Abstract
Purpose of Review
The present narrative systematic review summarizes current knowledge on germline gene mutations predisposing to solid tumors in adolescents and young adults (AYAs).
Recent Findings
AYAs with cancer represent a particular group of patients with specific challenging characteristics and yet unmet needs. A significant percentage of AYA patients carry pathogenic or likely pathogenic variants (PV/LPVs) in cancer predisposition genes. Nevertheless, knowledge on spectrum, frequency, and clinical implications of germline variants in AYAs with solid tumors is limited.
Summary
The identification of PV/LPV in AYA is especially critical given the need for appropriate communicative strategies, risk of second primary cancers, need for personalized long-term surveillance, potential reproductive implications, and cascade testing of at-risk family members. Moreover, these gene alterations may potentially provide novel biomarkers and therapeutic targets that are lacking in AYA patients. Among young adults with early-onset phenotypes of malignancies typically presenting at later ages, the increased prevalence of germline PV/LPVs supports a role for genetic counseling and testing irrespective of tumor type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adolescents and young adults (AYAs) with cancer represent a particular group of patients whose specific challenging characteristics are currently recognized by the scientific community. According to the most recent definition [1, 2], the AYA population is defined as those subjects diagnosed with cancer at ages 15 through 39. This represents a heterogeneous and challenging group of patients with increasing cancer incidence, modest survival gains compared with other age groups, and unique and often unmet needs [3, 4]. Tumor in AYAs shows substantial differences in etiology, cancer type, molecular profile, psychosocial implications, prognosis, and long-term treatment side effects from cancer affecting other age groups [4,5,6,7], and relevant differences persist also across AYA age groups themselves [8].
Various AYA oncology programs have been developed in the last year in several parts of the world (involving numerous organizations, healthcare providers, academic societies, and governments) [7, 9]. In particular, the European adult and pediatric oncology societies—the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE)—established a joint Working Group dedicated to AYA, with the aims of increasing awareness among the scientific community, exchanging knowledge, and foreseeing integrated programs to improve the standard of care for AYA with cancer across Europe [4•]. In the wake of this experience, also in Italy, a collaboration between pediatric and adult oncologists on the AYA theme has been recently formalized, and in April 2021, the national adult medical oncology society (AIOM—Associazione Italiana di Oncologia Medica) joined the pediatric hematology-oncology group (AIEOP—Associazione Italiana Ematologia Oncologia Pediatrica) in the creation of a formal AIEOP-AIOM Working Group dedicated to AYAs. Among the different initiatives of this group, it has been decided to focus on the specific need of counseling and genetic testing, as essential part of cancer journey of AYA patients and their family. To implement this aspect in Italian centers, we present a review of the cancer predisposition genes in this age range. Since the new-born AIEOP-AIOM Working Group involves pediatricians specialized in hematology-oncology and medical oncologists, who in Italy treat solid neoplasms, the work focuses on solid cancers rather than hematologic disorders. This topic appears relevant because it is known that a significant percentage of AYA patients carry pathogenic or likely pathogenic variants in cancer predisposition genes. A recent analysis of 1,507 patients with solid tumors showed 12% of germline pathogenic and/or likely pathogenic variants (PV/LPVs) in known cancer-predisposing genes [10••]. Nevertheless, this study also included children and only AYAs under 29 years of age. Previous studies showed that 7–8% of patients diagnosed <20 years of age have PV/LPV in known cancer predisposition genes, with adrenocortical carcinoma (50%) and high-grade glioma (25%) having the highest percentage of variants [11,12,13,14].
The presence of a germline PV/LPV in known cancer-predisposing genes brings several implications of utmost importance. First, germline variants may provide novel biomarkers and therapeutic targets that are lacking in AYA patients, as recently happened with the introduction of PARP inhibitors [15•]. Second, mutation carriers present elevated risk of secondary neoplasms that need specific surveillance programs [16]. Third, the identification of a hereditary cancer syndrome has an impact on all the relatives carrying the same mutation in terms of primary and secondary prevention. Despite all these implications, knowledge on spectrum, frequency, and implications of germline variants in AYAs with solid tumors is limited. Therefore, the objective of this systematic review was to summarize current knowledge regarding genes predisposing to solid tumors in AYA patients.
Methods
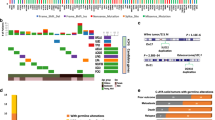
The search was carried out in the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed (accessed date 6 April 2021)). Key search terms used were as follows: “germline” AND “cancer” OR “neoplasm” AND “adolescent” OR “young adult” OR “AYA.” The eligibility criteria for articles reviewed included all types of articles published from January 2018 on AYA patients with a diagnosis of solid neoplasm that was associated with a germline PV/PLV in any cancer susceptibility gene. Then, reference lists were examined. Studies published in a language other than English were excluded. The systematic search identified the main predisposition genes described in the present review and summarized in Fig. 1.
Cancer Predisposition Genes in AYA
Genes Involved in the DNA Double-Strand Break Repair Mechanism
ATM (Ataxia Telangiectasia Mutated)
Homozygous or compound heterozygous ATM mutations cause ataxia telangiectasia, a syndrome characterized by progressive cerebellar ataxia, oculomotor apraxia, immunodeficiency, and general increased risk of malignancies [17] with an overall cumulative incidence of cancer by age 40 of 38.2% [18]. On the other hand, heterozygous ATM germline PV/LPV can be found in 0.35–1% of the general population [19] and are associated with an increased risk for breast cancer (BC) at a higher median age of onset (46.9 years) [20]. Maxwell et al. [21] identified 8 patients with a ATM germline PV/LPV out of 278 (2.9%) BRCA1/2-negative patients with BC diagnosed at less than 40 years of age. Moreover, a significant association between ATM heterozygous PV/LPV and ovarian cancer [22, 23], pancreatic cancer [24], and prostate cancer [25] has been described.
ATR (Ataxia Telangiectasia and Rad3 Related)
Mutations in ATR gene are rare. Homozygous hypomorphic mutations in ATR have been detected in Seckel syndrome, characterized by developmental delay and premature aging [26]. A different kind of genetic disorder due to heterozygous mutations in ATR comprises skin telangiectasis; mild developmental anomalies of the hair, teeth, and nails; and an increased risk of oropharyngeal cancer typically in the third decade of life or thereafter [27]. Other malignancies reported included nonmelanoma skin cancer, breast cancer, and cervical cancer, although data on these associations are still conflicting [28, 29].
BARD1 (BRCA1 Associated RING Domain 1)
BARD1 is a BC moderate-risk gene [30, 31] with a lifetime risk approximately doubled than that in the general population. Deleterious BARD1 germline variants are significantly associated with early-onset BC. The mean age at first BC diagnosis in BARD1 mutation carriers was 42.3 years (range 24–60 years) in a German cohort [32]. Particularly, two recent studies confirmed that BARD1 PV/LPVs are enriched among triple-negative BC patients compared to other BC subtypes [33, 34].
BRCA1 (BReast CAncer Gene 1) and BRCA2 (Breast Cancer Gene 2)
Germline mutations in the tumor suppressor genes BRCA1 and BRCA2 account for most cases of hereditary breast and ovarian cancer syndrome [35]. The population frequency of BRCA1/2 PV/LPV has been historically estimated 1:400, except for populations with high-frequency founder mutations, such as the Ashkenazi Jewish population [36]. However, recent unselected population-based genomic screening efforts have found a higher, almost doubled prevalence (1:190), predominantly in European ancestry individuals [37]. The cumulative BC risk to age 40 years in women was estimated 24% (95%CI, 21–29%) for BRCA1 and 13% (95%CI, 9–19%) for BRCA2 carriers [38], whereas the cumulative BC risk to age 40 in men was 0.12% (95%CI, 0.012–0.58%) for BRCA1 and 1.2% (95%CI, 0.3–3.6%) for BRCA2 carriers [39]. OC risk is almost null until 30 years and remains low from 31 to 40 years: 2% (95%CI, 1–3%) for BRCA1 and 0% (95%CI, 0–2%) for BRCA2 carriers [38]. In AYA with PV/LPV in BRCA1/2, the risk for prostate and pancreatic cancer is negligible [40, 41]. On these bases, surveillance in female BRCA carriers should start at 25 years of age with breast MRI, and at the same time, preventive surgeries can be discussed [42].
BRIP1 (BRCA1 Interacting Protein C-terminal Helicase 1)
Pathogenic mutations in BRIP1 have been described for the first time in two patients with early-onset BC [43]. Weber-Lassalle et al. [44] observed a barely significant association of BRIP1 PV/LPV mutations with BC in the subgroup of patients with an age at first diagnosis < 61 years. However, the role of pathogenic BRIP1 mutations in BC risk remains conflicting [31, 45]. OC risk is almost null until 40 years [46].
RAD51C and RAD51D (RAD51 Recombinase Paralog C and Paralog D)
Genetic testing through multigene cancer panel revealed an association between PV/LPV in RAD51C and RAD51D and the increased risk of OC [47, 48]. The estimated cumulative risk of developing OC to age 40 years was 0.2% (95%CI, 0.08–0.4%) for a woman with a RAD51C PV/LPV and 0.1% (95%CI, 0.06–0.3%) for a woman with a RAD51D PV/LPV. The relative risk of OC in the third decade was 2.85 (95%CI, 0.46–17.70) in carriers of RAD51C PV/LPV and 3.60 (0.78 to 16.75) in carriers of RAD51D PV/LPV [49]. Recent findings highlighted the evidence of an association between protein-truncating variants of RAD51C/D and BC risk [31, 50]. The estimated cumulative risk of developing BC to age 40 years was 1% (95%CI, 0.7–1%) for a woman with a RAD51C PV/LPV and 0.9% (95%CI, 0.6–1%) for a woman with a RAD51D PV/LPV. The relative risk of BC in the third decade was 3.25 (95%CI, 1.60–6.62) in carriers of RAD51C PV/LPV; in carriers of RAD51D PV/LPV, the relative risk of BC between 20 and 39 years of age was 2.25 (95%CI, 1.25–4.04) [49].
CHEK2 (Checkpoint Kinase 2)
CHEK2 has the highest mutation prevalence in individuals of European descent, while the spectrum and frequency of pathogenic variants vary among specific European populations [51]. Different case-control studies had revealed a significant association between CHEK2 1100delC mutation and early-onset BC [52]. In a Swedish cohort, the mean age at diagnosis of CHEK2 1100delC carriers was 12 years lower than that of non-carriers (46 vs 58 years, p=0.001) [52] and this has been recently confirmed in an Italian cohort of CHEK2 mutation carriers (median age at first BC onset 46.1 years) [20]. Relative risk of developing BC to age 35 years was 2.59 (95%CI, 1.23–5.47) for CHEK2 1100delC carriers, whereas the cumulative risk to age 40 years was < 5% [51]. Greville-Heygate et al. [53] detected 53 (2.3%) patients carrying a germline CHEK2 PV/LPV out of 2344 women with early-onset BC and CHEK2-associated tumors showed a worse prognosis. Moreover, a case-control enrichment analysis recently provided evidence for CHEK2 as a novel moderate-penetrance testicular germ cell tumor susceptibility gene [54]. Finally, pathogenic CHEK2 variants were associated with an increased risk of other malignancies including colon, prostate, kidney, bladder, and thyroid cancers, according to specific mutations (frameshift or missense substitutions) at a more mature age [55].
