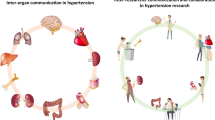
Abstract
Purpose of Review
The study aims to capture the history and lineage of hypertension researchers from the University of Toledo in Ohio and showcase their collective scientific contributions dating from their initial discoveries of the physiology of adrenal and renal systems and genetics regulating blood pressure (BP) to its more contemporary contributions including microbiota and metabolomic links to BP regulation.
Recent Findings
The University of Toledo College of Medicine and Life Sciences (UTCOMLS), previously known as the Medical College of Ohio, has contributed significantly to our understanding of the etiology of hypertension. Two of the scientists, Patrick Mulrow and John Rapp from UTCOMLS, have been recognized with the highest honor, the Excellence in Hypertension award from the American Heart Association for their pioneering work on the physiology and genetics of hypertension, respectively. More recently, Bina Joe has continued their legacy in the basic sciences by uncovering previously unknown novel links between microbiota and metabolites to the etiology of hypertension, work that has been recognized by the American Heart Association with multiple awards. On the clinical research front, Christopher Cooper and colleagues lead the CORAL trials and contributed importantly to the investigations on renal artery stenosis treatment paradigms. Hypertension research at this institution has not only provided these pioneering insights, but also grown careers of scientists as leaders in academia as University Presidents and Deans of Medical Schools. Through the last decade, the university has expanded its commitment to Hypertension research as evident through the development of the Center for Hypertension and Precision Medicine led by Bina Joe as its founding Director.
Summary
Hypertension being the top risk factor for cardiovascular diseases, which is the leading cause of human mortality, is an important area of research in multiple international universities. The UTCOMLS is one such university which, for the last 6 decades, has made significant contributions to our current understanding of hypertension. This review is a synthesis of this rich history. Additionally, it also serves as a collection of audio archives by more recent faculty who are also prominent leaders in the field of hypertension research, including John Rapp, Bina Joe, and Christopher Cooper, which are cataloged at Interviews.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Founding and Biomedical Research at the Medical College of Ohio (MCO)
The University of Toledo College of Medicine and Life Sciences (UTCOMLS) (https://www.utoledo.edu/med/) was originally known as the Medical College of Ohio (MCO). MCO was founded in 1964 but its first class of medical students began their studies in 1969 [1]. MCO has a history of innovative researchers in the cardiovascular field since its founding in 1964 (Figs. 1, 2, and Table 1). MCO was Northwest Ohio’s first independent medical school since the closure of the Toledo Medical College (1882–1914) (https://www.utoledo.edu/library/canaday/findingaids1/UM_68.pdf).
The foundations of basic cardiovascular research at MCO were laid by Murray Saffran, Ph.D., a renowned neuroendocrinologist [2,3,4,5,6] who arrived in 1969 and was the founder of the Biochemistry Department. Before arriving at MCO, Saffran worked at McGill University, Montreal, Canada, where he helped uncover the role of cortisol as a regulator of the body’s response to stress [2,3,4,5,6]. At MCO, Saffran continued his endocrinology research and explored the role of insulin in the vascular complications of diabetes [7,8,9,10,11]. Throughout the 1970s, he laid the foundation for a world-class cardiovascular and biochemical research department.
Also in 1970, George D. Ludwig, M.D., from the University of Pennsylvania joined MCO in 1970 as the founding chairman of the Department of Medicine. Although Ludwig’s expertise was on metabolic, endocrine, and molecular diseases with contributions in the areas of the abnormal heme, porphyrin, indoles, calcium-phosphate metabolism, parathyroid diseases, and inborn errors of metabolism, he is most remembered as a pioneer of the clinical application of the ultrasound technology. Tragically, Ludwig died of a cerebral hemorrhage in 1973, bringing a brilliant career to an untimely end at the age of 51 (https://www.ob-ultrasound.net/ludwig.html).
Meanwhile, Murray Saffran recruited Maurice Manning, Ph.D., from McGill University; Amir Askari, Ph.D., from Cornell University; and Patrick Mulrow, Ph.D., from Yale University (Fig. 1). Mulrow played a critical role in commencing hypertension research at MCO.
Manning began working in MCO in 1969 and was a faculty member for MCO’s inaugural class. He has made seminal contributions to advance the pharmacology of oxytocin and vasopressin [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. During the course of his research, he has donated thousands of samples of oxytocin and vasopressor analogs from his laboratory to other researchers across the world and is internationally known for his expertise in peptide biochemistry. To-date, Manning remains as an Emeritus faculty member of UTCOMLS.
Na+/K+ ATPase Pump Physiology
Amir Askari, Ph.D., pursued pioneering research related to cardiac glycoside-sensitive proteins and their effect on the cardiac Na+/K+ ATPase pump [41,42,43]. Askari investigated the pharmacologic properties of digitalis and how the drug affected the activity of the Na+/K+ ATPase pump. In 1990, Zijian Xie arrived at Toledo as a post-doctoral fellow in Askari’s laboratory. Xie continued working in MCO and further advanced research in Na+/K+ ATPase and cell membrane signal transduction [43,44,45,46,47,48, 49•]. Though the physiologic function of the pump has been studied for decades, the signaling capabilities of the pump were identified as a new function independent of the pump activity to maintain intracellular sodium and potassium homeostasis [49•, 50]. The research at Toledo to investigate how the Na+/K+ ATPase pump affected neighboring membrane proteins and signaling cascades was a unique aspect of its role in cardiovascular health. Unfortunately, both Askari and Xie passed away in 2020.
From Endocrinology to Cardiovascular Research
Patrick Mulrow, M.D. is a Cornell and Stanford trained endocrine physician well known for his research on the renin-angiotensin aldosterone system (RAAS). Mulrow’s research was instrumental in proving that angiotensin II stimulated aldosterone secretion, and not adrenocorticotrophic hormone [51,52,53,54,55,56]. Mulrow trained at the University of Pennsylvania and was recruited to Toledo in 1971 by Ludwig (Table 1). At Toledo, Mulrow conducted hypertension research in collaboration with Roberto Franco-Saenz, M.D., for 25 years [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79]. Dr. Franco-Saenz was an enthusiastic physician and scientist. Many of his publications in Toledo were focused on the renin-angiotensin system and mechanisms of hypertension. In 1975, Mulrow became Chair of Medicine at MCO (Fig. 2). As Chair from 1975 to 1995, Mulrow continued his physiology research and expanded hypertension research in the department (Table 1). Mulrow and Franco-Saenz discovered that atrial natriuretic factor, a heart hormone, inhibited aldosterone production. Mulrow’s research along with his penchant for leadership in a variety of scientific organizations such as chairman of the American Heart Association’s (AHA) Council for High Blood Pressure Research, chairman of the Medical Research Council in Canada, president of the Central Society for Clinical Research, member of the US National Research Council, Member of the Board of Directors of the Inter‐American Society of Hypertension, and involvement with the National High Blood Pressure Education Program (NHBPEP) gained national and international visibility to MCO.
Clinical Hypertension Research
Mulrow had a productive career at MCO in both cardiovascular and renal hypertension research. He found that NSAIDs like indomethacin or naproxen could inhibit renin secretion and lead to kidney damage in animals as well as patients with volume depletion [80, 81]. This was an early link to an NIH-funded National Analgesic Nephropathy Study, which would later be led by the nephrologists Joseph Shapiro, M.D., who came to MCO in 1995 and William Henrich, M.D., who worked at MCO from 1995 to 1999 [82,83,84,85,86,87,88,89,90]. Henrich is now President of the University of Texas Health Science Center, San Antonio, TX.
In 1999, Shapiro was appointed the Chair of Medicine at MCO. He continued to foster the research environment that Mulrow had established over the last 3 decades and researched on various topics pertinent to hypertension [91, 92, 93••, 94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119]. Shapiro served as Chair until 2012, and subsequently moved as the Dean at Marshall University College of Medicine.
Between 2004 and 2008, MCO went through two name changes from the Medical University of Ohio to what is now referred to as the University of Toledo College of Medicine and Life Sciences. Therefore, references beyond these years are likely to not refer to MCO anymore.
Renal research at UTCOMLS had a high point in 2014 with the publication of The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial [93••]. The CORAL trial, led by Christopher Cooper, Lance Dworkin, William Henrich, and Joseph Shapiro, evaluated the clinical outcomes of renal artery stents in patients with renal artery stenosis. Renal artery stenosis is present in 1–5% of patients with hypertension and can occur in the setting of other cardiovascular diseases and comorbidities. Results of the trial indicated that renal artery stents did not significantly decrease the incidence of adverse cardiovascular events [93••]. This study was published in the New England Journal of Medicine in 2014 and was one of the top 5 cardiovascular studies in that year [93••]. Results of the CORAL trial provided important data regarding the clinical management of these patients and were incorporated into the 2017 American College of Cardiology (ACC)/AHA Hypertension Guidelines [120] for the treatment of hypertensive patients. Cooper, who is now the Dean of the College of Medicine and Life Sciences stated, “I think I’ve done a tiny little piece of unraveling the biology of people. I think the better we understand the biology of people, the better we can take care of the people we serve.”
Learning the Genetics of Hypertension
In addition to the physiological and clinical studies, this institution is internationally reputed for its pioneering contributions to the dissection of genetic elements causing hypertension in experimental rats [121, 122••]. This new area of research was initiated by John Rapp, who was recruited to MCO by Mulrow. Rapp had acquired unique rat strains called the Dahl rats [123]. The Dahl rats are named after Lewis Kitchener Dahl, M.D., a physiologist who discovered the association between salt intake and hypertension. Dahl selectively bred the rats for salt sensitivity (S) and salt resistance (R), with the goal being to determine if there were genetic differences between these two strains of rats [124,125,126,127,128,129,130]. For details on Dahl and the history of these strains, readers are referred to the 2014 Dahl lecture award article [123]. It is important to note that their findings preceded the now common knowledge that a high-salt diet is associated with hypertension. In 1976, Mulrow recruited Rapp, who brought his Dahl rats to MCO. MCO was a new medical college at the time. The major focus of Rapp’s research at Toledo was uncovering the causal genes for the pathogenesis of salt-sensitive hypertension. Throughout the 1970s, he was primarily focused on the inbreeding of the S and R rat strains. This helped maintain genetic uniformity and the stability of the traits in each strain.
Rapp laid the groundwork for understanding their genetics by inbreeding these rats to develop the Dahl salt-sensitive (S) and Dahl salt-resistant (R) rats [131]. These are to-date among the most widely used inbred models for studies on hypertension as they are the only rat models with direct clinical relevance to salt-sensitive hypertension. The original colonies of the Dahl S and R strains are maintained in-house at the University of Toledo College of Medicine and Life Sciences. The inbred strains are now officially designated as SS/Jr and SR/Jr for salt-sensitive and salt-resistant, and Jr stands for John Rapp. These rats have been recently licensed to Charles River Laboratories and registered at the rat genome database as SS/Jr/Tol rats (https://rgd.mcw.edu/rgdweb/report/strain/main.html?id=724573).
