014.06.018.
Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–42. https://doi.org/10.1586/ecp.11.1.
Dexamethasone in adults with bacterial meningitis | NEJM. Accessed February 18, 2022. https://doi.org/10.1056/nejmoa021334.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432.
Saul TG, Ducker TB, Salcman M, Carro E. Steroids in severe head injury: a prospective randomized clinical trial. J Neurosurg. 1981;54(5):596–600. https://doi.org/10.3171/jns.1981.54.5.0596.
Braakman R, Schouten HJ, Blaauw-van Dishoeck M, Minderhoud JM. Megadose steroids in severe head injury. Results of a prospective double-blind clinical trial. J Neurosurg. 1983;58(3):326–330. https://doi.org/10.3171/jns.1983.58.3.0326.
Gaab MR, Trost HA, Alcantara A, et al. Ultrahigh dexamethasone in acute brain injury. Results from a prospective randomized double-blind multicenter trial (GUDHIS). German Ultrahigh Dexamethasone Head Injury Study Group. Zentralbl Neurochir. 1994;55(3):135–143.
Giannotta SL, Weiss MH, Apuzzo ML, Martin E. High dose glucocorticoids in the management of severe head injury. Neurosurgery. 1984;15(4):497–501. https://doi.org/10.1227/00006123-198410000-00004.
Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364(9442):1321–8. https://doi.org/10.1016/S0140-6736(04)17188-2.
• Edwards P, Arango M, Balica L et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365(9475):1957 1959. https://doi.org/10.1016/S0140-6736(05)66552-X. High dose steroids have shown to increase 2 weeks and 6 months mortality in TBI.
Sandercock PA, Soane T. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst Rev. 2011;(9):CD000064. https://doi.org/10.1002/14651858.CD000064.pub2.
Feigin VL, Anderson N, Rinkel GJE, Algra A, van Gijn J, Bennett DA. Corticosteroids for aneurysmal subarachnoid haemorrhage and primary intracerebral haemorrhage. Cochrane Database Syst Rev. 2005;(3):CD004583. https://doi.org/10.1002/14651858.CD004583.pub2.
Mistry AM, Mistry EA, Ganesh Kumar N, Froehler MT, Fusco MR, Chitale RV. Corticosteroids in the management of hyponatremia, hypovolemia, and vasospasm in subarachnoid hemorrhage: a meta-analysis. Cerebrovasc Dis. 2016;42(3–4):263–71. https://doi.org/10.1159/000446251.
Poungvarin N, Bhoopat W, Viriyavejakul A, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316(20):1229–33. https://doi.org/10.1056/NEJM198705143162001.
Mentzelopoulos SD, Malachias S, Chamos C, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310(3):270–9. https://doi.org/10.1001/jama.2013.7832.
Andersen LW, Isbye D, Kjærgaard J, et al. Effect of vasopressin and methylprednisolone vs placebo on return of spontaneous circulation in patients with in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2021;326(16):1586–94. https://doi.org/10.1001/jama.2021.16628.
Shah K, Mitra AR. Use of corticosteroids in cardiac arrest—a systematic review and meta-analysis. Crit Care Med. 2021;49(6):e642–50. https://doi.org/10.1097/CCM.0000000000004941.
Li Y, Zhang J, Cai N, He F. Efficacy and safety of corticosteroid therapy in patients with cardiac arrest: a systematic review of randomised controlled trials. Eur J Clin Pharmacol. 2020;76(12):1631–8. https://doi.org/10.1007/s00228-020-02964-3.
Chou SHY. Subarachnoid hemorrhage. Continuum (Minneap Minn). 2021;27(5):1201–45. https://doi.org/10.1212/CON.0000000000001052.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. https://doi.org/10.1038/nrneurol.2013.246.
Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol. 1985;17(2):137–40. https://doi.org/10.1002/ana.410170206.
Egge A, Waterloo K, Sjøholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery. 2001;49(3):593–605; discussion 605–606. https://doi.org/10.1097/00006123-200109000-00012.
Lennihan L, Mayer SA, Fink ME, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage. Stroke. 2000;31(2):383–91. https://doi.org/10.1161/01.STR.31.2.383.
Muench E, Horn P, Bauhuf C, et al. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Crit Care Med. 2007;35(8):1844–1851; quiz 1852. https://doi.org/10.1097/01.CCM.0000275392.08410.DD.
Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60. https://doi.org/10.1161/STR.0000000000000069.
Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85(12):1779–89. https://doi.org/10.1016/j.resuscitation.2014.08.011.
Sandroni C, D’Arrigo S, Cacciola S, et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46(10):1803–51. https://doi.org/10.1007/s00134-020-06198-w.
Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47(4):369–421. https://doi.org/10.1007/s00134-021-06368-4.
Beitler JR, Ghafouri TB, Jinadasa SP, et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am J Respir Crit Care Med. 2017;195(9):1198–206. https://doi.org/10.1164/rccm.201609-1771OC.
Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62(10):1743–8. https://doi.org/10.1212/01.wnl.0000125184.88797.62.
Esdaille CJ, Coppler PJ, Faro JW, et al. Duration and clinical features of cardiac arrest predict early severe cerebral edema. Resuscitation. 2020;153:111–8. https://doi.org/10.1016/j.resuscitation.2020.05.049.
Balu R, Rajagopalan S, Baghshomali S, et al. Cerebrovascular pressure reactivity and intracranial pressure are associated with neurologic outcome after hypoxic-ischemic brain injury. Resuscitation. 2021;164:114–21. https://doi.org/10.1016/j.resuscitation.2021.04.023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
We report no conflicts of interests, and none of the authors has received direct funding for this study. Abhijit Lele receives ongoing salary support from LifeCenter Northwest. Vasisht Srinivasan receives grant support from the Centers for Disease Control and Prevention via the Influenza and Other Viruses in the Acute Ill (IVY) network. Nicholas Johnson receives funding from the National Institutes of Health, from the Centers for Disease Control and Prevention, the Department of Defense, and the UW Royalty Research Fund for unrelated work. The remaining authors report no financial disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Critical Care Neurology
Rights and permissions
About this article
Cite this article
Matin, N., Sarhadi, K., Crooks, C.P. et al. Brain-Lung Crosstalk: Management of Concomitant Severe Acute Brain Injury and Acute Respiratory Distress Syndrome. Curr Treat Options Neurol 24, 383–408 (2022). https://doi.org/10.1007/s11940-022-00726-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-022-00726-3