PALB2 (Partner and Localizer of BRCA2)
PALB2 is a BC susceptibility gene [56]. The risk of BC for women with a PALB2 PV/LPV was 8 to 9 times as high among those younger than 40 years of age compared with the general population with a cumulative risk estimated to be 14% (95%CI, 9–20) by 50 years of age [57]. Some studies highlight a possible association between PALB2 mutations and OC and pancreatic cancer [58].
FANCA (Fanconi Anemia Complementation Group A) Family
Fanconi anemia (FA) is a rare autosomal recessive genetic disorder that comprises a broad spectrum of clinical features of variable penetrance, mainly progressive bone marrow failure, congenital abnormalities, and cancer predisposition [59]. Until now, 22 genes have been described as FA genes: FANCA, FANCB, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG/XRCC9, FANCI, FANCJ/BRIP1, FANCL/PHF9, FANCM, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4, FANCQ/ERCC4, FANCR/RAD51, FANCS/BRCA1, FANCT/UBE2T, FANCU/XRCC2, FANCV/REV7, and FANCW/RFWD3 [60]. FA patients develop acute myeloid leukemia at an incidence 700-fold higher compared to the general population. The median age at diagnosis is 19 (range 16–27) years, with a cumulative incidence of 10% by 50 years of age. FA patients who overcome severe bone marrow failure following a successful bone marrow transplant are still likely to develop solid tumors (head and neck, esophageal, gastrointestinal, vulvar, and anal cancers) at an incidence approximately 50-fold higher, with a median onset age of 30 (range 4–44) years and a cumulative risk of 10% by 40 years of age [61, 62]. Finally, heterozygous mutations in FA genes (e.g., BRCA1, BRCA2, BRIP1, PALB2, and RAD51C) are associated with hereditary breast and/or ovarian cancer predisposition (paragraphs above) [63].
Genes Involved in the Nucleotide Excision Repair (NER) Mechanism
ERCC4 (Human Excision Repair Cross-complementing Rodent Repair Deficiency, Complementation Group 4)
ERCC4 mutations are associated with three clinically distinct disorders: xeroderma pigmentosum, XFE progeroid syndrome, and Fanconi anemia [64, 65]. In xeroderma pigmentosum, there is a 1000-fold increased frequency of early-onset basal cell or squamous cell carcinomas and melanomas of the skin, often with multiple primary tumors, by age 20. The median age at first skin neoplasm diagnosis is 8 years, nearly 50 years younger than that found in the general population. A 5% risk of malignant melanoma is reported [66].
POLE (DNA Polymerase Epsilon)
Germline missense pathogenic variants in the exonuclease domain of polymerases epsilon (POLE) predispose to multiple colorectal adenomas and carcinomas, causing the so-called polymerase proofreading–associated polyposis (MIM 615083; 612591) [67]. Evidence of extracolonic tumors has been reported, including endometrial, brain, breast, ovarian, stomach, pancreas, and skin tumors, among others [68,69,70].
Genes Involved in the Mismatch Mediated Repair (MMR) Mechanism
MLH1 (MutL Homolog 1), MSH2 (MutL Homolog 2), MSH6 (MutS Homolog 6), PMS2 (PMS1 Homolog 2)
Germline heterozygous PV/LPVs in MLH1, MSH2, MSH6, or PMS2 cause Lynch syndrome (LS). LS, also called hereditary nonpolyposis colorectal cancer (HNPCC), leads to various types of tumors, including most of all colorectal (CRC) and endometrial cancers, but also ovarian, stomach, small bowel, urinary tract, biliary tract, brain, skin (sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas), pancreatic, and prostate cancers [71]. The MLH1 variant is correlated with the highest risk of developing CRC with a cumulative incidence at 40 years of age of 15.3% in females and 18.9% in males, and a cumulative incidence of endometrial cancer of 1.9% at 40 years [72]. PV/LPVs in MSH2 are correlated with the second highest risk of CRC and the highest risk of developing other non-colorectal cancers, with cumulative cancer incidences at 40 years of 6.9% for CRC in females, 9.9% for CRC in males, and 2.3% for endometrial cancer [72]. Cancers in MSH6 mutation carriers occur later than those in the MLH1 or MSH2 PV/LPVs, with cumulative CRC incidences at 40 of 2.5% (females) and 6.3% (males), and 2.3% for endometrial cancer. The cumulative incidence of overall cancers for PMS2 mutation carriers is the lowest among the four genes, with cumulative cancer incidences of 0% at the age of 40. On these bases, the Lynch syndrome surveillance should start at 20–25 years of age with colonoscopy and, in selected individuals, upper endoscopy, urinalysis, and physical and neurologic examination may be considered starting between 25 and 40 years of age. Hysterectomy and bilateral salpingo-oophorectomy should be individualized [73, 74].
RECQL (RecQ Like Helicase)
RECQL was first identified as a novel breast cancer susceptibility gene in 2015, by two independent research groups [75, 76]. In a cohort of early-onset BC patients from Poland (<40 years), the increased risk of BC in carriers of the RECQL mutation was found to be 1.9 (95%CI 0.27–13.6) [77]. Nevertheless, several subsequent studies have failed to support the association [78]. No high-quality penetrance study showed statistical significance for additional diseases beyond BC.
Genes Involved in the MAP Kinase Pathway
PTPN11, SOS1, RAF1, RIT1, KRAS, NRAS, BRAF, MAP2K1, RRAS, RASA2, A2ML1, SOS2, LZTR1
The RASopathies are a collective group of phenotypically related conditions caused by germline PV/LPV in genes within the Ras/mitogen-activated protein kinase (Ras/MAPK) signaling pathway. RASopathy conditions, such as Noonan syndrome (NS; MIM# 163950), cardiofaciocutaneous syndrome (CFC; MIM# 115150), and Costello syndrome (CS; MIM# 218040), typically present with multiple phenotypic features, including poor growth, cardiac anomalies, ectodermal abnormalities, neurodevelopmental deficits, and increased tumor risk [79, 80]. NS is caused by germline mutations of PTPN11 (50%); SOS1 (13%); RAF1 (5%); RIT1 (5%); or more rarely, KRAS, NRAS, BRAF, MAP2K1, RRAS, RASA2, A2ML1, SOS2, or LZTR1 [81]. Children with NS are at an approximately 8-fold increased risk for a spectrum of different cancers [82]. These include (but are not limited to) gliomas such as dysembryoplastic neuroepithelial tumors, acute lymphoblastic leukemia, neuroblastoma, and rhabdomyosarcoma [80, 82,83,84].
CFC syndrome is due to germline mutation of KRAS, MAP2K1, MAP2K2, or BRAF [81]. Affected persons have NS features and tend to have significant mental and neurologic impairment, more severe ectodermal involvement, and characteristic facies. Several cases of childhood cancer have been reported, and the cancer risk may be mildly increased [80, 82].
The CS is due to germline mutations of HRAS [85]. In addition to NS features, CS patients have mental deficits, poor feeding, hypertrophic cardiomyopathy, tachycardia, typical skin and hair, a coarse face, and a high childhood cancer risk, especially for embryonal rhabdomyosarcoma (ERMS), NBL, and early-onset bladder cancer. The occurrence of bladder carcinoma in adolescents is distinctly unusual as this is typically a neoplasm of older adults and is not seen with increased frequency in other tumor predisposition syndromes. The cumulative incidence of cancer is 15% by age 20 years [80, 82, 86, 87]. The HRAS G12A mutation appears to be associated with the highest cancer risk [88].
NF1 (Neurofibromatosis type 1) and NF2 (Neurofibromatosis type 2)
Neurofibromatosis (NF1) is a dominantly inherited syndrome with variable disease manifestations, affecting multiple organs, childhood development, and neurocognitive status. NF1 causes significantly increased malignancy risks compared with the general population. Specifically, mutation in NF1 gene is associated with highly elevated risk of malignant peripheral nerve sheath tumors (MPNST), rhabdomyosarcoma, and primary brain tumors. Because of the risk of MPNST being associated with high internal tumor burden, whole-body MRI should be considered between ages 16 and 20 years [89]. Furthermore, women with NF1 ages 30 to 50 showed an increased breast cancer risks of 4- to 5-fold [90].
Neurofibromatosis type 2 (NF2, also known as central neurofibromatosis) is an autosomal dominant disorder that is distinct from NF1 on both genetic and clinical grounds. Around 30% of NF2 presents symptomatically in childhood and nearly 50% by 20 years of age. A hallmark of NF2 is the occurrence of bilateral schwannomas that affect the vestibular branch of the eighth cranial nerve (acoustic neuromas). NF2 patients are also at elevated risk for meningiomas, spinal schwannomas, and ependymomas [89].
Genes Involved in the Cell Cycle Regulation
CDKN2A (Cyclin-Dependent Kinase Inhibitor 2A)
CDKN2A germline mutations are associated with a 65-fold increase in the risk of melanoma development [91], and these have been identified in approximately 20–40% of families showing a predisposition to melanoma [92, 93]. Mean age at melanoma diagnosis is earlier in CDKN2A mutation carriers than in the general population (mean of 35 years vs 59 years). Lifetime penetrance of CDKN2A mutations is 0.58 in Europe, 0.76 in the USA, and 0.91 in Australia [94]. Accordingly, the median age of melanoma diagnosis was also younger in Australian melanoma-prone families compared to European families [95]. Importantly, CDKN2A mutation carriers have been reported to be at increased risk of developing other early-onset cancers, including breast, lung, pancreatic, and nonmelanoma skin cancers and soft tissue sarcomas [96]. These additional cancer risks are not consistently observed, and this may indicate that the risk of other cancers varies with the specific PV/LPV [97, 98].
TP53 (Tumor Protein 53)
Germline PV/LPVs of TP53 gene have a high penetrance and cause Li-Fraumeni Syndrome (LFS). LFS is associated with increased risk of breast cancer, soft tissue sarcoma, osteosarcoma, leukemia, brain tumors, adrenocortical carcinoma, and other cancers [99, 100]. The cancer risk imparted by TP53 mutations is evident at an early age, with female carriers having a cumulative 49% risk of developing cancer by the age of 30, and men having a 21% cancer risk at the same age [101]. Indeed, regardless of familial history, the rate of disease associated with germline TP53 PV/LPV has been estimated to be between 3.8 and 7.7% in females with breast carcinoma before 31 years of age [102]. On these bases, the Li-Fraumeni syndrome surveillance should start from the birth with US of abdomen and pelvis, neurologic examination, and possibly whole-body and brain MRI, at 18 years of age with dermatologic examination, at 20 with breast MRI, and at 25 with colonoscopy and upper endoscopy [74].
BAP1 (BRCA1-Associated Protein 1)
Heterozygous germline mutations of BAP1 confer increased susceptibility for the development of several tumors, mostly uveal and cutaneous melanomas, epithelioid atypical Spitz tumors, and mesotheliomas but also other neoplasms, including renal cell carcinoma, lung adenocarcinoma, and meningioma (BAP1-TPDS, OMIM 614327) [103]. However, the complete tumor spectrum associated with germline BAP1 mutations is still uncertain.