Rapp hypothesized that a few major genes would be involved in the pathogenesis of hypertension and was focused on the reproducibility of his results. He performed breeding experiments to determine which chromosomes and what portions of the chromosomes were involved in the genetic predisposition to salt-sensitive hypertension. It was important to identify segments of chromosomes where a gene might be located. This research occurred before the human genome project, and consequently, widespread sequencing was not available.
Using these rats, Rapp created maps of chromosomes to find the areas of the chromosomes that were segregated by blood pressure, known as quantitative trait loci (QTLs). His outstanding work on discovering multiple such QTLs [121] was lauded by the AHA Council for High Blood Pressure Research, which decorated him with its highest honor, the Excellence in Hypertension Research Award (which was then referred to as the Novartis award). He became Chair of the Physiology department in 1994 and helped reshape the focus of the department to cardiovascular genetics. Dr. Rapp had two trainees that would go on to make significant contributions to the field of hypertension in their own right: Bina Joe and Michael Garrett.
In 1995, Rapp recruited Garrett to Toledo as a research assistant for his laboratory, who worked with Rapp to advance the genetic analysis of renal disease in the Dahl S rat. Joe was recruited by Rapp as Research Faculty in 2001. Prior to coming to Toledo, Joe, who graduated from Mysore University in India, was a Fogarty fellow at the Intramural division of the NIH. While at the NIH, she was conducting research on the genetics of rheumatoid arthritis using experimental congenic rat models. After many years of collaboration in Toledo, Rapp, Garrett, and Joe identified multiple genetic loci that causally regulated blood pressure in the Dahl S rat [121, 132,133,134,135,136,137,138,139,140,141,142,143,144,145]. They continued to work together until Rapp retired in 2004. In 2007, Garrett left Toledo, moved to the Medical College of Wisconsin, and moved again to the University of Mississippi Medical Center, where he is a Professor. He continues to work on the Dahl S rats to delineate the genetics of kidney disease [146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166]. The Joe lab in Toledo further advanced this positional cloning research by combining it with gene-editing technology to identify many protein coding genes and non-coding genes as BP QTLs [132,133,134,135,136,137,138,139,140,141,142,143,144,145].
In tracing this “genealogy,” it is important to mention that there are multiple varieties of the Dahl S rats which are genetically distinct and present with varying extents of hypertension [167]. Researchers who intend to use the original stock of Dahl S rats, which were inbred by Rapp, may please note that these are currently available through two academic sources, the Joe laboratory at the University of Toledo (https://www.utoledo.edu/med/depts/physpharm/faculty/joe.html) and the Garrett laboratory at the University of Mississippi Medical Center (https://www.umc.edu/som/Departments%20and%20Offices/SOM%20Departments/Pharmacology%20and%20Toxicology/Faculty/Michael-Garrett.html). Besides these 2 sources, a commercial source is the Charles River Laboratory as the Dahl S rat was recently licensed to the Charles River Laboratory by Rapp, which was facilitated by Joe.
From Genetics to the Gut Microbiota in Hypertension
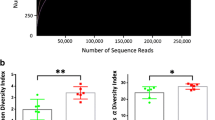
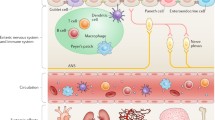
Close to a decade after the human and rat genome sequences were decoded, the Joe lab was successful in positional cloning of genetic loci regulating BP in the Dahl rat [122••, 135, 136, 168, 169]. However, genome-wide association studies in humans as well as QTL mapping studies in rats shed light on the landscape of the genetics of hypertension to be much larger than what was originally anticipated. More than 1500 loci in humans [170] and > 500 loci (https://rgd.mcw.edu/) [122••] in rats were located as potential regions of the mammalian genome to harbor BP regulatory genes. As such, contemplating clinical targets to render genetic corrections was not feasible. The value of identifying the polymorphisms was therefore pivoted by human geneticists towards the development of a polygenic risk score for predictive individualized medicine. Meanwhile, intrigued by a Science publication in 2010 that mice lacking Tlr5 [171], a bacterial flagellin receptor, developed microbial dysbiosis and metabolic syndrome, the Joe laboratory hypothesized that beyond the genetics of hypertension, microbiota, which are sensitive to salt and antibiotics, is a factor contributing to BP regulation. The hypothesis was indeed proven correct by the demonstration via cecal transplantation studies that the Dahl S rats increased BP when gavaged with cecal microbiota from the Dahl salt-resistant (R) rat [172••, 173]. Since the publication of this pioneering study in 2015, the University of Toledo is currently regarded as the site of discovery of the important link between gut microbiota and hypertension [172••, 174,175,176,177,178,179,180,181,182,183,184,185,186,187,188]. More recently, Joe et al. have employed germ-free rats and reported that gut microbiota is obligatory to blood pressure homeostasis [179].
Yet another significant contribution from the Joe laboratory is the discovery of the strong link between metabolism [189•], especially the inverse relationship between the ketone body, betahydroxybutyrate (BHB), and hypertension [190]. Both renal and vascular mechanisms have been identified via BHB facilitating the inhibition of the Nlrp3 inflammasome and vasodilatory function, respectively [191,192,193].
Overall, these impactful contributions were recognized by the Hypertension research community in the form of the Lewis Dahl Lectureship award in 2014 [123] and the Harriet Dustan Award in 2019 [189•] to Joe. It is notable that in both categories, Joe is globally, the first woman of color awardee of both of these awards.
Current Hypertension Research
Building on this strong foundation of both basic and clinical sciences, in 2011, the University-wide Research Council approved the Center for Hypertension and Precision Medicine. Most importantly, in 2015, fueled by a significant 50-year legacy model affiliation with a local Health organization, ProMedica (https://www.promedica.org/service-to-the-community/ut-academic-affiliation), the Dean of the College of Medicine, Cooper, and the Director of Hypertension Research, Joe, both Distinguished University Professors of the University, have expanded talent acquisition for hypertension research. Notable recruits include Matam Vijay-Kumar, who initially discovered the link between Tlr5 receptors and blood pressure [171, 172••, 173,174,175,176,177,178,179,180, 183,184,185, 187, 188, 189•, 191, 192, 194,195,196,197,198,199,200,201,202,203,204,205], [181, 196]; Jasenka Zubcevic [206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244], who focuses on the gut-brain axis; Jennifer Hill, whose work is on prenatal environmental effects on hypertension [182, 186, 189•, 196]; Tao Yang, who is studying microbiota as causes for drug resistance in hypertensives [175, 179, 181, 187, 189•, 192, 209, 210, 223, 229,230,231,232,233,234, 239]; Charles Thodeti and Guillermo Vazquez, who focus on transient receptor potential channels [245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283]; Islam Osman, who is a vascular physiologist [284,285,286,287,288,289,290,291,292,293,294,295,296]; Lauren Koch, who has developed unique rat models with distinct aerobic exercise endurance capacities: low- and high-capacity runners (LCR and HCR) to study the relationship between exercise and hypertension [188, 189•, 199]; Sailaja Paruchuri, who works on lipid mediators [245, 246, 248,249,250,251,252, 255, 257, 258, 261, 263,264,265]; and Piu Saha, who is working in immunological aspects promoting hypertension in rat genetic models [174, 175, 179, 180, 187, 191, 194, 195, 197, 200, 201, 205]. From the Department of Medicine, Cooper and his colleagues including Lance Dworkin, David Kennedy, Steven Haller, and Rujun Gong continue their investigations on the renal physiology of BP control [101, 102, 104, 112, 297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339]. From the College of Pharmacy, members of the Center for Hypertension and Precision Medicine include Wissam Aboualaiwi [340,341,342,343,344,345,346,347,348,349,350] in the Department of Pharmacology and Experimental Therapeutics who investigates drug targets and the physiology of cilia in polycystic kidney disease and hypertension. Further, in 2018, UTCOMLS identified Hypertension as a strategically focused spotlight area of unique distinction.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McCray V. Toledo's medical college reaches golden milestone. In: The Blade, 2014. https://www.toledoblade.com/Education/2014/05/25/Toledo-s-medical-college-reaches-golden-milestone.html.
Saffran M. Activation of ACTH release by neurohypophysial peptides. Can J Biochem Physiol. 1959;37(2):319–29. PMID: 13618788.
Saffran M, Schally AV. The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol. 1955;33(3):408–15. PMID: 14364332.
Saffran M, Schally AV. Effect of histamine, hog vasopressin, and corticotropin-releasing factor (CRF) on ACTH release in vitro. Proc Soc Exp Biol Med. 1956;92(3):636–7. PMID: 13359490.
Saffran M, Schally AV, Benfey BG. Stimulation of the release of corticotropin from the adenohypophysis by a neurohypophysial factor. Endocrinology. 1955;57(4):439–44. PMID: 13261946.
Saffran M, Vogt M. Depletion of pituitary corticotrophin by reserpine and by a nitrogen mustard. Br J Pharmacol Chemother. 1960;15:165–9. PMID: 14440746 PMC: PMC1481994.
Leighton RF, et al. The Toledo exercise and diet study. Results at 26 weeks. Arch Intern Med. 1990;150(5):1016–20. PMID: 2184789.
Saffran M. Is insulin a factor in the genesis of the vascular complications of diabetes. Trends Endocrinol Metab. 1989;1(2):56–9. PMID: 18411090.
Saffran M. Where the hormones, there moan I. Steroids. 1991;56(6):298–310. PMID: 1926225.
Saffran M. Banting and Best and those who went before. Hosp Pract (Off Ed). 1992;27(5):123–6, 129–32. PMID: 1577882.
Saffran M, et al. Oral insulin in diabetic dogs. J Endocrinol. 1991;131(2):267–78. PMID: 1744572.
Cheng LL, et al. Design of potent and selective agonists for the human vasopressin V1b receptor based on modifications of [deamino-cys1]arginine vasopressin at position 4. J Med Chem. 2004;47(9):2375–88. PMID: 15084136.
Kruszynski M, et al. Invertebrate neuropeptides resembling vasotocin and some analogues: synthesis and pharmacological properties. Experientia. 1990;46(7):771–3. PMID: 2373207.
Lammek B, et al. 2-O-alkyltyrosine derivatives of 1-deamino-arginine-vasopressin: highly specific and potent antidiuretic agonists. J Med Chem. 1989;32(1):244–7. PMID: 2909737.
Manning M. Impact of the Merrifield solid phase method on the design and synthesis of selective agonists and antagonists of oxytocin and vasopressin: a historical perspective. Biopolymers. 2008;90(3):203–12. PMID: 17610261.
Manning M, et al. Novel approach to the design of synthetic radioiodinated linear V1A receptor antagonists of vasopressin. Int J Pept Protein Res. 1992;40(3–4):261–7. PMID: 1478783.
Manning M, Chan WY, Sawyer WH. Design of cyclic and linear peptide antagonists of vasopressin and oxytocin: current status and future directions. Regul Pept. 1993;45(1–2):279–83. PMID: 8511357.