The prevalence of germline BAP1 alterations in unselected patients with metastatic uveal melanoma ranges from 2 to 8% [104], whereas the prevalence in patients with mesothelioma was 4.4% [105]. BAP1 mutation carriers showed a lower age at diagnosis in comparison with the general population, and median age of onset associated with null variants was younger than that with missense variants (in null variants: 53 years for uveal melanoma, 55 years for mesothelioma, 39 years for cutaneous melanoma, 50 years for renal tumors, and 44 years for nonmelanoma skin cancer) [106].
CDC73 (Cell Division Cycle 73)
Because of its incomplete penetrance, patients with germline CDC73 mutation can present with a spectrum of phenotypes including seemingly sporadic parathyroid cancer (CDC73 PV/LPVs have been identified in 20–29% of parathyroid carcinomas), familial isolated hyperparathyroidism (FIHP) with or without parathyroid cancer, or full expression of hyperparathyroidism-jaw tumor syndrome (HPT-JT) [107, 108]. HPT-JT is a rare autosomal dominant syndrome with typical onset in late adolescence or early adulthood that causes familial hyperparathyroidism associated with ossifying fibromas of the maxillofacial bones and increased risk of parathyroid carcinoma. Renal abnormalities occur in 15% of patients and include Wilms’ tumors, hamartomas, renal cell carcinoma, and polycystic disease [109]. Uterine tumors affect up to 75% of female HPT-JT patients and may be benign or malignant (e.g., adenosarcomas) [110].
Genes Encoding for Transmembrane Receptors
EGFR (Epidermal Growth Factor Receptor)
EGFR germline mutations, including the mutations p.T790M and p.R776H in exon 20 and p.V843I in exon 21, are associated with genetic susceptibility to lung cancer [111,112,113,114]. Overall, the estimated risk of developing lung cancer among nonsmoking EGFR T790M carriers is 31%, compared with a 0.2% risk in a general population of nonsmokers and an approximately 23% risk in a general population of smokers [115]. Diagnosis of lung adenocarcinoma was accelerated 9.0 years (95%CI, 0.5–16.5 years) by EGFR germline PV/LPV [116, 117].
Furthermore, EGFR could be a novel underlying germline predisposition factor for adrenocortical carcinoma (ACC) especially in the AYA population [117].
EPCAM (Epithelial Cell Adhesion Molecule)
Deletions of EPCAM can cause Lynch syndrome through epigenetic silencing of MSH2 in EPCAM-expressing tissues, resulting in tissue-specific MSH2 deficiency. Carriers of an EPCAM deletion had a 75% cumulative risk of colorectal cancer before the age of 70 years (mean age at diagnosis 43 years). Women with EPCAM deletions had a 12% cumulative risk of endometrial cancer (mean age at diagnosis 47 years) [118].
KIT (Receptor Tyrosine Kinase)
Somatic mutations of KIT are frequently found in mastocytosis and gastrointestinal stromal tumor (GIST), while germline mutations of KIT are rare, and only found in few cases of familial GIST and mastocytosis [119]. GISTs are reported predominantly in patients who are 40 to 70 years old but in rare cases may occur in younger persons. Beghini et al. studied an Italian family in which 4 members over 3 generations, including a father and son, had multiple hyperpigmented spots. At 18 years of age, the father developed multiple GISTs with diffuse hyperplasia of the myenteric plexus. The proband was the 14-year-old son whose hyperpigmented lesions were found to be cutaneous mastocytosis [120].
RET (REarranged During Transfection)
Inherited mutations in the RET proto-oncogene, which encodes a receptor tyrosine kinase, predispose individuals to the multiple endocrine neoplasia type 2 (MEN 2) cancer syndromes. The major component tumor of these syndromes is medullary thyroid carcinoma (MTC) [121]. Different mutations in the RET gene produce varying phenotypes for the disease, including age of onset and aggressiveness of MTC, and the presence or absence of other endocrine neoplasms, such as pheochromocytoma or hyperparathyroidism.
RET mutations can be classified into 3 groups based on aggressiveness of MTC or level of risk. Level 1 RET mutations (codons 609, 768s790, 791, 804, and 891) are the lowest risk for aggressive MTC marked by later onset of tumor development and a more indolent biological course. Patients with level 1 mutations rarely develop tumors before the age of 10 years of age. Level 2 RET mutations (codons 611, 618, 620, and 634 mutations) are considered high risk for aggressive MTC. Patients with level 2 RET mutations should undergo thyroidectomy before age 5 years. Level 3 RET mutations (codons 883, 918, and 922) are the most aggressive of all the RET mutations. Patients with level 3 mutations can have metastasis in the first years of life [122].
PDGFRA (Platelet-Derived Growth Factor Receptor Alpha)
Familial gastrointestinal stromal tumor (GIST) is a rare autosomal dominant genetic disorder associated with KIT and PDGFRA germline mutations. Structure and organization of both human PDGFRA and KIT genes are very similar and could derive from a common ancestral gene. PDGF receptor α is member of the protein tyrosine kinase family subclass III, similar to that of the KIT protein. Chompret et al. described a French family in which 5 individuals had GISTs (age at onset 40–61 years) with germline mutation in PDGFRA gene [123]. A 22-year-old patient with multiple GISTs and small intestinal polyps, fibroid tumors, and lipomas was also described in association with V561D germline PDGFRA mutation [124]. A unique phenotype including coarse facies and skin, broad hands and feet, and previously undescribed premature tooth loss was described in a family with four first-degree relatives that harbor a PDGFRA exon 18 (D846V) germline mutation. The index patient presented with multiple small bowel inflammatory fibroid polyps (IFPs) and has a gastric gastrointestinal stromal tumor (GIST) [125].
Genes Involved in the Metabolic Mitochondrial Pathway
SDHx (SDHA, SDHB, SDHC, and SDHD) (Succinate DeHydrogenase Complex)
Germline alterations in the SDHB, SDHC, and SDHD genes and, to a lesser extent, the SDHA gene predispose to hereditary phaeochromocytoma and/or paraganglioma (PPGL) [126]. Germline mutations in SDHx genes are responsible for approximately 20% of cases of PPGL and can also be associated with the presence of other SDHx-related tumors including renal cell carcinoma (RCC), GIST, and thyroid and pituitary tumors.
FH (Fumarate Hydratase)
FH inactivating mutations can cause hereditary leiomyomatosis and renal cell cancer (HLRCC), a hereditary cancer syndrome that follows an autosomal dominant inheritance pattern with incomplete penetrance [127]. The age of onset for HLRCC is typically around adolescence to adult with penetrance increasing with age [128]. It is characterized by the development of multiple tumor types including skin leiomyomas, uterine fibroids, and HLRCC kidney tumors with morphological and clinical features similar to those of type 2 papillary renal cell carcinoma (PRCC2) [127]. In addition to the 3 main tumor types, bladder cancer and Leydig cell tumors of the testis have also been reported in HLRCC patients [129, 130].
Genes Involved in the PIK3, AKT mTOR/AMPK Pathway
TSC1 (Tuberous Sclerosis Complex 1), TSC2 (Tuberous Sclerosis Complex 2)
TSC is an autosomal dominant disorder caused by the mutation of one of two tumor suppressor genes, TSC1 or TSC2, and characterized by skin manifestations and formation of multiple tumors in different organs, mainly in the central nervous system. The phenotypic expression can vary over the years, with neurological and cutaneous manifestations being more prevalent in childhood, and kidney and pulmonary involvement more characteristic of adulthood. There is a 6–14% incidence of childhood brain tumors in patients with TSC, of which more than 90% are subependymal giant cell astrocytomas [131]. TSC is associated with a cumulative renal cancer incidence of 2.2–4.4%, higher than the estimated incidence in the general population [131]; the average age at diagnosis is 28 years, with occasional early childhood cases [132].
TMEM127 (TransMEMbrane Protein 127)
Loss-of-function alterations in the tumor suppressor TMEM127 have been detected in familial pheochromocytomas and paragangliomas (PCC/PGL) and associated with increased risk for RCC [133, 134].
STK11 (Serine/Threonine Kinase 11)
Peutz-Jeghers (PJ) syndrome is an autosomal dominant disorder caused by germline mutations of the STK11 gene and characterized by melanocytic macules of the lips, multiple gastrointestinal hamartomatous polyps, and an increased risk for various neoplasms, including gastrointestinal cancer [135, 136]. Cumulative risk for all cancer was 93% from ages 15 to 64 years old with a significant increase for esophagus, stomach, small intestine, colon, pancreas, lung, breast, uterus, and ovary malignancies [136].
PTEN (Phosphatase and Tensin Homolog)
Individuals with germline mutations of the PTEN tumor suppressor gene have diverse phenotypic features affecting multiple systems, with the primary clinical concern of high lifetime risks of cancer. Elevated risks of breast, thyroid, endometrial, colorectal, and kidney cancers and melanoma were found. The particularly elevated penetrance of breast cancer in females with PTEN mutations is noted, beginning around age 30 and rising to an estimated 85% lifetime risk. PTEN-related endometrial cancer risk begins at age 25 rising to 30% by age 60, whereas for thyroid cancer, risk begins at birth and continues lifelong. Risks of colorectal and kidney cancers begin around age 40, with a lifetime risk of 9% and 34% respectively. For melanoma, the earliest reported age of onset was 3 years [137].
Genes Involved in the Wnt/β-Catenin Pathway
APC (Adenomatous Polyposis Coli)
The APC gene encodes a tumor suppressor protein that acts as an antagonist of the Wnt signaling pathway. Defects in this gene cause familial adenomatous polyposis (FAP), an autosomal dominant pre-malignant disease that usually progresses to colorectal cancer. Additionally, in infants and toddlers, there is an increased risk of hepatoblastoma, while in teenagers and adults, duodenal carcinomas, desmoid tumors, thyroid cancer, and medulloblastoma are more common in FAP than in the general population [138, 139].
CDH1 (CaDHerin 1)
CDH1 is a tumor suppressor gene that is required to maintain cell adhesion, cell polarity, and cell survival signaling. Mutation or transcriptional silencing of the CDH1 gene is associated with hereditary diffuse gastric cancer. These patients have a 70% lifetime risk of gastric cancer in males and 56% in females with a median age of 38 years (range 14–69 years). Women additionally have a 42% lifetime risk of lobular breast cancer. The current management for identified carriers includes prophylactic total gastrectomy between the ages of 20 and 40 years, and the initiation of high-risk breast cancer screening with annual mammography and MRI at ages 30–35 years for female carriers [140, 141].
Genes Involved in the BMP Signaling Pathway
BMPR1A (Bone Morphogenetic Protein Receptor Type 1A), SMAD4 (Mothers Against Decapentaplegic Homolog 4)
Germline mutations in SMAD4 and BMPR1A genes have been identified to cause juvenile polyposis syndrome (JPS). It is a rare autosomal dominant hereditary disorder characterized by the development of multiple distinct juvenile polyps in the gastrointestinal tract with an increased risk of colorectal cancer [142]. The reported age at diagnosis of JPS was similar for both SMAD4 and BMPR1A pathogenic variant carriers (median 28 and 25 years, respectively). The incidence of colorectal cancer is 17–22% by age 35 years and approaches 68% by age 60 years. The median age at diagnosis is 42 years. The incidence of gastric cancer is 21% in those with gastric polyps [143].