Manning M, et al. Effects of a D-Cys6/L-Cys6 interchange in nonselective and selective vasopressin and oxytocin antagonists. J Med Chem. 1995;38(10):1762–9. PMID: 7752199.
Manning M, et al. Advances in the design of selective antagonists, potential tocolytics, and radioiodinated ligands for oxytocin receptors. Adv Exp Med Biol. 1995;395:559–83. PMID: 8714021.
Manning M, et al. An exploration of the effects of L- and D-tetrahydroisoquinoline-3-carboxylic acid substitutions at positions 2, 3 and 7 in cyclic and linear antagonists of vasopressin and oxytocin and at position 3 in arginine vasopressin. J Pept Sci. 1995;1(1):66–79. PMID: 9222985.
Manning M, et al. Position three in vasopressin antagonist tolerates conformationally restricted and aromatic amino acid substitutions: a striking contrast with vasopressin agonists. J Pept Sci. 1997;3(1):31–46. PMID: 9230469.
Manning M, et al. Design of peptide oxytocin antagonists with strikingly higher affinities and selectivities for the human oxytocin receptor than atosiban. J Pept Sci. 2005;11(10):593–608. PMID: 15880385.
Manning M, et al. Novel linear antagonists of the antidiuretic (V2) and vasopressor (V1) responses to vasopressin. Int J Pept Protein Res. 1988;32(6):455–67. PMID: 3246475.
Manning M, et al. Solid-phase synthesis of 16 potent (selective and nonselective) in vivo antagonists of oxytocin. J Med Chem. 1989;32(2):382–91. PMID: 2913298.
Manning M, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol, 2012. 24(4):609–28. PMID: 22375852 PMC: PMC3490377.
Manning M, et al. C-terminal deletions in agonistic and antagonistic analogues of vasopressin that improve their specificities for antidiuretic (V2) and vasopressor (V1) receptors. J Med Chem. 1987;30(12):2245–52. PMID: 2960812.
Manning M, et al. Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with Atosiban. Int J Pept Protein Res. 1995;46(3–4):244–52. PMID: 8537178.
Manning M, et al. Potent V2/V1a vasopressin antagonists with C-terminal ethylenediamine-linked retro-amino acids. J Med Chem. 1992;35(21):3895–904. PMID: 1433200.
Manning M, et al. No requirements of cyclic conformation of antagonists in binding to vasopressin receptors. Nature. 1987;329(6142):839–40. PMID: 2959865.
Manning M, Sawyer WH. Discovery, development, and some uses of vasopressin and oxytocin antagonists. J Lab Clin Med. 1989;114(6):617–32. PMID: 2687422.
Manning M, Sawyer WH. Design, synthesis and some uses of receptor-specific agonists and antagonists of vasopressin and oxytocin. J Recept Res. 1993;13(1–4):195–214. PMID: 8383753.
Manning M, et al. Synthesis and some pharmacological properties of potent and selective antagonists of the vasopressor (V1-receptor) response to arginine-vasopressin. J Med Chem. 1992;35(2):382–8. PMID: 1531076.
Manning M, et al. Receptor-specific antagonists of vasopressin and oxytocin. A current perspective. Ann NY Acad Sci. 1993;689:219–32. PMID: 8396867.
Manning M, et al. Synthesis and structure-activity investigation of novel vasopressin hypotensive peptide agonists. J Pept Sci. 1999;5(11):472–90. PMID: 10587312.
Manning M, et al. Discovery and design of novel vasopressin hypotensive peptide agonists. J Recept Signal Transduct Res. 1999;19(1–4):631–44. PMID: 10071789.
Manning M, et al. Design of oxytocin antagonists, which are more selective than atosiban. J Pept Sci. 2001;7(9):449–65. PMID: 11587184.
Manning M, et al. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. PMID: 18655903.
Manning M, et al. Design of potent and selective linear antagonists of vasopressor (V1-receptor) responses to vasopressin. J Med Chem. 1990;33(11):3079–86. PMID: 2231609.
Stoev S, et al. Design and synthesis of potent, highly selective vasopressin hypotensive agonists. J Pept Sci. 2006;12(9):592–604. PMID: 16625682.
Stoev S, et al. An investigation of position 3 in arginine vasopressin with aliphatic, aromatic, conformationally-restricted, polar and charged amino acids. J Pept Sci. 1999;5(3):141–53. PMID: 10323558.
Askari A, Kakar SS, Huang WH. Ligand binding sites of the ouabain-complexed (Na+ + K+)-ATPase. J Biol Chem. 1988;263(1):235–42. PMID: 2826440.
Kakar SS, Huang WH, Askari A. Properties of the Na+, K+, and ATP binding sites of the ouabain-complexed (Na+ + K+)-ATPase: implications for the mechanism of ouabain action. Prog Clin Biol Res. 1988;268A:211–8. PMID: 2843864.
Liu J, et al. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275(36):27838–44. PMID: 10874029.
Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275(36):27832–7. PMID: 10874030.
Kometiani P, et al. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases.J Biol Chem, 1998. 273(24):15249–56. PMID: 9614140.
Liu L, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003;284(6):C1550–60. PMID: 12606314.
Mohammadi K, et al. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem. 2001;276(45):42050–6. PMID: 11562372.
Mohammadi K, et al. Positive inotropic effect of ouabain on isolated heart is accompanied by activation of signal pathways that link Na+/K+-ATPase to ERK1/2. J Cardiovasc Pharmacol. 2003;41(4):609–14. PMID: 12658063.
• Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem, 2002;269(10):2434–9. PMID: 12027880. This article is signficant because it describes the signaling pathways that the Na+/K+-ATPase plasma membrane enzyme uses to communicate with intracellular organelles outside of its role as an energy tranducing ion pump.
Askari A. The other functions of the sodium pump. Cell Calcium. 2019;84: 102105. PMID: 31733624.
Brecher AS, et al. Regulation of adrenal renin messenger ribonucleic acid by dietary sodium chloride. Endocrinology. 1989;124(6):2907–13. PMID: 2470583.
Gupta P, Franco-Saenz R, Mulrow PJ. Regulation of the adrenal renin angiotensin system in cultured bovine zona glomerulosa cells: effect of catecholamines. Endocrinology. 1992;130(4):2129–34. PMID: 1312445.
Mulrow, P.J., Adrenal renin: a possible local regulator of aldosterone production. Yale J Biol Med, 1989. 62(5):503–10. PMID: 2697984 PMC: PMC2589164.
Mulrow PJ. Adrenal renin: regulation and function. Front Neuroendocrinol. 1992;13(1):47–60. PMID: 1468598.
Mulrow PJ, et al. Adrenal renin: a possible local hormonal regulator of aldosterone production. Cardiovasc Drugs Ther. 1988;2(4):463–71. PMID: 3154627.
Tokita Y, et al. Adrenal renin is released into the circulation of the hypertensive transgenic rat TGR (mRen-2)27. Trans Assoc Am Physicians. 1992;105:123–32. PMID: 1308989.
Figueroa O, et al. Changes in cholesterol levels after coronary artery bypass surgery. Am J Med Sci. 1992;303(2):73–7. PMID: 1539612.
Franco-Saenz R, et al. Effect of atrial natriuretic factor on renin and aldosterone. J Cardiovasc Pharmacol. 1989;13(Suppl 6):S31–5. PMID: 2473345.
Franco-Saenz R, Harper D, Mulrow PJ. Effect of posture on the plasma levels of atrial natriuretic factor. Clin Exp Hypertens A. 1989;11(2):337–47. PMID: 2650932.
Franco-Saenz R, Somani P, Mulrow PJ. Effect of atrial natriuretic peptide (8–33-Met ANP) in patients with hypertension. Am J Hypertens. 1992;5(5 Pt 1):266–75. PMID: 1533767.
Gupta P, et al. Transforming growth factor-beta 1 inhibits aldosterone and stimulates adrenal renin in cultured bovine zona glomerulosa cells. Endocrinology. 1992;131(2):631–6. PMID: 1322277.
Gupta P, Franco-Saenz R, Mulrow PJ. Transforming growth factor-beta 1 inhibits aldosterone biosynthesis in cultured bovine zona glomerulosa cells. Endocrinology. 1993;132(3):1184–8. PMID: 8440178.
Lotshaw DP, Franco-Saenz R, Mulrow PJ. Atrial natriuretic peptide inhibition of calcium ionophore A23187-stimulated aldosterone secretion in rat adrenal glomerulosa cells. Endocrinology. 1991;129(5):2305–10. PMID: 1657569.
Lotshaw DP, Franco-Saenz R, Mulrow PJ. Guanabenz-induced inhibition of aldosterone secretion from isolated rat adrenal glomerulosa cells. Am J Med Sci. 1991;301(1):15–20. PMID: 1847275.
Mulrow PJ. Sabbatical leave: an important mechanism for revitalizing faculty. J Lab Clin Med. 1989;113(5):537–40. PMID: 2715679.
Mulrow PJ. Production of renin by adrenal glomerulosa cells. Trans Am Clin Climatol Assoc. 1989;100:126–31. PMID: 3077571 PMC: PMC2376478.
Mulrow PJ. The intrarenal renin-angiotensin system. Curr Opin Nephrol Hypertens. 1993;2(1):41–4. PMID: 7922165.
Mulrow PJ, et al. Inhibition of aldosterone secretion by atrial natriuretic peptide. Ann N Y Acad Sci. 1987;512:438–48. PMID: 2831784.
Mulrow PJ, et al. Inhibitors of aldosterone secretion. J Steroid Biochem. 1987;27(4–6):941–6. PMID: 2826913.
Oda H, et al. Local generation of angiotensin II as a mechanism of aldosterone secretion in rat adrenal capsules. Proc Soc Exp Biol Med. 1991;196(2):175–7. PMID: 1846675.
Shier DN, et al. Production of renin, angiotensin II, and aldosterone by adrenal explant cultures: response to potassium and converting enzyme inhibition. Endocrinology. 1989;125(1):486–91. PMID: 2544410.
Takagi M, et al. Effect of atrial natriuretic peptide on renin release in a superfusion system of kidney slices and dispersed juxtaglomerular cells. Endocrinology. 1988;122(4):1437–42. PMID: 2831030.
Takagi M, et al. Effects of dibutyryl adenosine 3′,5′-monophosphate, angiotensin II, and atrial natriuretic factor on phosphorylation of a 17,600-dalton protein in adrenal glomerulosa cells. Endocrinology. 1988;123(5):2419–23. PMID: 2844512.
Takagi M, et al. Effect of atrial natriuretic factor on calcium fluxes in adrenal glomerulosa cells. Hypertension. 1988;11(5):433–9. PMID: 2966768.
Tokita Y, et al. Effects of nephrectomy and adrenalectomy on the renin-angiotensin system of transgenic rats TGR(mRen2)27. Endocrinology. 1994;134(1):253–7. PMID: 8275941.
Wang Y, et al. Regulation of renin gene expression in rat adrenal zona glomerulosa cells. Hypertension. 1992;20(6):776–81. PMID: 1333446.