Genes Encoding for Proteins of the SWI/SNF Complex
SMARCB1 (SWI/SNF-Related, Matrix-Associated, Actin-Dependent Regulator of Chromatin, Subfamily b, Member 1), SMARCA4 (SWI/SNF-Related, Matrix-Associated, Actin-Dependent Regulator of Chromatin, Subfamily A, Member 4)
Carriers of heterozygous constitutional mutations of SMARCB1 or SMARCA4 are prone to develop rhabdoid tumors (RT), which have been clinically named as rhabdoid tumor predisposition syndromes 1 (RTS1) and 2 (RTS2), respectively [144, 145]. The manifestation of RTS1 occurs at a very early age, with a median of 5.5 months in children with SMARCB1 germline mutations, but is very rare in older children or adults [146].
RTPS2 is a complex familial disorder with an autosomal dominant pattern of inheritance with variable penetrance predisposing to formation of tumors that develop in the brain, spine, lung, bladder, pelvis, kidney, or ovary of young children or adults [147]. Genetic profiling has demonstrated these mutations in small cell carcinoma of the ovary and hypercalcemic type (SCCOHT) and SMARCA4-deficient undifferentiated uterine sarcoma, as well as atypical teratoid rhabdoid tumors, malignant rhabdoid tumors, and aggressive SMARCA4-deficient thoracic sarcomas [148, 149].
Other Genes
COL7A1 (Collagen Type VII Alpha 1 Chain)
Mutations in COL7A1 cause the severe inherited blistering disorder recessive dystrophic epidermolysis bullosa (RDEB) affecting skin and mucosae, associated with a greatly increased risk of skin cancer [150]. In the severe generalized subtype of RDEB (Hallopeau-Siemens RDEB), recurrent blistering leads to extensive scarring with a cumulative risk of squamous cell carcinoma (SCC) of 70% by age 45 [151].
DICER1 (Double-Stranded RNA-Specific Endoribonuclease)
The DICER1 syndrome (OMIM 606241) is an autosomal dominant cancer predisposition disorder that is associated with a variety of benign and malignant tumors, including pleuropulmonary blastoma, cystic nephroma, Sertoli-Leydig cell tumors, multinodular goiter, thyroid cancer, rhabdomyosarcoma, and pineoblastoma [152].
EXT2 (Exostosin Glycosyltransferase 2)
Germline mutations in EXT2 are causative for hereditary multiple exostoses (HME), also called multiple osteochondromas (MO). The main complication in HME is malignant transformation of an osteochondroma (exostosis) into chondrosarcoma, which is estimated to occur in 1–3% of the HME cases [153, 154].
GJB2 (Gap Junction Protein Beta 2)
GJB2 is mostly known for being associated with syndromic hearing loss, for example, keratitis-ichthyosis-deafness (KID). It has been reported that these KID patients with germline GJB2 mutation have increased risks of developing epithelial malignancies, for example, 19% occurrence of squamous cell carcinoma of the skin and oral mucosa compared to the normal population [10••].
HABP2 (Hyaluronan Binding Protein 2)
HABP2 G534E variant functions as a dominant-negative tumor suppressor gene and is a susceptibility gene for familial papillary thyroid cancer [155]. Nevertheless, this association was not confirmed in several subsequent studies [156].
MUTYH (MutY DNA Glycosylase)
Biallelic pathogenic variants in the MUTYH gene cause MUTYH-associated polyposis (MAP). MAP is characterized by the presence of 15–100 colorectal polyps and an increased risk of colorectal adenomas and carcinomas [157]. Patients are diagnosed with MAP at a mean age of 45–50 years and median age of CCR onset is 48 [158]. Moreover, carriers of biallelic mutation have an increased risk of ovarian cancer, urinary bladder cancer, cancer of the upper gastrointestinal tracts, breast cancer, endometrial cancer, and skin cancer. Carriers of monoallelic mutation have an approximately 2.5-fold increased risk of CRC compared with the general population [159], but the risk of developing extraintestinal cancer in heterozygotes is still unclear [157].
VHL (Von Hippel-Lindau)
Germline inactivation of the VHL tumor suppressor gene causes the von Hippel-Lindau hereditary cancer syndrome (MIM 193300). Common VHL-associated clinical manifestations include central nervous system hemangioblastoma, renal cell carcinoma or renal cyst, retinal angioma, pancreatic tumor or cyst, pheochromocytoma and paragangliomas, endolymphatic sac tumor, and epididymis or broad ligament cystadenoma [160]. Patients may be affected by cancers from childhood and throughout their lifetime [161]. In a recent analysis of a large cohort of Chinese VHL families, the mean ages at onset for central nervous system hemangioblastoma and renal cell carcinoma were 41.1 ± 9.1 (range = 29–62) and 37.4 ± 12.9 (range = 23–65), respectively [160].
Genetic Counseling and Testing in AYA
Genetic counseling involves three consecutive steps: pre-test counseling, genetic testing, and post-test counseling [42, 162]. Each of these stages should be carried out by a healthcare professional with expertise and experience in cancer risk assessment and management of individuals with an inherited predisposition to cancer [163]. The first step includes an evaluation of patient’s needs and concerns, a detailed collection of personal and family cancer history, a discussion on the possible testing results, subsequent management options, inherited cancer risk to relatives and the privacy of genetic information, and, finally, a written informed consent [164, 165]. The pre-test counseling also guides the clinician towards the most appropriate test to order. The indication to genetic testing is based on the features of an individual’s personal or family medical history. Particularly, age at onset of the tumor, recurrence of specific cancers in the same person or family (e.g., breast, ovarian or colon cancers), unusual cases of cancer (e.g., male breast cancer), the presence of less frequent tumor histotypes (e.g., medullary thyroid carcinoma or triple-negative breast cancer), or birth defects that are known to be associated with inherited cancer syndromes (e.g., neurofibromas). For the most known syndromes, testing criteria are published and universally recognized, such as the Amsterdam criteria for the Lynch syndrome [166] or the Chrompret criteria for Li-Fraumeni syndrome [167]. However, for most of syndromes, testing criteria vary among institutions.
The introduction of multigene testing based on next-generation sequencing (NGS) technology allowed to simultaneously analyze a set of cancer predisposition genes. It is sufficient for cancer risk assessment to evaluate genes of established clinical utility that are suggested by the patient’s personal and/or family history [163]. This approach may be more efficient and cost-effective than the previous tests for single syndrome. Notably, when a pathogenic variant of a predisposition gene is identified on tumor genetic testing, a confirmatory germline testing is recommended. Germline genetic testing should be performed by laboratories equipped to provide analytically and clinically valid results [163]. When results of genetic testing are ready, in a post-test counseling, clinicians should discuss results, related risks, and medical management options in the context of personal and family history.
Patients of reproductive age should also be advised about prenatal diagnosis and assisted reproduction and partners should be tested in case of identification of PV/LPV in genes associated with rare autosomal recessive conditions, such as Fanconi anemia [42, 168]. Moreover, the importance of sharing these results with family members should be discussed so they may benefit from this information [163].
Emotional distress following testing is influenced by factors including disease characteristics (e.g., severity, preventability), amount of uncertainty remaining after testing [169], and ethical and religious beliefs. Often, individuals with hereditary cancer predisposition syndromes worry about passing the condition down to their children and trouble starting a family because of employment and insurance discrimination [170]. Questions about availability of prenatal genetic testing, and occurrence, timing, severity, course, and preventability of cancer may reduce or elevate distress in AYA patients. Young age, perception of high risk, pre-existing psychological distress, a passive way of coping, little social support, and family members with cancer were predictive of psychological problems and/or reduced quality of life [171].
Predictive genetic testing in minors, including adolescents, for conditions for which there are limited preventative or therapeutic measures has traditionally been deferred [42, 172, 173]. Parents or guardians should be informed about the risks and benefits of testing, and in case of testing, their permission should be obtained [174]. Ideally and when appropriate, the assent of the minor should be obtained as well. Then, the patient needs to be informed of the test results at an appropriate age. Finally, parents or guardians should be advised that, under most circumstances, a request by a mature adolescent for test results should be honored [173].
As clinical screening recommendations for many AYA CPS are now available, it is important to consider the family’s lived experiences and their perceived challenges and benefits associated with cancer screening. Exploration of these issues allows the identification of appropriate supports, which may be important to ensure ongoing adherence with surveillance recommendations. The psychosocial impact specifically related to cancer surveillance in adolescents with CPS may be particularly problematic from a psychosocial perspective, given that adolescents are in a key developmental stage of life during which uncertainty about their health is less easily managed [175]. A study looking at the experience of adolescents with hereditary cancer predisposition found that self-concept is influenced, but not defined by tumor risk, and that having this diagnosis allowed for new perspectives on health and illness [176]. The family narrative, or the experiences the family has had with the CPS, has been shown to be an important predictor of one’s personal risk perception [177•]. Therefore, the opportunity to receive accurate, updated medical information at regular intervals is important, particularly as adolescents reach an age when they will assume responsibility for their own health [178].
Conclusions
The identification of germline pathogenic variants in AYA (summarized in Fig. 1) is especially critical given risk of second primary cancers, need for appropriate long-term surveillance, potential reproductive implications, and cascade testing of at-risk family members, and potentially provides novel biomarkers and therapeutic targets. Among young adults with early-onset phenotypes of malignancies typically presenting at later ages, the increased prevalence of germline PVs supports a role for genetic testing irrespective of tumor type.
Availability of Data and Material
Not applicable
Code Availability
Not applicable
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
In: Closing the Gap: Research and care imperatives for adolescents and young adults with cancer. https://www.cancer.gov/types/aya/research/ayao-august-2006.pdf. Accessed 27 Sep 2021.
Saloustros E, Stark DP, Michailidou K, Mountzios G, Brugieres L, Peccatori FA, Jezdic S, Essiaf S, Douillard JY, Bielack S. The care of adolescents and young adults with cancer: results of the ESMO/SIOPE survey. ESMO Open. 2017;2(4):e000252. https://doi.org/10.1136/esmoopen-2017-000252.
Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–59. https://doi.org/10.3322/caac.21637.
• Ferrari A, Stark D, Peccatori FA, Fern L, Laurence V, Gaspar N, et al. Adolescents and young adults (AYA) with cancer: a position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open. 2021;6(2):100096. https://doi.org/10.1016/j.esmoop.2021.100096 This manuscript reflects the position of ESMO (European Society for Medical Oncology)-SIOPE (European Society for Paediatric Oncology) AYA Working Group regarding current AYA cancer care, the challenges to be addressed, and possible solutions.
Tricoli JV, Blair DG, Anders CK, Bleyer WA, Boardman LA, Khan J, et al. Biologic and clinical characteristics of adolescent and young adult cancers: Acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma, and sarcoma. Cancer. 2016;122(7):1017–28. https://doi.org/10.1002/cncr.29871.
Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. Biology and Clinical Trials Subgroups of the US National Cancer Institute Progress Review Group in Adolescent and Young Adult Oncology. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288–98. https://doi.org/10.1038/nrc2349.
Ferrari A, Barr RD. International evolution in AYA oncology: Current status and future expectations. Pediatr Blood Cancer. 2017;64(9). https://doi.org/10.1002/pbc.26528.
Barr RD, Ferrari A, Ries L, Whelan J, Bleyer WA. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr. 2016;170(5):495–501. https://doi.org/10.1001/jamapediatrics.2015.4689.
Ferrari A, Thomas D, Franklin AR, Hayes-Lattin BM, Mascarin M, van der Graaf W, Albritton KH. Starting an adolescent and young adult program: some success stories and some obstacles to overcome. J Clin Oncol. 2010;28(32):4850–7. https://doi.org/10.1200/JCO.2009.23.8097.
•• Akhavanfard S, Padmanabhan R, Yehia L, Cheng F, Eng C. Comprehensive germline genomic profiles of children, adolescents and young adults with solid tumors. Nat Commun. 2020;11(1):2206. https://doi.org/10.1038/s41467-020-16067-1 This article investigates germline genomic signatures of 1507 patients with solid tumors diagnosed under 29 years of age showing that 12% of these patients carried germline pathogenic and/or likely pathogenic variants in known cancer-predisposing genes.
Gröbner SN, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva VA, et al. The landscape of genomic alterations across childhood cancers. Nature. 2018;555(7696):321–7. https://doi.org/10.1038/nature25480.
Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med. 2015;373(24):2336–46. https://doi.org/10.1056/NEJMoa1508054.
Parsons DW, Roy A, Yang Y, Wang T, Scollon S, Bergstrom K, et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children With Solid Tumors. JAMA Oncol. 2016;2(5):616–24. https://doi.org/10.1001/jamaoncol.2015.5699.
Mody RJ, Wu YM, Lonigro RJ, Cao X, Roychowdhury S, Vats P, et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA. 2015;314(9):913–25. https://doi.org/10.1001/jama.2015.10080.
• Thavaneswaran S, Rath E, Tucker K, Joshua AM, Hess D, Pinese M, Ballinger ML, Thomas DM. Therapeutic implications of germline genetic findings in cancer. Nat Rev Clin Oncol. 2019;16(6):386–96. https://doi.org/10.1038/s41571-019-0179-3This review summarizes the literature on the therapeutic potential of alterations in the germline genome.
Wang Z, Wilson CL, Easton J, Thrasher A, Mulder H, Liu Q, et al. Genetic Risk for Subsequent Neoplasms Among Long-Term Survivors of Childhood Cancer. J Clin Oncol. 2018;36(20):2078–87. https://doi.org/10.1200/JCO.2018.77.8589.
Chun HH, Gatti RA. Ataxia-telangiectasia, an evolving phenotype. DNA Repair (Amst). 2004;3(8-9):1187–96. https://doi.org/10.1016/j.dnarep.2004.04.010.
Suarez F, Mahlaoui N, Canioni D, Andriamanga C, Dubois d'Enghien C, Brousse N, et al. Incidence, presentation, and prognosis of malignancies in ataxia-telangiectasia: a report from the French national registry of primary immune deficiencies. J Clin Oncol. 2015;33(2):202–8. https://doi.org/10.1200/JCO.2014.56.5101.
Prokopcova J, Kleibl Z, Banwell CM, Pohlreich P. The role of ATM in breast cancer development. Breast Cancer Res Treat. 2007;104(2):121–8. https://doi.org/10.1007/s10549-006-9406-6.
Toss A, Tenedini E, Piombino C, Venturelli M, Marchi I, Gasparini E, et al. Clinicopathologic Profile of Breast Cancer in Germline ATM and CHEK2 Mutation Carriers. Genes (Basel). 2021;12(5):616. https://doi.org/10.3390/genes12050616.
Maxwell KN, Wubbenhorst B, D'Andrea K, Garman B, Long JM, Powers J, et al. Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet Med. 2015;17(8):630–8. https://doi.org/10.1038/gim.2014.176.
Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016;2(4):482–90. https://doi.org/10.1001/jamaoncol.2015.5495.
Kurian AW, Ward KC, Howlader N, Deapen D, Hamilton AS, Mariotto A, et al. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J Clin Oncol. 2019;37(15):1305–15. https://doi.org/10.1200/JCO.18.01854.
Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA. 2018;319(23):2401–9. https://doi.org/10.1001/jama.2018.6228.
Pilié PG, Johnson AM, Hanson KL, Dayno ME, Kapron AL, Stoffel EM, Cooney KA. Germline genetic variants in men with prostate cancer and one or more additional cancers. Cancer. 2017;123(20):3925–32. https://doi.org/10.1002/cncr.30817.
O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33(4):497–501. https://doi.org/10.1038/ng1129.
Tanaka A, Weinel S, Nagy N, O'Driscoll M, Lai-Cheong JE, Kulp-Shorten CL, et al. Germline mutation in ATR in autosomal-dominant oropharyngeal cancer syndrome. Am J Hum Genet. 2012;90(3):511–7. https://doi.org/10.1016/j.ajhg.2012.01.007.
Heikkinen K, Mansikka V, Karppinen SM, Rapakko K, Winqvist R. Mutation analysis of the ATR gene in breast and ovarian cancer families. Breast Cancer Res. 2005;7(4):R495–501. https://doi.org/10.1186/bcr1037.
Durocher F, Labrie Y, Soucy P, Sinilnikova O, Labuda D, Bessette P, et al. Mutation analysis and characterization of ATR sequence variants in breast cancer cases from high-risk French Canadian breast/ovarian cancer families. BMC Cancer. 2006;6:230. https://doi.org/10.1186/1471-2407-6-230.
Suszynska M, Kozlowski P. Summary of BARD1 Mutations and Precise Estimation of Breast and Ovarian Cancer Risks Associated with the Mutations. Genes (Basel). 2020;11(7):798. https://doi.org/10.3390/genes11070798.
Breast Cancer Association Consortium, Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C, Wahlström C, et al. Breast Cancer Risk Genes - Association Analysis in More than 113,000 Women. N Engl J Med. 2021;384(5):428–39. https://doi.org/10.1056/NEJMoa1913948.
Weber-Lassalle N, Borde J, Weber-Lassalle K, Horváth J, Niederacher D, Arnold N, et al. Germline loss-of-function variants in the BARD1 gene are associated with early-onset familial breast cancer but not ovarian cancer. Breast Cancer Res. 2019;21(1):55. https://doi.org/10.1186/s13058-019-1137-9.
Hu C, Polley EC, Yadav S, Lilyquist J, Shimelis H, Na J, et al. The Contribution of Germline Predisposition Gene Mutations to Clinical Subtypes of Invasive Breast Cancer From a Clinical Genetic Testing Cohort. J Natl Cancer Inst. 2020;112(12):1231–41. https://doi.org/10.1093/jnci/djaa023.
Rofes P, Del Valle J, Torres-Esquius S, Feliubadaló L, Stradella A, Moreno-Cabrera JM, et al. BARD1 Pathogenic Variants are Associated with Triple-Negative Breast Cancer in a Spanish Hereditary Breast and Ovarian Cancer Cohort. Genes (Basel). 2021;12(2):150. https://doi.org/10.3390/genes12020150.
Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. 1998 Sep 4 [Updated 2016 Dec 15]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®. Seattle: University of Washington; 1993-2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1247/
Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer. 2000;83(10):1301–8. https://doi.org/10.1054/bjoc.2000.1407.
Manickam K, Buchanan AH, Schwartz MLB, Hallquist MLG, Williams JL, Rahm AK, et al. Exome Sequencing-Based Screening for BRCA1/2 Expected Pathogenic Variants Among Adult Biobank Participants. JAMA Netw Open. 2018;1(5):e182140. https://doi.org/10.1001/jamanetworkopen.2018.2140.
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317(23):2402–16. https://doi.org/10.1001/jama.2017.7112.
Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(23):1811–4. https://doi.org/10.1093/jnci/djm203.
Nyberg T, Frost D, Barrowdale D, Evans DG, Bancroft E, Adlard J, et al. Prostate Cancer Risks for Male BRCA1 and BRCA2 Mutation Carriers: A Prospective Cohort Study. Eur Urol. 2020;77(1):24–35. https://doi.org/10.1016/j.eururo.2019.08.025.
Toss A, Venturelli M, Molinaro E, Pipitone S, Barbieri E, Marchi I, et al. Hereditary Pancreatic Cancer: A Retrospective Single-Center Study of 5143 Italian Families with History of BRCA-Related Malignancies. Cancers (Basel). 2019;11(2):193. https://doi.org/10.3390/cancers11020193.
NCCN Guidelines. In: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Accessed 27 Sep 2021.
Cantor S, Drapkin R, Zhang F, Lin Y, Han J, Pamidi S, Livingston DM. The BRCA1-associated protein BACH1 is a DNA helicase targeted by clinically relevant inactivating mutations. Proc Natl Acad Sci U S A. 2004;101(8):2357–62. https://doi.org/10.1073/pnas.0308717101.
Weber-Lassalle N, Hauke J, Ramser J, Richters L, Groß E, Blümcke B, et al. BRIP1 loss-of-function mutations confer high risk for familial ovarian cancer, but not familial breast cancer. Breast Cancer Res. 2018;20(1):7. https://doi.org/10.1186/s13058-018-0935-9.
Easton DF, Lesueur F, Decker B, Michailidou K, Li J, Allen J, et al. No evidence that protein truncating variants in BRIP1 are associated with breast cancer risk: implications for gene panel testing. J Med Genet. 2016;53(5):298–309. https://doi.org/10.1136/jmedgenet-2015-103529.
Ramus SJ, Song H, Dicks E, Tyrer JP, Rosenthal AN, Intermaggio MP, et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women With Ovarian Cancer. J Natl Cancer Inst. 2015;107(11):djv214. https://doi.org/10.1093/jnci/djv214.
Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43(9):879–82. https://doi.org/10.1038/ng.893.
Loveday C, Turnbull C, Ruark E, Xicola RM, Ramsay E, Hughes D, et al. Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat Genet. 2012;44(5):475–6; author reply 476. https://doi.org/10.1038/ng.2224.
Yang X, Song H, Leslie G, Engel C, Hahnen E, Auber B, et al. Ovarian and Breast Cancer Risks Associated With Pathogenic Variants in RAD51C and RAD51D. J Natl Cancer Inst. 2020;112(12):1242–50. https://doi.org/10.1093/jnci/djaa030.
Schmidt MK, Hogervorst F, van Hien R, Cornelissen S, Broeks A, Adank MA, et al. Age- and Tumor Subtype-Specific Breast Cancer Risk Estimates for CHEK2*1100delC Carriers. J Clin Oncol. 2016;34(23):2750–60. https://doi.org/10.1200/JCO.2016.66.5844.
Desrichard A, Bidet Y, Uhrhammer N, Bignon YJ. CHEK2 contribution to hereditary breast cancer in non-BRCA families. Breast Cancer Res. 2011;13(6):R119. https://doi.org/10.1186/bcr3062.
Margolin S, Eiberg H, Lindblom A, Bisgaard ML. CHEK2 1100delC is prevalent in Swedish early onset familial breast cancer. BMC Cancer. 2007;17(7):163. https://doi.org/10.1186/1471-2407-7-163.
Greville-Heygate SL, Maishman T, Tapper WJ, Cutress RI, Copson E, Dunning AM, et al. Pathogenic Variants in CHEK2 Are Associated With an Adverse Prognosis in Symptomatic Early-Onset Breast Cancer. JCO Precis Oncol. 2020;4:PO.19.00178. https://doi.org/10.1200/PO.19.00178.