Yamaguchi T, Franco-Saenz R, Mulrow PJ. Effect of angiotensin II on renin production by rat adrenal glomerulosa cells in culture. Hypertension. 1992;19(3):263–9. PMID: 1312512.
Yamaguchi T, et al. Role of the adrenal renin-angiotensin system on adrenocorticotropic hormone- and potassium-stimulated aldosterone production by rat adrenal glomerulosa cells in monolayer culture. Hypertension. 1990;16(6):635–41. PMID: 2174021.
Yamaguchi T, et al. Zonal distribution and regulation of adrenal renin in a transgenic model of hypertension in the rat. Endocrinology. 1992;131(4):1955–62. PMID: 1396339.
Izumi Y, Franco-Saenz R, Mulrow PJ. Effects of prostaglandin synthesis inhibitors on the renin-angiotensin system and renal function. Hypertension. 1985;7(5):791–6. PMID: 3861577.
Suzuki S, et al. Urinary prostaglandin E2 excretion in chronic renal disease. Prostaglandins Med. 1980;4(5):377–82. PMID: 7403335.
Dheenan S, Henrich WL. Preventing dialysis hypotension: a comparison of usual protective maneuvers. Kidney Int. 2001;59(3):1175–81. PMID: 11231376.
Dheenan S, et al. Effect of sertraline hydrochloride on dialysis hypotension. Am J Kidney Dis. 1998;31(4):624–30. PMID: 9531178.
Franco-Saenz R, et al. Regulation of the genes for 11beta-hydroxysteroid dehydrogenase type 1 and type 2 in the kidney of the Dahl rat. J Hypertens. 1999;17(8):1089–93. PMID: 10466463.
Henrich WL. Approach to volume control, cardiac preservation, and blood pressure control in the pre-end-stage renal disease patient. J Am Soc Nephrol. 1998;9(12 Suppl):S63–5. PMID: 11443770.
Henrich WL. Analgesic nephropathy. Trans Am Clin Climatol Assoc. 1998;109:147–58; discussion 158–9. PMID: 9601134 PMC: PMC2194329.
Henrich WL. Renal artery disease in the elderly. Geriatr Nephrol Urol. 1999;9(2):81–6. PMID: 10518251.
Henrich WL, et al. Analgesics and the kidney: summary and recommendations to the Scientific Advisory Board of the National Kidney Foundation from an Ad Hoc Committee of the National Kidney Foundation. Am J Kidney Dis. 1996;27(1):162–5. PMID: 8546133.
Venkatesan J, Henrich WL. Anemia, hypertension, and myocardial dysfunction in end-stage renal disease. Semin Nephrol. 1997;17(4):257–69. PMID: 9241712.
Venkatesan J, Henrich WL. Cardiac disease in chronic uremia: management. Adv Ren Replace Ther. 1997;4(3):249–66. PMID: 9239429.
Barbato JC, et al. Rapid effects of aldosterone and spironolactone in the isolated working rat heart. Hypertension. 2002;40(2):130–5. PMID: 12154102.
Barbato JC, et al. Mechanisms for aldosterone and spironolactone-induced positive inotropic actions in the rat heart. Hypertension. 2004;44(5):751–7. PMID: 15466666.
•• Cooper CJ, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370(1):13–22. PMID: 24245566 PMC: PMC4815927. This randomized, control study compared the outcomes for medical therapy alone with medical therapy plus renal-artery stenting in patients with atherosclerotic renal-artery stenosis and elevated blood pressure, chronic kidney disease, or both.
Drummond CA, et al. Reduction of Na/K-ATPase affects cardiac remodeling and increases c-kit cell abundance in partial nephrectomized mice. Am J Physiol Heart Circ Physiol. 2014;306(12):H1631–43. PMID: 24748592 PMC: PMC4059984.
Elkareh J, et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49(1):215–24. PMID: 17145984.
El-Okdi N, et al. Effects of cardiotonic steroids on dermal collagen synthesis and wound healing. J Appl Physiol 1985;2008.105(1):30–6. PMID: 18483172 PMC: PMC2494826.
Fedorova LV, et al. Peroxisome proliferator-activated receptor delta agonist, HPP593, prevents renal necrosis under chronic ischemia. PLoS One. 2013;8(5):e64436. PMID: 23691217 PMC: PMC3654981.
Gupta S, et al. Ouabain and insulin induce sodium pump endocytosis in renal epithelium. Hypertension. 2012;59(3):665–72. PMID: 22311908 PMC: PMC3336087.
Haller ST, et al. Passive immunization against marinobufagenin attenuates renal fibrosis and improves renal function in experimental renal disease. Am J Hypertens. 2014;27(4):603–9. PMID: 24014658 PMC: PMC3958603.
Haller ST, et al. Effect of CD40 and sCD40L on renal function and survival in patients with renal artery stenosis. Hypertension, 2013;61(4):894–900. PMID: 23399713 PMC: PMC3783956.
Haller ST, et al.k Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure. Am J Hypertens. 2012;25(6):690–6. PMID: 22378033 PMC: PMC3355226.
Joe B, Shapiro JI. Molecular mechanisms of experimental salt-sensitive hypertension. J Am Heart Assoc. 2012;1(3):e002121. PMID: 23130148 PMC: PMC3487327.
Kennedy D, et al. Effect of chronic renal failure on cardiac contractile function, calcium cycling, and gene expression of proteins important for calcium homeostasis in the rat. J Am Soc Nephrol. 2003;14(1):90–7. PMID: 12506141.
Kennedy DJ, et al. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am J Physiol Renal Physiol. 2008;294(2):F450–4. PMID: 18032546 PMC: PMC2742580.
Kennedy DJ, et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47(3):488–95. PMID: 16446397.
Krol DM, et al. A mobile medical care approach targeting underserved populations in post-Hurricane Katrina Mississippi. J Health Care Poor Underserved. 2007;18(2):331–40. PMID: 17483561.
Liu J, et al. Reactive oxygen species modulation of Na/K-ATPase regulates fibrosis and renal proximal tubular sodium handling. Int J Nephrol. 2012;381320. PMID: 22518311 PMC: PMC3299271.
Liu J, et al. Impairment of Na/K-ATPase signaling in renal proximal tubule contributes to Dahl salt-sensitive hypertension. J Biol Chem. 2011;286(26):22806–13. PMID: 21555512 PMC: PMC3123048.
Murphy TP, et al. Roll-in experience from the Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study. J Vasc Interv Radiol. 2014;25(4):511–20. PMID: 24325931 PMC: PMC4815916.
Murphy TP, et al. Renal artery stent outcomes: effect of baseline blood pressure, stenosis severity, and translesion pressure gradient. J Am Coll Cardiol. 2015;66(22):2487–94. PMID: 26653621 PMC: PMC4819253.
Oweis S, et al. Cardiac glycoside downregulates NHE3 activity and expression in LLC-PK1 cells. Am J Physiol Renal Physiol. 2006;290(5):F997-1008. PMID: 16352745.
Priyadarshi S, et al. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney Int. 2003;63(5):1785–90. PMID: 12675854.
Reddy BK, et al. Compliance with antihypertensive therapy after renal artery stenting. Biol Res Nurs. 2003;5(1):37–46. PMID: 12886669.
Sodhi K, et al. PPARdelta binding to heme oxygenase 1 promoter prevents angiotensin II-induced adipocyte dysfunction in Goldblatt hypertensive rats. Int J Obes (Lond). 2014;38(3):456–65. PMID: 23779049 PMC: PMC3950586.
Tian J, et al. Renal ischemia regulates marinobufagenin release in humans. Hypertension. 2015;56(5):914–9. PMID: 20823380 PMC: PMC2959137.
Tian J, et al. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension. 2009;54(6):1313–20. PMID: 19884563 PMC: PMC2783263.
Tzamaloukas AH, et al. Management of severe hyponatremia: infusion of hypertonic saline and desmopressin or infusion of vasopressin inhibitors? Am J Med Sci. 2014;348(5):432–9. PMID: 25247759 PMC: PMC4206391.
Xie JX, Shapiro AP, Shapiro JI. The trade-off between dietary salt and cardiovascular disease; a role for Na/K-ATPase signaling. Front Endocrinol (Lausanne). 2014;5:97. PMID: 25101054 PMC: PMC4101451.
Yan Y, et al. Involvement of reactive oxygen species in a feed-forward mechanism of Na/K-ATPase-mediated signaling transduction. J Biol Chem. 2013;288(47):34249–34258. PMID: 24121502 PMC: PMC3837165.
Whelton PK, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2018;138(17):e426–83. PMID: 30354655.
Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev. 2000;80(1):135–72. PMID: 10617767.
•• Padmanabhan S, Joe B. Towards precision medicine for hypertension: a review of genomic, epigenomic, and microbiomic effects on blood pressure in experimental rat models and humans. Physiol Rev. 2017;97(4):1469–1528. PMID: 28931564 PMC: PMC6347103. This review is an extensive compilation of more recent discoveries of the early 21st century in the field of genetics, epigenetics and microbiotal contributions.
Joe B. Dr Lewis Kitchener Dahl, the Dahl rats, and the “inconvenient truth” about the genetics of hypertension. Hypertension. 2015;65(5):963–9. PMID: 25646295 PMC: PMC4393342.
Dahl LK, Heine M, Tassinari L. Effects of chronia excess salt ingestion. Evidence that genetic factors play an important role in susceptibility to experimental hypertension. J Exp Med. 1962;115:1173–90. PMID: 13883089 PMC: PMC2137393.
Dahl LK, Heine M, Tassinari L. Effects of chronic excess salt ingestion. Vascular reactivity in two strains of rats with opposite genetic susceptibility to experimental hypertension. Circulation. 1964;30:SUPPL 2:11–22. PMID: 14187059.
Dahl LK, Knudsen KD, Iwai J. Genetic influence of the kidney in hypertension-prone rats. Circ Res. 1970;27:Suppl 2:277+. PMID: 5506146.
Dahl LK, Schackow E. Effects of chronic excess salt ingestion: experimental hypertension in the rat. Can Med Assoc J. 1964;90:155–60. PMID: 14111710 PMC: PMC1922502.
Iwai J, Knudsen KD, Dahl LK. Genetic influence on the renin-angiotensin system. Evidence for a renin inhibitor in hypertension-prone rats. J Exp Med. 1970;131(3):543–57. PMID: 4312940 PMC: PMC2138815.
Knudsen KD, et al. Genetic influence on the development of renoprival hypertension in parabiotic rats. Evidence that a humoral hypertensinogenic factor is produced in kidney tissue of hypertension-prone rats. J Exp Med. 1969;130(6):1353–65. PMID: 5352784 PMC: PMC2138691.
Wolf G, Dahl LK, Miller NE. Voluntary sodium chloride intake of two strains of rats with opposite genetic susceptibility to experimental hypertension. Proc Soc Exp Biol Med. 1965;120(2):301–5. PMID: 5860031.
Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension. 1985;7(3 Pt 1):340–9. PMID: 3997219.