AlDubayan SH, Pyle LC, Gamulin M, Kulis T, Moore ND, Taylor-Weiner A, et al. Regeneron Genetics Center (RGC) Research Team. Association of Inherited Pathogenic Variants in Checkpoint Kinase 2 (CHEK2) With Susceptibility to Testicular Germ Cell Tumors. JAMA Oncol. 2019;5(4):514–22. https://doi.org/10.1001/jamaoncol.2018.6477.
Cybulski C, Górski B, Huzarski T, Masojć B, Mierzejewski M, Debniak T, et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004;75(6):1131–5. https://doi.org/10.1086/426403.
Rahman N, Seal S, Thompson D, Kelly P, Renwick A, Elliott A. et al; Breast Cancer Susceptibility Collaboration (UK), Easton DF, Stratton MR. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39(2):165–7. https://doi.org/10.1038/ng1959.
Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkäs K, Roberts J, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371(6):497–506. https://doi.org/10.1056/NEJMoa1400382.
Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324(5924):217. https://doi.org/10.1126/science.1171202.
Mamrak NE, Shimamura A, Howlett NG. Recent discoveries in the molecular pathogenesis of the inherited bone marrow failure syndrome Fanconi anemia. Blood Rev. 2017;31(3):93–9. https://doi.org/10.1016/j.blre.2016.10.002.
Asur RS, Kimble DC, Lach FP, Jung M, Donovan FX, Kamat A, et al. Somatic mosaicism of an intragenic FANCB duplication in both fibroblast and peripheral blood cells observed in a Fanconi anemia patient leads to milder phenotype. Mol Genet Genomic Med. 2018;6(1):77–91. https://doi.org/10.1002/mgg3.350.
Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150(2):179–88. https://doi.org/10.1111/j.1365-2141.2010.08212.x.
Alter BP. Fanconi anemia and the development of leukemia. Best Pract Res Clin Haematol. 2014;27(3-4):214–21. https://doi.org/10.1016/j.beha.2014.10.002.
Del Valle J, Rofes P, Moreno-Cabrera JM, López-Dóriga A, Belhadj S, Vargas-Parra G, Teulé À, Cuesta R, Muñoz X, Campos O, Salinas M, de Cid R, Brunet J, González S, Capellá G, Pineda M, Feliubadaló L, Lázaro C. Exploring the Role of Mutations in Fanconi Anemia Genes in Hereditary Cancer Patients. Cancers (Basel). 2020;12(4):829. https://doi.org/10.3390/cancers12040829.
Kashiyama K, Nakazawa Y, Pilz DT, Guo C, Shimada M, Sasaki K, et al. Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia. Am J Hum Genet. 2013;92(5):807–19. https://doi.org/10.1016/j.ajhg.2013.04.007.
Mori T, Yousefzadeh MJ, Faridounnia M, Chong JX, Hisama FM, Hudgins L. et al; University of Washington Center for Mendelian Genomics, Niedernhofer LJ, Oshima J. ERCC4 variants identified in a cohort of patients with segmental progeroid syndromes. Hum Mutat. 2018;39(2):255–65. https://doi.org/10.1002/humu.23367.
Cleaver JE. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nat Rev Cancer. 2005;5(7):564–73. https://doi.org/10.1038/nrc1652.
Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45(2):136–44. https://doi.org/10.1038/ng.2503.
Hansen MF, Johansen J, Bjørnevoll I, Sylvander AE, Steinsbekk KS, Sætrom P, Sandvik AK, Drabløs F, Sjursen W. A novel POLE mutation associated with cancers of colon, pancreas, ovaries and small intestine. Familial Cancer. 2015;14(3):437–48. https://doi.org/10.1007/s10689-015-9803-2.
Rohlin A, Zagoras T, Nilsson S, Lundstam U, Wahlström J, Hultén L, et al. A mutation in POLE predisposing to a multi-tumour phenotype. Int J Oncol. 2014;45(1):77–81. https://doi.org/10.3892/ijo.2014.2410.
Aoude LG, Heitzer E, Johansson P, Gartside M, Wadt K, Pritchard AL, et al. POLE mutations in families predisposed to cutaneous melanoma. Familial Cancer. 2015;14(4):621–8. https://doi.org/10.1007/s10689-015-9826-8.
Li X, Liu G, Wu W. Recent advances in Lynch syndrome. Exp Hematol Oncol. 2021;10(1):37. https://doi.org/10.1186/s40164-021-00231-4.
Dominguez-Valentin M, Sampson JR, Seppälä TT, Ten Broeke SW, Plazzer JP, Nakken S, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med. 2020;22(1):15–25. https://doi.org/10.1038/s41436-019-0596-9.
NCCN Guidelines. In: Genetic/Familial High-Risk Assessment: Colorectal. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Accessed 27 Sep 2021.
Piombino C, Cortesi L, Lambertini M, Punie K, Grandi G, Toss A. Secondary Prevention in Hereditary Breast and/or Ovarian Cancer Syndromes Other Than BRCA. J Oncol. 2020;14(2020):6384190. https://doi.org/10.1155/2020/6384190.
Cybulski C, Carrot-Zhang J, Kluźniak W, Rivera B, Kashyap A, Wokołorczyk D, et al. Germline RECQL mutations are associated with breast cancer susceptibility. Nat Genet. 2015;47(6):643–6. https://doi.org/10.1038/ng.3284.
Sun J, Wang Y, Xia Y, Xu Y, Ouyang T, Li J, et al. Mutations in RECQL Gene Are Associated with Predisposition to Breast Cancer. PLoS Genet. 2015;11(5):e1005228. https://doi.org/10.1371/journal.pgen.1005228.
Rogoża-Janiszewska E, Malińska K, Cybulski C, Jakubowska A, Gronwald J, Huzarski T, On Behalf Of The Polish Hereditary Breast Cancer Consortium, et al. Prevalence of Recurrent Mutations Predisposing to Breast Cancer in Early-Onset Breast Cancer Patients from Poland. Cancers (Basel). 2020;12(8):2321. https://doi.org/10.3390/cancers12082321.
Bowden AR, Tischkowitz M. Clinical implications of germline mutations in breast cancer genes: RECQL. Breast Cancer Res Treat. 2019;174(3):553–60. https://doi.org/10.1007/s10549-018-05096-6.
Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet. 2013;14:355–69. https://doi.org/10.1146/annurev-genom-091212-153523.
Kratz CP, Rapisuwon S, Reed H, Hasle H, Rosenberg PS. Cancer in Noonan, Costello, cardiofaciocutaneous and LEOPARD syndromes. Am J Med Genet C: Semin Med Genet. 2011;157C(2):83–9. https://doi.org/10.1002/ajmg.c.30300.
Villani A, Greer MC, Kalish JM, Nakagawara A, Nathanson KL, Pajtler KW, et al. Recommendations for Cancer Surveillance in Individuals with RASopathies and Other Rare Genetic Conditions with Increased Cancer Risk. Clin Cancer Res. 2017;23(12):e83–90. https://doi.org/10.1158/1078-0432.CCR-17-0631.
Kratz CP, Franke L, Peters H, Kohlschmidt N, Kazmierczak B, Finckh U, et al. Cancer spectrum and frequency among children with Noonan, Costello, and cardio-facio-cutaneous syndromes. Br J Cancer. 2015;112(8):1392–7. https://doi.org/10.1038/bjc.2015.75.
McWilliams GD, SantaCruz K, Hart B, Clericuzio C. Occurrence of DNET and other brain tumors in Noonan syndrome warrants caution with growth hormone therapy. Am J Med Genet A. 2016;170A(1):195–201. https://doi.org/10.1002/ajmg.a.37379.
Jongmans MC, van der Burgt I, Hoogerbrugge PM, Noordam K, Yntema HG, Nillesen WM, et al. Cancer risk in patients with Noonan syndrome carrying a PTPN11 mutation. Eur J Hum Genet. 2011;19(8):870–4. https://doi.org/10.1038/ejhg.2011.37.
Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37(10):1038–40. https://doi.org/10.1038/ng1641.
McCormick EM, Hopkins E, Conway L, Catalano S, Hossain J, Sol-Church K, et al. Assessing genotype-phenotype correlation in Costello syndrome using a severity score. Genet Med. 2013;15(7):554–7. https://doi.org/10.1038/gim.2013.6.
Gripp KW. Tumor predisposition in Costello syndrome. Am J Med Genet C: Semin Med Genet. 2005;137C(1):72–7. https://doi.org/10.1002/ajmg.c.30065.
Kerr B, Delrue MA, Sigaudy S, Perveen R, Marche M, Burgelin I, et al. Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet. 2006;43(5):401–5. https://doi.org/10.1136/jmg.2005.040352.
Evans DGR, Salvador H, Chang VY, Erez A, Voss SD, Schneider KW, et al. Cancer and Central Nervous System Tumor Surveillance in Pediatric Neurofibromatosis 1. Clin Cancer Res. 2017;23(12):e46–53. https://doi.org/10.1158/1078-0432.CCR-17-0589.
Uusitalo E, Rantanen M, Kallionpää RA, Pöyhönen M, Leppävirta J, Ylä-Outinen H, et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J Clin Oncol. 2016;34(17):1978–86. https://doi.org/10.1200/JCO.2015.65.3576.
Helgadottir H, Höiom V, Tuominen R, Nielsen K, Jönsson G, Olsson H, Hansson J. Germline CDKN2A Mutation Status and Survival in Familial Melanoma Cases. J Natl Cancer Inst. 2016;10:108(11). https://doi.org/10.1093/jnci/djw135.
Hansson J. Familial cutaneous melanoma. Adv Exp Med Biol. 2010;685:134–45. https://doi.org/10.1007/978-1-4419-6448-9_13.
Kefford R, Bishop JN, Tucker M, Bressac-de Paillerets B, Bianchi-Scarrá G, Bergman W, et al. Melanoma Genetics Consortium. Genetic testing for melanoma. Lancet Oncol. 2002;3(11):653–4. https://doi.org/10.1016/s1470-2045(02)00894-x.
Bishop DT, Demenais F, Goldstein AM, Bergman W, Bishop JN, Bressac-de Paillerets B, et al. Melanoma Genetics Consortium. Geographical variation in the penetrance of CDKN2A mutations for melanoma. J Natl Cancer Inst. 2002;94(12):894–903. https://doi.org/10.1093/jnci/94.12.894.
Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, et al. Lund Melanoma Study Group; Melanoma Genetics Consortium (GenoMEL). Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet. 2007;44(2):99–106. https://doi.org/10.1136/jmg.2006.043802.
Middlebrooks CD, Stacey ML, Li Q, Snyder C, Shaw TG, Richardson-Nelson T, et al. Analysis of the CDKN2A Gene in FAMMM Syndrome Families Reveals Early Age of Onset for Additional Syndromic Cancers. Cancer Res. 2019;79(11):2992–3000. https://doi.org/10.1158/0008-5472.CAN-18-1580.
Ming Z, Lim SY, Rizos H. Genetic Alterations in the INK4a/ARF Locus: Effects on Melanoma Development and Progression. Biomolecules. 2020;10(10):1447. https://doi.org/10.3390/biom10101447.
Astiazaran-Symonds E, Goldstein AM. A systematic review of the prevalence of germline pathogenic variants in patients with pancreatic cancer. J Gastroenterol. 2021;56(8):713–21. https://doi.org/10.1007/s00535-021-01806-y.
McBride KA, Ballinger ML, Killick E, Kirk J, Tattersall MH, Eeles RA, et al. Li-Fraumeni syndrome: cancer risk assessment and clinical management. Nat Rev Clin Oncol. 2014;11(5):260–71. https://doi.org/10.1038/nrclinonc.2014.41.
Frebourg T, Bajalica Lagercrantz S, Oliveira C, Magenheim R, Evans DG. European Reference Network GENTURIS. Guidelines for the Li-Fraumeni and heritable TP53-related cancer syndromes. Eur J Hum Genet. 2020;28(10):1379–86. https://doi.org/10.1038/s41431-020-0638-4.
Hwang SJ, Lozano G, Amos CI, Strong LC. Germline p53 mutations in a cohort with childhood sarcoma: sex differences in cancer risk. Am J Hum Genet. 2003;72(4):975–83. https://doi.org/10.1086/374567.
Fortuno C, James PA, Spurdle AB. Current review of TP53 pathogenic germline variants in breast cancer patients outside Li-Fraumeni syndrome. Hum Mutat. 2018;39(12):1764–73. https://doi.org/10.1002/humu.23656.
Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology. 2013;45(2):116–26. https://doi.org/10.1097/PAT.0b013e32835d0efb.
Njauw CN, Kim I, Piris A, Gabree M, Taylor M, Lane AM, et al. Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PLoS One. 2012;7(4):e35295. https://doi.org/10.1371/journal.pone.0035295.
Zauderer MG, Jayakumaran G, DuBoff M, Zhang L, Francis JH, Abramson DH, et al. Prevalence and Preliminary Validation of Screening Criteria to Identify Carriers of Germline BAP1 Mutations. J Thorac Oncol. 2019;14(11):1989–94. https://doi.org/10.1016/j.jtho.2019.07.002.
Walpole S, Pritchard AL, Cebulla CM, Pilarski R, Stautberg M, Davidorf FH, et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. J Natl Cancer Inst. 2018;110(12):1328–41. https://doi.org/10.1093/jnci/djy171.
Grigorie D, Sucaliuc A, Ciuffi S, Franceschelli F, Marini F, Ioachim D, Terzea D, Brandi MLL. High risk of parathyroid carcinoma and genetic screening in the first diagnosed romanian family with hyperparathyroidism-jaw tumor syndrome and a germline mutation of the cdc73 gene. Acta Endocrinol (Buchar). 2019;15(3):398–403. https://doi.org/10.4183/aeb.2019.398.
Li Y, Zhang J, Adikaram PR, Welch J, Guan B, Weinstein LS, et al. Genotype of CDC73 germline mutation determines risk of parathyroid cancer. Endocr Relat Cancer. 2020;27(9):483–94. https://doi.org/10.1530/ERC-20-0149.
Hyde SM, Rich TA, Waguespack SG, et al. CDC73-Related Disorders. 2008 Dec 31 [Updated 2018 Apr 26]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®. Seattle: University of Washington; 1993-2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK3789/
Bradley KJ, Hobbs MR, Buley ID, Carpten JD, Cavaco BM, Fares JE, et al. Uterine tumours are a phenotypic manifestation of the hyperparathyroidism-jaw tumour syndrome. J Intern Med. 2005;257(1):18–26. https://doi.org/10.1111/j.1365-2796.2004.01421.x.
Ohtsuka K, Ohnishi H, Kurai D, Matsushima S, Morishita Y, Shinonaga M, Goto H, Watanabe T. Familial lung adenocarcinoma caused by the EGFR V843I germ-line mutation. J Clin Oncol. 2011;29(8):e191–2. https://doi.org/10.1200/JCO.2010.31.4492.
Prudkin L, Tang X, Wistuba II. Germ-line and somatic presentations of the EGFR T790M mutation in lung cancer. J Thorac Oncol. 2009;4(1):139–41. https://doi.org/10.1097/JTO.0b013e3181915f92.
Tibaldi C, Giovannetti E, Vasile E, Boldrini L, Gallegos-Ruiz MI, Bernardini I, et al. Inherited germline T790M mutation and somatic epidermal growth factor receptor mutations in non-small cell lung cancer patients. J Thorac Oncol. 2011;6(2):395–6. https://doi.org/10.1097/JTO.0b013e3182059a6f.
van Noesel J, van der Ven WH, van Os TA, Kunst PW, Weegenaar J, Reinten RJ, Kancha RK, Duyster J, van Noesel CJ. Activating germline R776H mutation in the epidermal growth factor receptor associated with lung cancer with squamous differentiation. J Clin Oncol. 2013;31(10):e161–4. https://doi.org/10.1200/JCO.2012.42.1586.
Gazdar A, Robinson L, Oliver D, Xing C, Travis WD, Soh J, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J Thorac Oncol. 2014;9(4):456–63. https://doi.org/10.1097/JTO.0000000000000130.
Reckamp KL, Behrendt CE, Slavin TP, Gray SW, Castillo DK, Koczywas MC, et al. Germline mutations and age at onset of lung adenocarcinoma. Cancer. 2021;127(15):2801–6. https://doi.org/10.1002/cncr.33573.
Akhavanfard S, Yehia L, Padmanabhan R, Reynolds JP, Ni Y, Eng C. Germline EGFR variants are over-represented in adolescents and young adults (AYA) with adrenocortical carcinoma. Hum Mol Genet. 2021;29(22):3679–90. https://doi.org/10.1093/hmg/ddaa268.
Kempers MJ, Kuiper RP, Ockeloen CW, Chappuis PO, Hutter P, Rahner N, et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. Lancet Oncol. 2011;12(1):49–55. https://doi.org/10.1016/S1470-2045(10)70265-5.
Ke H, Kazi JU, Zhao H, Sun J. Germline mutations of KIT in gastrointestinal stromal tumor (GIST) and mastocytosis. Cell Biosci. 2016;18(6):55. https://doi.org/10.1186/s13578-016-0120-8.
Beghini A, Tibiletti MG, Roversi G, Chiaravalli AM, Serio G, Capella C, Larizza L. Germline mutation in the juxtamembrane domain of the kit gene in a family with gastrointestinal stromal tumors and urticaria pigmentosa. Cancer. 2001;92(3):657–62. https://doi.org/10.1002/1097-0142(20010801)92:3<657::aid-cncr1367>3.0.co;2-d.
Wells SA Jr, Pacini F, Robinson BG, Santoro M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab. 2013;98(8):3149–64. https://doi.org/10.1210/jc.2013-1204.
Krampitz GW, Norton JA. RET gene mutations (genotype and phenotype) of multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma. Cancer. 2014;120(13):1920–31. https://doi.org/10.1002/cncr.28661.
Chompret A, Kannengiesser C, Barrois M, Terrier P, Dahan P, Tursz T, et al. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004;126(1):318–21. https://doi.org/10.1053/j.gastro.2003.10.079.
Pasini B, Matyakhina L, Bei T, Muchow M, Boikos S, Ferrando B, et al. Multiple gastrointestinal stromal and other tumors caused by platelet-derived growth factor receptor alpha gene mutations: a case associated with a germline V561D defect. J Clin Endocrinol Metab. 2007;92(9):3728–32. https://doi.org/10.1210/jc.2007-0894.
Manley PN, Abu-Abed S, Kirsch R, Hawrysh A, Perrier N, Feilotter H, et al. Familial PDGFRA-mutation syndrome: somatic and gastrointestinal phenotype. Hum Pathol. 2018;76:52–7. https://doi.org/10.1016/j.humpath.2018.02.014.
Ben Aim L, Pigny P, Castro-Vega LJ, Buffet A, Amar L, Bertherat J, et al. Targeted next-generation sequencing detects rare genetic events in pheochromocytoma and paraganglioma. J Med Genet. 2019;56(8):513–20. https://doi.org/10.1136/jmedgenet-2018-105714.
Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, et al. Multiple Leiomyoma Consortium. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30(4):406–10. https://doi.org/10.1038/ng849.
Wong MH, Tan CS, Lee SC, Yong Y, Ooi AS, Ngeow J, Tan MH. Potential genetic anticipation in hereditary leiomyomatosis-renal cell cancer (HLRCC). Familial Cancer. 2014;13(2):281–9. https://doi.org/10.1007/s10689-014-9703-x.
Kopp RP, Stratton KL, Glogowski E, Schrader KA, Rau-Murthy R, Russo P, et al. Utility of prospective pathologic evaluation to inform clinical genetic testing for hereditary leiomyomatosis and renal cell carcinoma. Cancer. 2017;123(13):2452–8. https://doi.org/10.1002/cncr.30605.
Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, Salovaara R, Herva R, Koivisto PA, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43(6):523–6. https://doi.org/10.1136/jmg.2005.036400.
Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–21. https://doi.org/10.1016/j.eururo.2011.06.049.
Washecka R, Hanna M. Malignant renal tumors in tuberous sclerosis. Urology. 1991;37(4):340–3. https://doi.org/10.1016/0090-4295(91)80261-5.
Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42(3):229–33. https://doi.org/10.1038/ng.533.
Qin Y, Deng Y, Ricketts CJ, Srikantan S, Wang E, Maher ER, Dahia PL. The tumor susceptibility gene TMEM127 is mutated in renal cell carcinomas and modulates endolysosomal function. Hum Mol Genet. 2014;23(9):2428–39. https://doi.org/10.1093/hmg/ddt638.
Wang ZJ, Taylor F, Churchman M, Norbury G, Tomlinson I. Genetic pathways of colorectal carcinogenesis rarely involve the PTEN and LKB1 genes outside the inherited hamartoma syndromes. Am J Pathol. 1998;153(2):363–6. https://doi.org/10.1016/S0002-9440(10)65579-4.
Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119(6):1447–53. https://doi.org/10.1053/gast.2000.20228.
Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18(2):400–7. https://doi.org/10.1158/1078-0432.CCR-11-2283.
Kanth P, Grimmett J, Champine M, Burt R, Samadder NJ. Hereditary Colorectal Polyposis and Cancer Syndromes: A Primer on Diagnosis and Management. Am J Gastroenterol. 2017;112(10):1509–25. https://doi.org/10.1038/ajg.2017.212.
de Marchis ML, Tonelli F, Quaresmini D, Lovero D, Della-Morte D, Silvestris F, Guadagni F, Palmirotta R. Desmoid Tumors in Familial Adenomatous Polyposis. Anticancer Res. 2017;37(7):3357–66. https://doi.org/10.21873/anticanres.11702.
Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015;1(1):23–32. https://doi.org/10.1001/jamaoncol.2014.168 Erratum in: JAMA Oncol. 2015 Apr;1(1):110.
Dossus L, Benusiglio PR. Lobular breast cancer: incidence and genetic and non-genetic risk factors. Breast Cancer Res. 2015;17:37. https://doi.org/10.1186/s13058-015-0546-7.
Schreibman IR, Baker M, Amos C, McGarrity TJ. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005;100(2):476–90. https://doi.org/10.1111/j.1572-0241.2005.40237.x.