Rapp JP, Joe B. Use of contiguous congenic strains in analyzing compound QTLs. Physiol Genomics. 2012;44(2):117–20. PMID: 22108210 PMC: PMC3289116.
Cheng X, et al. Genetic predisposition for increased red blood cell distribution width is an early risk factor for cardiovascular and renal comorbidities. Dis Model Mech. 2020;13(5). PMID: 32238420 PMC: PMC7325433.
Cheng X, et al. Pleiotropic effect of a high resolution mapped blood pressure QTL on tumorigenesis. PLoS One. 2016;11(4):e0153519. PMID: 27073989 PMC: PMC4830557.
Gopalakrishnan K, et al. Defining a rat blood pressure quantitative trait locus to a <81.8 kb congenic segment: comprehensive sequencing and renal transcriptome analysis. Physiol Genomics. 2010;42A(2):153–61. PMID: 20716646 PMC: PMC2957796.
Kumarasamy S, et al. Targeted disruption of regulated endocrine-specific protein ( Resp18) in Dahl SS/Mcw rats aggravates salt-induced hypertension and renal injury. Physiol Genomics. 2018;50(5):369–375. PMID: 29570433 PMC: PMC6008117.
Lee SJ, et al. Substitution mapping in dahl rats identifies two distinct blood pressure quantitative trait loci within 1.12- and 1.25-mb intervals on chromosome 3. Genetics. 2006;174(4):2203–13. PMID: 17028336 PMC: PMC1698641.
Mell B, et al. Multiple blood pressure loci with opposing blood pressure effects on rat chromosome 1 in a homologous region linked to hypertension on human chromosome 15. Hypertens Res. 2015;38(1):61–7. PMID: 25231251.
Mell B, Cheng X, Joe B. QTL mapping of rat blood pressure loci on RNO1 within a homologous region linked to human hypertension on HSA15. PLoS One. 2019;14(8):e0221658. PMID: 31442284 PMC: PMC6707578.
Nie Y, et al. High-resolution mapping of a novel rat blood pressure locus on chromosome 9 to a region containing the Spp2 gene and colocalization of a QTL for bone mass. Physiol Genomics. 2016;48(6):409–19. PMID: 27113531 PMC: PMC4891933.
Pillai R, et al. Isolation and high-throughput sequencing of two closely linked epistatic hypertension susceptibility loci with a panel of bicongenic strains. Physiol Genomics. 2013;45(16):729–36. PMID: 23757393 PMC: PMC3742915.
Saad Y, et al. Congenic mapping of a blood pressure QTL region on rat chromosome 10 using the Dahl salt-sensitive rat with introgressed alleles from the Milan normotensive strain. Mamm Genome. 2008;19(2):85–91. PMID: 18175179.
Saad Y, et al. Congenic interval mapping of RNO10 reveals a complex cluster of closely-linked genetic determinants of blood pressure. Hypertension. 2007;50(5):891–8. PMID: 17893371.
Toland EJ, et al. Blood pressure and proteinuria effects of multiple quantitative trait loci on rat chromosome 9 that differentiate the spontaneously hypertensive rat from the Dahl salt-sensitive rat. J Hypertens. 2008;26(11):2134–41. PMID: 18854752.
Waghulde H, et al. Fine mapping of epistatic genetic determinants of blood pressure on rat chromosome 5. J Hypertens. 2018;36(7):1486–91. PMID: 29634662.
Cobb MB, et al. Nephron-deficient HSRA rats exhibit renal injury with age but have limited renal damage from streptozotocin-induced hyperglycemia. Am J Physiol Renal Physiol. 2021;320(6):F1093-F1105. PMID: 33843272 PMC: PMC8285653.
Dreisbach AW, et al. Urinary CYP eicosanoid excretion correlates with glomerular filtration in African-Americans with chronic kidney disease. Prostaglandins Other Lipid Mediat. 2014;113–115: 45–51. PMID: 25151892 PMC: PMC4396182.
Ishimwe JA, Garrett MR, Sasser JM. 1,3-Butanediol attenuates hypertension and suppresses kidney injury in female rats. Am J Physiol Renal Physiol. 2020;319(1):F106-F114. PMID: 32508113 PMC: PMC7468835.
Jia Z, et al. Allelic variants in Arhgef11 via the rho-rock pathway are linked to epithelial-mesenchymal transition and contributes to kidney injury in the Dahl salt-sensitive rat. PLoS One. 2015;10(7):e0132553. PMID: 26172442 PMC: PMC4501567.
Johnson AC, et al. A mutation in the start codon of gamma-crystallin D leads to nuclear cataracts in the Dahl SS/Jr-Ctr strain. Mamm Genome. 2013;24(3–4):95–104. PMID: 23404175 PMC: PMC3628938.
Johnson AC, et al. Loss of Arhgef11 in the Dahl salt-sensitive rat protects against hypertension-induced renal injury. Hypertension. 2020;75(4):1012–1024. PMID: 32148127 PMC: PMC8167818.
McPherson KC, et al. Altered renal hemodynamics is associated with glomerular lipid accumulation in obese Dahl salt-sensitive leptin receptor mutant rats. Am J Physiol Renal Physiol. 2020;318(4):F911-F921. PMID: 32068459 PMC: PMC7191445.
McPherson KC, et al. Early development of podocyte injury independently of hyperglycemia and elevations in arterial pressure in nondiabetic obese Dahl SS leptin receptor mutant rats. Am J Physiol Renal Physiol. 2016;311(4):F793-F804. PMID: 27465994 PMC: PMC5142236.
Muroya Y, et al. Deficiency in the formation of 20-hydroxyeicosatetraenoic acid enhances renal ischemia-reperfusion injury. J Am Soc Nephrol. 2015;26(10):2460–9. PMID: 25644108 PMC: PMC4587700.
Peterson SM, et al. Estimation of nephron number in whole kidney using the acid maceration method. J Vis Exp. 2019(147). PMID: 31180342.
Sawyer RT, et al. Renoprotective effects of C-peptide in the Dahl salt-sensitive rat. Am J Physiol Renal Physiol. 2012;303(6):F893–9. PMID: 22811482 PMC: PMC3468525.
Slaughter TN, et al. Characterization of the development of renal injury in type-1 diabetic Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2013;305(7):R727–34. PMID: 23926133 PMC: PMC3798803.
Terstappen F, et al. Sodium thiosulfate in the pregnant Dahl salt-sensitive rat, a model of preeclampsia. Biomolecules. 2020;10(2). PMID: 32075042 PMC: PMC7072460.
Turbeville HR, et al. Nitric oxide and oxidative stress pathways do not contribute to sex differences in renal injury and function in Dahl SS/Jr rats. Physiol Rep. 2020;8(13):e14440. PMID: 32652814 PMC: PMC7354091.
Turbeville HR, et al. Superimposed preeclampsia exacerbates postpartum renal injury despite lack of long-term blood pressure difference in the Dahl salt-sensitive rat. Hypertension. 2019;73(3):650–658. PMID: 30612494 PMC: PMC6374193.
Wang X, Garrett MR. Nephron number, hypertension, and CKD: physiological and genetic insight from humans and animal models. Physiol Genomics. 2017;49(3):180–192. PMID: 28130427 PMC: PMC5374451.
Wang X, et al. Spontaneous one-kidney rats are more susceptible to develop hypertension by DOCA-NaCl and subsequent kidney injury compared with uninephrectomized rats. Am J Physiol Renal Physiol. 2016;310(10):F1054–64. PMID: 26936874 PMC: PMC5002061.
Wang X, et al. Nephron deficiency and predisposition to renal injury in a novel one-kidney genetic model. J Am Soc Nephrol. 2015; 26(7):1634–46. PMID: 25349207 PMC: PMC4483580.
Westbrook L, et al. Genetic susceptibility and loss of Nr4a1 enhances macrophage-mediated renal injury in CKD. J Am Soc Nephrol. 2014;25(11):2499–510. PMID: 24722447 PMC: PMC4214519.
Williams JM, et al. Genetic variants in Arhgef11 are associated with kidney injury in the Dahl salt-sensitive rat. Hypertension. 2012;60(5):1157–68. PMID: 22987919 PMC: PMC3505884.
Zhou Y, et al. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science, 2017; 358(6368):1332–1336. PMID: 29217578 PMC: PMC6014699.
Rapp JP, Garrett MR. Will the real Dahl S rat please stand up? Am J Physiol Renal Physiol. 2019;317(5):F1231-F1240. PMID: 31545925 PMC: PMC6879929.
Gopalakrishnan K, et al. Targeted disruption of Adamts16 gene in a rat genetic model of hypertension. Proc Natl Acad Sci USA. 2012;109(50):20555–9. PMID: 23185005 PMC: PMC3528556.
Joe B, et al. Positional identification of variants of Adamts16 linked to inherited hypertension. Hum Mol Genet. 2009;18(15):2825–38. PMID: 19423552 PMC: PMC2706685.
Evangelou E, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50(10):1412–1425. PMID: 30224653 PMC: PMC6284793.
Vijay-Kumar M, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–31. PMID: 20203013 PMC: PMC4714868.
•• Mell B, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47(6):187–97. PMID: 25829393 PMC: PMC4451389. This is the pioneering study at the University of Toledo College of Medicine that defined the link between microbiota and hypertension in a genetic model of hypertension.
Galla S, et al. Microbiotal-host interactions and hypertension. Physiology (Bethesda). 2017;32(3):224–233. PMID: 28404738 PMC: PMC6347099.
Abokor AA, et al. Immunoglobulin A, an active liaison for host-microbiota homeostasis. Microorganisms. 2021;9(10). PMID: 34683438 PMC: PMC8539215.
Chakraborty S, et al. Diurnal timing dependent alterations in gut microbial composition are synchronously linked to salt-sensitive hypertension and renal damage. Hypertension. 2020;76(1):59–72. PMID: 32450738 PMC: PMC7289684.
Galla S, et al. Disparate effects of antibiotics on hypertension. Physiol Genomics, 2018; 50(10):837–845. PMID: 30095376 PMC: PMC6230872.
Galla S, et al. Exposure to amoxicillin in early life is associated with changes in gut microbiota and reduction in blood pressure: findings from a study on rat dams and offspring. J Am Heart Assoc. 2020; 9(2):e014373. PMID: 31928175 PMC: PMC7033837.
Golonka RM, et al. Altered nutrient status reprograms host inflammation and metabolic health via gut microbiota. J Nutr Biochem. 2020;80:108360. PMID: 32163821 PMC: PMC7242157.
Joe B, et al. Microbiota introduced to germ-free rats restores vascular contractility and blood pressure. Hypertension. 2020;76(6):1847–1855. PMID: 33070663 PMC: PMC7666075.
McCarthy CG, et al. Innate immune cells and hypertension: neutrophils and neutrophil extracellular traps (NETs). Compr Physiol. 2021;11(1):1575–89. PMID: 33577121.
Sharma RK, et al. Pulmonary arterial hypertension-associated changes in gut pathology and microbiota. ERJ Open Res. 2020; 6(3). PMID: 32743008 PMC: PMC7383054. A.C. Oliveira has nothing to disclose. Conflict of interest: T. Yang has nothing to disclose. Conflict of interest: S. Kim has nothing to disclose. Conflict of interest: J. Zubcevic has nothing to disclose. Conflict of interest: V. Aquino has nothing to disclose. Conflict of interest: G.O. Lobaton has nothing to disclose. Conflict of interest: R. Goel has nothing to disclose. Conflict of interest: E.M. Richards has nothing to disclose. Conflict of interest: M.K. Raizada reports grants from the NIH outside the submitted work.
Sherman SB, et al. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9(5):400–421. PMID: 29469650 PMC: PMC6219642.
Singh V, et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab. 2015;22(6):983–96. PMID: 26525535 PMC: PMC4670569.
Singh V, et al. Lipocalin 2 deficiency-induced gut microbiota dysbiosis evokes metabolic syndrome in aged mice. Physiol Genomics. 2020;52(8):314–321. PMID: 32628083 PMC: PMC7473890.
Singh V, et al. Vancomycin prevents fermentable fiber-induced liver cancer in mice with dysbiotic gut microbiota. Gut Microbes. 2020;11(4):1077–1091. PMID: 32223398 PMC: PMC7524287.
Wang M, et al. Microbial reconstitution reverses early female puberty induced by maternal high-fat diet during lactation. Endocrinology. 2020;161(2). PMID: 31912132 PMC: PMC7035910.
Yang T, et al. Gnotobiotic rats reveal that gut microbiota regulates colonic mRNA of Ace2, the receptor for SARS-CoV-2 infectivity. Hypertension. 2020;76(1):e1-e3. PMID: 32426999 PMC: PMC7379164.
Zhang Y, et al. Vertical selection for nuclear and mitochondrial genomes shapes gut microbiota and modifies risks for complex diseases. Physiol Genomics. 2020;52(1):1–14. PMID: 31762410 PMC: PMC8526334.
• Chakraborty S, et al. Metabolites and hypertension: insights into hypertension as a metabolic disorder: 2019 Harriet Dustan Award. Hypertension. 2020;75(6):1386–1396. PMID: 32336227 PMC: PMC7225070. This review highlights the links between energy metabolism of the host and microbiota and its dysfunction as a potential cause for hypertension.
Place DE, Kanneganti TD. Fueling ketone metabolism quenches salt-induced hypertension. Trends Endocrinol Metab. 2019;30(3):145–7. PMID: 30670332.
Chakraborty S, et al. Salt-responsive metabolite, beta-hydroxybutyrate, attenuates hypertension. Cell Rep. 2018;25(3):677–689 e4. PMID: 30332647 PMC: PMC6542293.
McCarthy CG, et al. Ketone body beta-hydroxybutyrate is an autophagy-dependent vasodilator. JCI Insight. 2021;6(20). PMID: 34499623 PMC: PMC8564907.
McCarthy CG, et al. Physiologic, metabolic, and toxicologic profile of 1,3-butanediol. J Pharmacol Exp Ther. 2021;379(3):245–52. PMID: 34521698.
Edwards JM, et al. FPR-1 (formyl peptide receptor-1) activation promotes spontaneous, premature hypertension in Dahl salt-sensitive rats. Hypertension. 2021;77(4):1191–1202. PMID: 33641367 PMC: PMC7946782.
Edwards JM, et al. Microbiota are critical for vascular physiology: germ-free status weakens contractility and induces sex-specific vascular remodeling in mice. Vascul Pharmacol. 2020;125–126:106633. PMID: 31843471 PMC: PMC7036036.
Golonka RM, et al. Impact of nutritional epigenetics in essential hypertension: targeting microRNAs in the gut-liver axis. Curr Hypertens Rep. 2021;23(5):28. PMID: 33961141 PMC: PMC8105193.
Golonka RM, et al. Harnessing innate immunity to eliminate SARS-CoV-2 and ameliorate COVID-19 disease. Physiol Genomics. 2020;52(5):217–221. PMID: 32275178 PMC: PMC7200864.
Jordan J, et al. Pressure from the bugs within. Hypertension. 2019;73(5):977–979. PMID: 30905193 PMC: PMC6478159.
Roy S, et al. Intrinsic exercise capacity and mitochondrial DNA lead to opposing vascular-associated risks. Function (Oxf). 2021;2(1):zqaa029. PMID: 33363281 PMC: PMC7749784.
Saha P, et al. Enterobactin induces the chemokine, interleukin-8, from intestinal epithelia by chelating intracellular iron. Gut Microbes. 2020;12(1):1–18. PMID: 33171063 PMC: PMC7671005.
Singh V, et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell. 2018;175(3):679–694 e22. PMID: 30340040 PMC: PMC6232850.
Singh V, et al. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun. 2015;6:7113. PMID: 25964185 PMC: PMC6336494.
Waghulde H, et al. Attenuation of microbiotal dysbiosis and hypertension in a CRISPR/Cas9 gene ablation rat model of GPER1. Hypertension. 2018;72(5):1125–1132. PMID: 30354811 PMC: PMC6208154.
Yeoh BS, et al. Epigallocatechin-3-gallate inhibition of myeloperoxidase and its counter-regulation by dietary iron and lipocalin 2 in murine model of gut inflammation. Am J Pathol. 2016;186(4):912–26. PMID: 26968114 PMC: PMC5848242.
Yeoh BS, et al. Deficiency of stearoyl-CoA desaturase-1 aggravates colitogenic potential of adoptively transferred effector T cells. Am J Physiol Gastrointest Liver Physiol. 2016;311(4):G713-G723. PMID: 27609767 PMC: PMC5142196.
Ahmari N, Hayward LF, Zubcevic J. The importance of bone marrow and the immune system in driving increases in blood pressure and sympathetic nerve activity in hypertension. Exp Physiol. 2020;105(11):1815–26. PMID: 32964557.
Ahmari N, et al. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. Am J Physiol Heart Circ Physiol. 2019;317(2):H279-H289. PMID: 31150271 PMC: PMC6732488.
Ahmari N, et al. Loss of bone marrow adrenergic beta 1 and 2 receptors modifies transcriptional networks, reduces circulating inflammatory factors, and regulates blood pressure. Physiol Genomics. 2016;48(7):526–36. PMID: 27235450.
Bartley A, et al. Increased abundance of Lactobacillales in the colon of beta-adrenergic receptor knock out mouse is associated with increased gut bacterial production of short chain fatty acids and reduced IL17 expression in circulating CD4(+) immune cells. Front Physiol. 2018;9:1593. PMID: 30483153 PMC: PMC6242911.
Buerger AN, et al. Gastrointestinal dysbiosis following diethylhexyl phthalate exposure in zebrafish (Danio rerio):altered microbial diversity, functionality, and network connectivity. Environ Pollut. 2020;265(Pt B):114496. PMID: 32806437.
Davis EA, et al. Ghrelin signaling affects feeding behavior, metabolism, and memory through the vagus nerve. Curr Biol. 2020;30(22):4510–4518 e6. PMID: 32946754 PMC: PMC7674191.
de Kloet AD, et al. Neuroimmune communication in hypertension and obesity: a new therapeutic angle. Pharmacol Ther. 2013;138(3):428–40. PMID: 23458610 PMC: PMC3646992.
Donertas Ayaz B, et al. Central administration of hydrogen sulfide donor NaHS reduces Iba1-positive cells in the PVN and attenuates rodent angiotensin II hypertension. Front Neurosci. 2021;15:690919. PMID: 34602965 PMC: PMC8479468.
Donertas Ayaz B, Zubcevic J. Gut microbiota and neuroinflammation in pathogenesis of hypertension: a potential role for hydrogen sulfide. Pharmacol Res. 2020;153:104677. PMID: 32023431 PMC: PMC7056572.
Huentelman MJ, et al. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44(6):903–6. PMID: 15492138.
Huentelman MJ, et al. Cloning and characterization of a secreted form of angiotensin-converting enzyme 2. Regul Pept. 2004;122(2):61–7. PMID: 15380922.
Jiron JM, et al. Comparison of isoflurane, ketamine-dexmedetomidine, and ketamine-xylazine for general anesthesia during oral procedures in rice rats (Oryzomys palustris). J Am Assoc Lab Anim Sci. 2019;58(1):40–49. PMID: 30572978 PMC: PMC6351055.
Jun JY, et al. Brain-mediated dysregulation of the bone marrow activity in angiotensin II-induced hypertension. Hypertension. 2012;60(5):1316–23. PMID: 23045460 PMC: PMC3482108.
Kuan SP, et al. Attenuated amiloride-sensitive current and augmented calcium-activated chloride current in marsh rice rat (Oryzomys palustris) airways. iScience. 2019;19:737–748. PMID: 31491720 PMC: PMC6731178.
Martyniuk CJ, et al. Genetic ablation of bone marrow beta-adrenergic receptors in mice modulates miRNA-transcriptome networks of neuroinflammation in the paraventricular nucleus. Physiol Genomics. 2020;52(4):169–177. PMID: 32089076 PMC: PMC7191424.
Perez PD, et al. Cocaine differentially affects synaptic activity in memory and midbrain areas of female and male rats: an in vivo MEMRI study. Brain Imaging Behav. 2018;12(1):201–216. PMID: 28236167 PMC: PMC6397741.
Santisteban MM, et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117(2):178–91. PMID: 25963715 PMC: PMC4490954.
Santisteban MM, et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120(2):312–323. PMID: 27799253 PMC: PMC5250568.
Santisteban MM, et al. Dysfunctional brain-bone marrow communication: a paradigm shift in the pathophysiology of hypertension. Curr Hypertens Rep. 2013;15(4):377–89. PMID: 23715920 PMC: PMC3714364.
Shan Z, et al. Chronic knockdown of the nucleus of the solitary tract AT1 receptors increases blood inflammatory-endothelial progenitor cell ratio and exacerbates hypertension in the spontaneously hypertensive rat. Hypertension. 2013;61(6):1328–33. PMID: 23547238 PMC: PMC4128231.
Sharma RK, et al. Involvement of neuroinflammation in the pathogenesis of monocrotaline-induced pulmonary hypertension. Hypertension. 2018;71(6):1156–1163. PMID: 29712738 PMC: PMC5945302.
Souders CL 2nd, Zubcevic J, Martyniuk CJ. Tumor necrosis factor alpha and the gastrointestinal epithelium: implications for the gut-brain axis and hypertension. Cell Mol Neurobiol. 2021. PMID: 33594519 PMC: PMC8364923.
Sun C, et al. Shift to an involvement of phosphatidylinositol 3-kinase in angiotensin II actions on nucleus tractus solitarii neurons of the spontaneously hypertensive rat. Circ Res. 2009;105(12):1248–55. PMID: 19850939 PMC: PMC2810537.
Yang T, et al. Shifts in the gut microbiota composition due to depleted bone marrow beta adrenergic signaling are associated with suppressed inflammatory transcriptional networks in the mouse colon. Front Physiol. 2017;8:220. PMID: 28446880 PMC: PMC5388758.