Brosens LA, van Hattem A, Hylind LM, Iacobuzio-Donahue C, Romans KE, Axilbund J, et al. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56(7):965–7. https://doi.org/10.1136/gut.2006.116913.
Foulkes WD, Clarke BA, Hasselblatt M, Majewski J, Albrecht S, McCluggage WG. No small surprise - small cell carcinoma of the ovary, hypercalcaemic type, is a malignant rhabdoid tumour. J Pathol. 2014;233(3):209–14. https://doi.org/10.1002/path.4362.
Schneppenheim R, Frühwald MC, Gesk S, Hasselblatt M, Jeibmann A, Kordes U, et al. Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet. 2010;86(2):279–84. https://doi.org/10.1016/j.ajhg.2010.01.013.
Kordes U, Gesk S, Frühwald MC, Graf N, Leuschner I, Hasselblatt M, et al. Clinical and molecular features in patients with atypical teratoid rhabdoid tumor or malignant rhabdoid tumor. Genes Chromosom Cancer. 2010;49(2):176–81. https://doi.org/10.1002/gcc.20729.
Solomon DA, Perry A. 22 - Familial Tumor Syndromes. In: Perry A, Brat DJ, editors. Practical Surgical Neuropathology: A Diagnostic Approach. 2nd ed. Netherlands: Elsevier; 2018. p. 505–45. ISBN 9780323449410. https://doi.org/10.1016/B978-0-323-44941-0.00022-9.
Connor YD, Miao D, Lin DI, et al. Germline mutations of SMARCA4 in small cell carcinoma of the ovary, hypercalcemic type and in SMARCA4-deficient undifferentiated uterine sarcoma: Clinical features of a single family and comparison of large cohorts. Gynecol Oncol. 2020;157(1):106–14. https://doi.org/10.1016/j.ygyno.2019.10.031.
Lin DI, Chudnovsky Y, Duggan B, Zajchowski D, Greenbowe J, Ross JS, et al. Comprehensive genomic profiling reveals inactivating SMARCA4 mutations and low tumor mutational burden in small cell carcinoma of the ovary, hypercalcemic-type. Gynecol Oncol. 2017 Dec;147(3):626–33. https://doi.org/10.1016/j.ygyno.2017.09.031.
Martins VL, Vyas JJ, Chen M, Purdie K, Mein CA, South AP, et al. Increased invasive behaviour in cutaneous squamous cell carcinoma with loss of basement-membrane type VII collagen. J Cell Sci. 2009;122(Pt 11):1788–99. https://doi.org/10.1242/jcs.042895.
Fine JD, Johnson LB, Weiner M, Li KP, Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986-2006. J Am Acad Dermatol. 2009;60(2):203–11. https://doi.org/10.1016/j.jaad.2008.09.035.
Kim J, Schultz KAP, Hill DA, Stewart DR. The prevalence of germline DICER1 pathogenic variation in cancer populations. Mol Genet Genomic Med. 2019;7(3):e555. https://doi.org/10.1002/mgg3.555.
Heddar A, Fermey P, Coutant S, Angot E, Sabourin JC, Michelin P, et al. Familial solitary chondrosarcoma resulting from germline EXT2 mutation. Genes Chromosom Cancer. 2017;56(2):128–34. https://doi.org/10.1002/gcc.22419.
Bovée JV, Cleton-Jansen AM, Wuyts W, Caethoven G, Taminiau AH, Bakker E, et al. EXT-mutation analysis and loss of heterozygosity in sporadic and hereditary osteochondromas and secondary chondrosarcomas. Am J Hum Genet. 1999;65(3):689–98. https://doi.org/10.1086/302532.
Gara SK, Jia L, Merino MJ, Agarwal SK, Zhang L, Cam M, et al. Germline HABP2 Mutation Causing Familial Nonmedullary Thyroid Cancer. N Engl J Med. 2015;373(5):448–55. https://doi.org/10.1056/NEJMoa1502449.
Hińcza K, Kowalik A, Kowalska A. Current Knowledge of Germline Genetic Risk Factors for the Development of Non-Medullary Thyroid Cancer. Genes (Basel). 2019;10(7):482. https://doi.org/10.3390/genes10070482.
Fabišíková K, Hamidová O, Behulová RL, Závodná K, Priščáková P, Repiská V. Case Report: The Role of Molecular Analysis of the MUTYH Gene in Asymptomatic Individuals. Front Genet. 2020;15(11):590486. https://doi.org/10.3389/fgene.2020.590486.
Nielsen M, Infante E, Brand R. MUTYH Polyposis. 2012 Oct 4 [Updated 2021 May 27]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®. Seattle: University of Washington Seattle; 1993-2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK107219/
Win AK, Dowty JG, Cleary SP, Kim H, Buchanan DD, Young JP, et al. Risk of colorectal cancer for carriers of mutations in MUTYH, with and without a family history of cancer. Gastroenterology. 2014;146(5):1208–11.e1-5. https://doi.org/10.1053/j.gastro.2014.01.022.
Hong B, Ma K, Zhou J, Zhang J, Wang J, Liu S, et al. Frequent Mutations of VHL Gene and the Clinical Phenotypes in the Largest Chinese Cohort With Von Hippel-Lindau Disease. Front Genet. 2019;10:867. https://doi.org/10.3389/fgene.2019.00867.
Wang JY, Peng SH, Ning XH, Li T, Liu SJ, Liu JY, Hong BA, Qi NN, Peng X, Zhou BW, Zhang JF, Cai L, Gong K. Shorter telomere length increases age-related tumor risks in von Hippel-Lindau disease patients. Cancer Med. 2017;6(9):2131–41. https://doi.org/10.1002/cam4.1134.
Lancaster JM, Powell CB, Chen LM, Richardson DL. SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3–7. https://doi.org/10.1016/j.ygyno.2014.09.009.
Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, et al. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol. 2015;33(31):3660–7. https://doi.org/10.1200/JCO.2015.63.0996.
Berliner JL, Fay AM, Cummings SA, Burnett B, Tillmanns T. NSGC practice guideline: risk assessment and genetic counseling for hereditary breast and ovarian cancer. J Genet Couns. 2013;22(2):155–63. https://doi.org/10.1007/s10897-012-9547-1.
Weitzel JN, Blazer KR, MacDonald DJ, Culver JO, Offit K. Genetics, genomics, and cancer risk assessment: State of the Art and Future Directions in the Era of Personalized Medicine. CA Cancer J Clin. 2011;61(5):327–59. https://doi.org/10.3322/caac.20128.
Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch Syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–6.
Mai PL, Malkin D, Garber JE, Schiffman JD, Weitzel JN, Strong LC, et al. Li-Fraumeni syndrome: report of a clinical research workshop and creation of a research consortium. Cancer Gene Ther. 2012;205:479–87.
Offit K, Levran O, Mullaney B, Mah K, Nafa K, Batish SD, et al. Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J Natl Cancer Inst. 2003;95(20):1548–51. https://doi.org/10.1093/jnci/djg072.
Baum A, Friedman AL, Zakowski SG. Stress and genetic testing for disease risk. Health Psychol. 1997;16(1):8–19. https://doi.org/10.1037//0278-6133.16.1.8.
Wauters A, Van Hoyweghen I. Global trends on fears and concerns of genetic discrimination: a systematic literature review. J Hum Genet. 2016;61(4):275–82. https://doi.org/10.1038/jhg.2015.151.
Gopie JP, Vasen HF, Tibben A. Surveillance for hereditary cancer: does the benefit outweigh the psychological burden?--A systematic review. Crit Rev Oncol Hematol. 2012;83(3):329–40. https://doi.org/10.1016/j.critrevonc.2012.01.004.
Botkin JR, Belmont JW, Berg JS, Berkman BE, Bombard Y, Holm IA, et al. Points to Consider: Ethical, Legal, and Psychosocial Implications of Genetic Testing in Children and Adolescents. Am J Hum Genet. 2015;97(1):6–21. https://doi.org/10.1016/j.ajhg.2015.05.022 Erratum in: Am J Hum Genet. 2015 Sep 3;97(3):501.
Committee on bioethics; committee on genetics, and; american college of medical genetics and; genomics social; ethical; legal issues committee. Ethical and policy issues in genetic testing and screening of children. Pediatrics. 2013;131(3):620–2. https://doi.org/10.1542/peds.2012-3680.
Informed consent, parental permission, and assent in pediatric practice. Committee on Bioethics, American Academy of Pediatrics. Pediatrics. 1995;95(2):314–7.
Grosfeld FJ, Lips CJ, Beemer FA, Ten Kroode HF. Who Is at Risk for Psychological Distress in Genetic Testing Programs for Hereditary Cancer Disorders? J Genet Couns. 2000;9(3):253–66. https://doi.org/10.1023/A:1009468005966.
Weber E, Shuman C, Wasserman JD, Barrera M, Patenaude AF, Fung K, et al. “A change in perspective”: Exploring the experiences of adolescents with hereditary tumor predisposition. Pediatr Blood Cancer. 2019;66(1):e27445. https://doi.org/10.1002/pbc.27445.
• Young AL, Butow PN, Vetsch J, Quinn VF, Patenaude AF, Tucker KM, Wakefield CE. Family Communication, Risk Perception and Cancer Knowledge of Young Adults from BRCA1/2 Families: a Systematic Review. J Genet Couns. 2017;26(6):1179–96. https://doi.org/10.1007/s10897-017-0125-4This systematic review summarizes the literature on BRCA1/2 family risk communication and hereditary cancer knowledge in the young adult population.
Forbes Shepherd R, Lewis A, Keogh LA, Werner-Lin A, Delatycki MB, Forrest LE. A Systematic Review of How Young People Live with Inherited Disease: What Can We Learn for Li-Fraumeni Syndrome? J Adolesc Young Adult Oncol. 2018;7(5):525–45. https://doi.org/10.1089/jayao.2018.0028.
Funding
Giuseppe Luigi Banna’s work is supported by “FPRC 5xmille Ministero Salute 2017 PTCRC-Intra 2020 CTU-Lung.”
Author information
Authors and Affiliations
Contributions
A.T. and P.Q. contributed in conception/design of the review; A.T. and P.Q. contributed in data acquisition; A.T., P.Q., M.M., G.B., M.Z., S.C., F.A.P., and A.F. contributed in drafting the work or revising it critically for important intellectual content; all the authors gave final approval for the version of the study to be published.
Corresponding author
Ethics declarations
Conflict of Interest
Angela Toss declares that she has no conflict of interest. Paola Quarello declares that she has no conflict of interest. Maurizio Mascarin has received compensation for advisory role from Merck Sharp & Dohme. Giuseppe Luigi Banna has received compensation for service as a consultant from AstraZeneca and Astellas, has received speaker’s honoraria from AstraZeneca and Astellas, and has received reimbursement for travel/accommodations from AstraZeneca. Marco Zecca declares that he has no conflict of interest. Saverio Cinieri declares that she has no conflict of interest. Fedro Alessandro Peccatori declares that he has no conflict of interest. Andrea Ferrari declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Oncology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toss, A., Quarello, P., Mascarin, M. et al. Cancer Predisposition Genes in Adolescents and Young Adults (AYAs): a Review Paper from the Italian AYA Working Group. Curr Oncol Rep 24, 843–860 (2022). https://doi.org/10.1007/s11912-022-01213-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01213-3