Yang T, et al. Sustained captopril-induced reduction in blood pressure is associated with alterations in gut-brain axis in the spontaneously hypertensive rat. J Am Heart Assoc. 2019;8(4):e010721. PMID: 30755073 PMC: PMC6405665.
Yang T, et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol (Oxf). 2019;226(2):e13256. PMID: 30656835 PMC: PMC7199780.
Yang T, et al. Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiol Rep. 2018;6(14):e13732. PMID: 30039527 PMC: PMC6056753.
Yang T, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40. PMID: 25870193 PMC: PMC4433416.
Yang T, Zubcevic J. Gut-brain axis in regulation of blood pressure. Front Physiol. 2017;8:845. PMID: 29118721 PMC: PMC5661004.
Zubcevic J, Baker A, Martyniuk CJ. Transcriptional networks in rodent models support a role for gut-brain communication in neurogenic hypertension: a review of the evidence. Physiol Genomics. 2017;49(7):327–38. PMID: 28550087.
Zubcevic J, et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63(3):542–50. PMID: 24366083 PMC: PMC3945212.
Zubcevic J, et al. Nucleus of the solitary tract (pro)renin receptor-mediated antihypertensive effect involves nuclear factor-kappaB-cytokine signaling in the spontaneously hypertensive rat. Hypertension. 2013;61(3):622–7. PMID: 23319541.
Zubcevic J, Potts JT. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp Physiol. 2010;95(9):909–18. PMID: 20591977.
Zubcevic J, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104–116. PMID: 31219753 PMC: PMC6588177.
Zubcevic J, et al. A single angiotensin II hypertensive stimulus is associated with prolonged neuronal and immune system activation in Wistar-Kyoto rats. Front Physiol. 2017;8:592. PMID: 28912720 PMC: PMC5583219.
Zubcevic J, et al. Functional neural-bone marrow pathways: implications in hypertension and cardiovascular disease. Hypertension. 2014;63(6):e129–39. PMID: 24688127 PMC: PMC4295780.
Zubcevic J, et al. Chronic blockade of phosphatidylinositol 3-kinase in the nucleus tractus solitarii is prohypertensive in the spontaneously hypertensive rat. Hypertension. 2009;53(1):97–103. PMID: 19015400 PMC: PMC2735392.
Zubcevic J, et al. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension. 2011;57(6):1026–33. PMID: 21536990 PMC: PMC3105900.
Zubcevic J, et al. MEMRI reveals altered activity in brain regions associated with anxiety, locomotion, and cardiovascular reactivity on the elevated plus maze in the WKY vs SHR rats. Brain Imaging Behav. 2018;12(5):1318–31. PMID: 29181695.
Adapala RK, et al. TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic Res Cardiol. 2020;115(2):14. PMID: 31925567 PMC: PMC7322630.
Adapala RK, et al. TRPV4 mechanotransduction in fibrosis. Cells. 2021;10(11). PMID: 34831281 PMC: PMC8619244.
Adapala RK, et al. PKCalpha mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301(3):H757–65. PMID: 21705673 PMC: PMC3302191.
Adapala RK, et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. 2016;35(3):314–22. PMID: 25867067 PMC: PMC4948740.
Adapala RK, et al. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol. 2013;54:45–52. PMID: 23142541 PMC: PMC3935769.
Al-Azzam N, et al. Transient Receptor Potential Vanilloid channel regulates fibroblast differentiation and airway remodeling by modulating redox signals through NADPH oxidase 4. Sci Rep. 2020;10(1):9827. PMID: 32555397 PMC: PMC7299963.
Cappelli HC, et al. Transient receptor potential vanilloid 4 channel deletion regulates pathological but not developmental retinal angiogenesis. J Cell Physiol. 2021;236(5):3770–3779. PMID: 33078410 PMC: PMC7920906.
Cappelli HC, et al. Mechanosensitive TRPV4 channels stabilize VE-cadherin junctions to regulate tumor vascular integrity and metastasis. Cancer Lett. 2019;442: 15–20. PMID: 30401632 PMC: PMC6924277.
DelloStritto DJ, et al. Differential regulation of TRPV1 channels by H2O2: implications for diabetic microvascular dysfunction. Basic Res Cardiol 2016;111(2):21. PMID: 26907473 PMC: PMC4831139.
DelloStritto DJ, et al. 4-Hydroxynonenal dependent alteration of TRPV1-mediated coronary microvascular signaling. Free Radic Biol Med. 2016;101:10–19. PMID: 27682362 PMC: PMC5490661.
Gombedza F, et al. Mechanosensitive transient receptor potential vanilloid 4 regulates Dermatophagoides farinae-induced airway remodeling via 2 distinct pathways modulating matrix synthesis and degradation. FASEB J. 2017;31(4):1556–1570. PMID: 28073835 PMC: PMC5349793.
Guarini G, et al. Disruption of TRPV1-mediated coupling of coronary blood flow to cardiac metabolism in diabetic mice: role of nitric oxide and BK channels. Am J Physiol Heart Circ Physiol. 2012;303(2):H216–23. PMID: 22610171.
Guarino BD, Paruchuri S, Thodeti CK. The role of TRPV4 channels in ocular function and pathologies. Exp Eye Res. 2020;201:108257. PMID: 32979394 PMC: PMC7736234.
Kanugula AK, et al. Novel noncanonical regulation of soluble VEGF/VEGFR2 signaling by mechanosensitive ion channel TRPV4. FASEB J. 2019;33(1):195–203. PMID: 29957061 PMC: PMC6355071.
Matthews BD, et al. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol (Camb). 2010;2(9):435–42. PMID: 20725677 PMC: PMC3147167.
Ohanyan VA, et al. Endothelin-mediated in vivo pressor responses following TRPV1 activation. Am J Physiol Heart Circ Physiol. 2011;301(3):H1135–42. PMID: 21705674.
Rahaman SO, et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest. 2014;124(12):5225–38. PMID: 25365224 PMC: PMC4348970.
Thodeti CK, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104(9):1123–30. PMID: 19359599 PMC: PMC2754067.
Thodeti CK, Paruchuri S, Meszaros JG. A TRP to cardiac fibroblast differentiation. Channels (Austin). 2013;7(3):211–4. PMID: 23511028 PMC: PMC3710348.
Thoppil RJ, et al. TRPV4 channel activation selectively inhibits tumor endothelial cell proliferation. Sci Rep. 2015;5:14257. PMID: 26388427 PMC: PMC4585691.
Thoppil RJ, et al. TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget. 2016;7(18):25849–61. PMID: 27029071 PMC: PMC5041949.
Ampem PT, Smedlund K, Vazquez G. Pharmacological evidence for a role of the transient receptor potential canonical 3 (TRPC3) channel in endoplasmic reticulum stress-induced apoptosis of human coronary artery endothelial cells. Vascul Pharmacol. 2016;76:42–52. PMID: 26215710 PMC: PMC4706806.
Dube PR, Birnbaumer L, Vazquez G. Evidence for constitutive bone morphogenetic protein-2 secretion by M1 macrophages: Constitutive auto/paracrine osteogenic signaling by BMP-2 in M1 macrophages. Biochem Biophys Res Commun. 2017;491(1):154–158. PMID: 28711495 PMC: PMC5572828.
Dube PR, et al. Reduced calcification and osteogenic features in advanced atherosclerotic plaques of mice with macrophage-specific loss of TRPC3. Atherosclerosis. 2018;270:199–204. PMID: 29290366 PMC: PMC5835414.
Kumarasamy S, et al. Deep transcriptomic profiling of M1 macrophages lacking Trpc3. Sci Rep. 2017;7 39867. PMID: 28051144 PMC: PMC5209678.
Smedlund K, Bah M, Vazquez G. On the role of endothelial TRPC3 channels in endothelial dysfunction and cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2012;10(3):265–74. PMID: 22827251 PMC: PMC3465809.
Smedlund K, Dube P, Vazquez G. Early steatohepatitis in hyperlipidemic mice with endothelial-specific gain of TRPC3 function precedes changes in aortic atherosclerosis. Physiol Genomics. 2016;48(8):644–9. PMID: 27449657 PMC: PMC5005460.
Smedlund K, Tano JY, Vazquez G. The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ Res. 2010;106(9):1479–88. PMID: 20360250.
Smedlund K, Vazquez G. Involvement of native TRPC3 proteins in ATP-dependent expression of VCAM-1 and monocyte adherence in coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(11):2049–55. PMID: 18787184.
Smedlund KB, Birnbaumer L, Vazquez, Increased size and cellularity of advanced atherosclerotic lesions in mice with endothelial overexpression of the human TRPC3 channel. Proc Natl Acad Sci USA. 2015;112(17):E2201–6. PMID: 25870279 PMC: PMC4418920.
Solanki S, et al. Reduced necrosis and content of apoptotic M1 macrophages in advanced atherosclerotic plaques of mice with macrophage-specific loss of Trpc3. Sci Rep. 2017;7:42526. PMID: 28186192 PMC: PMC5301208.
Solanki S, et al. Reduced endoplasmic reticulum stress-induced apoptosis and impaired unfolded protein response in TRPC3-deficient M1 macrophages. Am J Physiol Cell Physiol. 2014;307(6):C521–31. PMID: 25031020 PMC: PMC4166736.
Tano JY, Lee RH, Vazquez G. Macrophage function in atherosclerosis: potential roles of TRP channels. Channels (Austin). 2012;6(3):141–8. PMID: 22909953 PMC: PMC3431590.
Tano JY, et al. Impairment of survival signaling and efferocytosis in TRPC3-deficient macrophages. Biochem Biophys Res Commun. 2011;410(3):643–7. PMID: 21684255.
Tano JY, Smedlund K, Vazquez G. Endothelial TRPC3/6/7 proteins at the edge of cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2010;8(1):76–86. PMID: 20214601.
Tano JY, et al. Bone marrow deficiency of TRPC3 channel reduces early lesion burden and necrotic core of advanced plaques in a mouse model of atherosclerosis. Cardiovasc Res. 2014;101(1):138–44. PMID: 24101197 PMC: PMC3868349.
Vazquez G. TRPC channels as prospective targets in atherosclerosis: terra incognita. Front Biosci (Schol Ed). 2012;4:157–66. PMID: 22202050.
Vazquez G, et al. On the roles of the transient receptor potential canonical 3 (TRPC3) channel in endothelium and macrophages: implications in atherosclerosis. Adv Exp Med Biol. 2016;898:185–99. PMID: 27161230.
Vazquez G, Tano JY, Smedlund K. On the potential role of source and species of diacylglycerol in phospholipase-dependent regulation of TRPC3 channels. Channels (Austin). 2010;4(3):232–40. PMID: 20458187.
Ahmed ASI, et al. Long noncoding RNA NEAT1 (nuclear paraspeckle assembly transcript 1) is critical for phenotypic switching of vascular smooth muscle cells. Proc Natl Acad Sci USA. 2018;115(37):E8660-E8667. PMID: 30139920 PMC: PMC6140535.
Dong K, et al. CARMN is an evolutionarily conserved smooth muscle cell-specific LncRNA that maintains contractile phenotype by binding myocardin. Circulation. 2021;144(23):1856–1875. PMID: 34694145 PMC: PMC8726016.
Fairaq A, et al. AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: implications toward suppression of neointimal hyperplasia. Pharmacol Res. 2017;119:289–302. PMID: 28237515 PMC: PMC5392421.
He X, et al. Deficiency of the novel high mobility group protein HMGXB4 protects against systemic inflammation-induced endotoxemia in mice. Proc Natl Acad Sci USA. 2021;118(7). PMID: 33563757 PMC: PMC7896282.
Liu J, et al. TEAD1 protects against necroptosis in postmitotic cardiomyocytes through regulation of nuclear DNA-encoded mitochondrial genes. Cell Death Differ. 2021;28(7):2045–2059. PMID: 33469230 PMC: PMC8257617.
Osman I, et al. YAP1/TEAD1 upregulate platelet-derived growth factor receptor beta to promote vascular smooth muscle cell proliferation and neointima formation. J Mol Cell Cardiol, 2021;156:20–32. PMID: 33753119 PMC: PMC8217227.
Osman I, Fairaq A, Segar L. Pioglitazone attenuates injury-induced neointima formation in mouse femoral artery partially through the activation of AMP-activated protein kinase. Pharmacology. 2017;100(1–2):64–73. PMID: 28482342 PMC: PMC5884074.
Osman I, et al. TEAD1 (TEA domain transcription factor 1) promotes smooth muscle cell proliferation through upregulating SLC1A5 (solute carrier family 1 member 5)-mediated glutamine uptake. Circ Res. 2019;124(9):1309–1322. PMID: 30801233 PMC: PMC6493685.
Osman I, et al. High fructose-mediated attenuation of insulin receptor signaling does not affect PDGF-induced proliferative signaling in vascular smooth muscle cells. Eur J Pharmacol. 2016;791:703–710. PMID: 27729247 PMC: PMC5107134.
Osman I, Segar L. Pioglitazone, a PPARgamma agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem Pharmacol. 2016;101:54–70. PMID: 26643070 PMC: PMC4753090.
Osman I, et al. GFAP (glial fibrillary acidic protein)-positive progenitor cells contribute to the development of vascular smooth muscle cells and endothelial cells—brief report. Arterioscler Thromb Vasc Biol. 2020;40(5):1231–1238. PMID: 32160776 PMC: PMC7180117.
Pyla R, et al. Metformin exaggerates phenylephrine-induced AMPK phosphorylation independent of CaMKKbeta and attenuates contractile response in endothelium-denuded rat aorta. Biochem Pharmacol. 2014;92(2):266–79. PMID: 25179145 PMC: PMC4252840.
Wen T, et al. Transcription factor TEAD1 is essential for vascular development by promoting vascular smooth muscle differentiation. Cell Death Differ. 2019;26(12):2790–2806. PMID: 31024075 PMC: PMC7224394.
Bao H, et al. Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells. FASEB J. 2019;33(12):14370–81. PMID: 31661633.
Chang M, et al. Melanocortin system in kidney homeostasis and disease: novel therapeutic opportunities. Front Physiol. 2021;12:651236. PMID: 33716796 PMC: PMC7943476.
Chen B, et al. Mineralocorticoid receptor: a hidden culprit for hemodialysis vascular access dysfunction. EBioMedicine. 2019;39:621–627. PMID: 30527626 PMC: PMC6354623.
Chen B, et al. Permissive effect of GSK3beta on profibrogenic plasticity of renal tubular cells in progressive chronic kidney disease. Cell Death Dis. 2021;12(5):432. PMID: 33931588 PMC: PMC8087712.
Fang Y, et al. The ketone body beta-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults. Kidney Int. 2021;100(5):1037–53. PMID: 34246657.
Fang Y, et al. The ageing kidney: molecular mechanisms and clinical implications. Ageing Res Rev. 2020;63:101151. PMID: 32835891 PMC: PMC7595250.
Guo J, et al. RNA-binding proteins tristetraprolin and human antigen R are novel modulators of podocyte injury in diabetic kidney disease. Cell Death Dis. 2020;11(6):413. PMID: 32487989 PMC: PMC7265504.
Guo J, Liu Z, Gong R. Long noncoding RNA: an emerging player in diabetes and diabetic kidney disease. Clin Sci (Lond). 2019;133(12):1321–39. PMID: 31221822.
Guo Q, et al. Brain natriuretic peptide mitigates TIMP2 induction and reinstates extracellular matrix catabolic activity via GSK3beta inhibition in glomerular podocytes exposed to a profibrogenic milieu. Am J Transl Res. 2019;11(2):964–973. PMID: 30899395 PMC: PMC6413260.
Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy. 2021;17(1):1–382. PMID: 33634751 PMC: PMC7996087.
Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. PMID: 26799652 PMC: PMC4835977.
Liang X, et al. Triptolide potentiates the cytoskeleton-stabilizing activity of cyclosporine A in glomerular podocytes via a GSK3beta dependent mechanism. Am J Transl Res. 2020;12(3):800–812. PMID: 32269713 PMC: PMC7137037.
Liang X, et al. Glycogen synthase kinase 3beta hyperactivity in urinary exfoliated cells predicts progression of diabetic kidney disease. Kidney Int. 2020;97(1):175–92. PMID: 31791666.
Lu M, et al. Activation of mineralocorticoid receptor by ecdysone, an adaptogenic and anabolic ecdysteroid, promotes glomerular injury and proteinuria involving overactive GSK3beta pathway signaling. Sci Rep. 2018;8(1):12225. PMID: 30111886 PMC: PMC6093907.
Lu M, et al. GSK3beta-mediated Keap1-independent regulation of Nrf2 antioxidant response: a molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 2019;26:101275. PMID: 31349118 PMC: PMC6669347.
Lu M, et al. Ecdysone elicits chronic renal impairment via mineralocorticoid-like pathogenic activities. Cell Physiol Biochem. 2018;49(4):1633–45. PMID: 30227391.
Qiao Y, et al. Melanocortin therapy ameliorates podocytopathy and proteinuria in experimental focal segmental glomerulosclerosis involving a podocyte specific non-MC1R-mediated melanocortinergic signaling. Clin Sci (Lond). 2020;134(7):695–710. PMID: 32167144.
Shaban E, et al. Targeting regulatory T cells for transplant tolerance: new insights and future perspectives. Kidney Dis (Basel). 2018;4(4):205–213. PMID: 30574497 PMC: PMC6276757.
Shaffner J, et al. Therapeutic targeting of SGLT2: a new era in the treatment of diabetes and diabetic kidney disease. Front Endocrinol (Lausanne). 2021;12:749010. PMID: 34790170 PMC: PMC8591164.
Sharif S, et al. Rationale and design of assessing the effectiveness of short-term low-dose lithium therapy in averting cardiac surgery-associated acute kidney injury: a randomized, double blinded, placebo controlled pilot trial. Front Med (Lausanne). 2021; 8:639402. PMID: 34195206 PMC: PMC8236527.
Shrivastava S, et al. Relapse of nephrotic syndrome after adrenocorticotropic hormone-induced remission: implications of adrenocorticotropic hormone antibodies. Am J Nephrol. 2020;51(5):390–394. PMID: 32187600 PMC: PMC7521046.
Xu Y, et al. Melanocortin 5 receptor signaling pathway in health and disease. Cell Mol Life Sci. 2020;77(19):3831–3840. PMID: 32248247 PMC: PMC7511443.
Zhang J, et al. Microdose lithium protects against pancreatic islet destruction and renal impairment in streptozotocin-elicited diabetes. Antioxidants (Basel). 2021;10(1). PMID: 33478120 PMC: PMC7835906.
Cooper CJ, et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117(21):2752–60. PMID: 18490527.
Drummond CA, et al. Cigarette smoking causes epigenetic changes associated with cardiorenal fibrosis. Physiol Genomics. 2016;48(12):950–960. PMID: 27789733 PMC: PMC5206391.
Drummond CA, et al. Na/K-ATPase signaling mediates miR-29b-3p regulation and cardiac fibrosis formation in mice with chronic kidney disease. PLoS One. 2018;13(5):e0197688. PMID: 29775473 PMC: PMC5959191.
Drummond CA, et al. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol Genomics. 2016;48(3):220–9. PMID: 26702050 PMC: PMC4773889.
Dube P, et al. Vascular calcification in chronic kidney disease: diversity in the vessel wall. Biomedicines. 2021;9(4). PMID: 33917965 PMC: PMC8068383.
Fedorova LV, et al. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. Am J Physiol Renal Physiol. 2009;296(4):F922–34. PMID: 19176701 PMC: PMC3973402.
Fedorova LV, et al. Mitochondrial impairment in the five-sixth nephrectomy model of chronic renal failure: proteomic approach. BMC Nephrol. 2013;14:209. PMID: 24090408 PMC: PMC3851543.
Kennedy DJ, et al. Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis. 2003;42(5):926–35. PMID: 14582036.
Kennedy DJ, et al. Telocinobufagin, a novel cardiotonic steroid, promotes renal fibrosis via Na(+)/K(+)-ATPase profibrotic signaling pathways. Int J Mol Sci. 2018;19(9). PMID: 30158457 PMC: PMC6164831.
Kennedy DJ, Malhotra D, Shapiro JI. Molecular insights into uremic cardiomyopathy: cardiotonic steroids and Na/K ATPase signaling. Cell Mol Biol (Noisy-le-grand). 2006;52(8):3–14. PMID: 17535729.
Khalaf FK, et al. Proinflammatory effects of cardiotonic steroids mediated by NKA alpha-1 (Na+/K+-ATPase alpha-1)/Src complex in renal epithelial cells and immune cells. Hypertension. 2019;74(1):73–82. PMID: 31132948 PMC: PMC6561827.
Khalaf FK, et al. Cardiotonic steroids and the sodium trade balance: new insights into trade-off mechanisms mediated by the Na(+)/K(+)-ATPase. Int J Mol Sci. 2018;19(9). PMID: 30200235 PMC: PMC6165267.
Khalaf FK, et al. Getting to the heart and soul of chronic kidney disease. J Am Heart Assoc. 2020;9(15):e017427. PMID: 32696700 PMC: PMC7792275.
Khalaf FK, et al. Epithelial and endothelial adhesion of immune cells is enhanced by cardiotonic steroid signaling through Na(+)/K(+)-ATPase-alpha-1. J Am Heart Assoc. 2020;9(3):e013933. PMID: 32013704 PMC: PMC7033897.
Mohammed CJ, et al. Circulating lactonase activity but not protein level of PON-1 predicts adverse outcomes in subjects with chronic kidney disease. J Clin Med. 2019;8(7). PMID: 31311140 PMC: PMC6678354.
Periyasamy SM, et al. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney Int. 2005;67(5):1868–77. PMID: 15840034.
Su RC, et al. Hyperglycemia induces key genetic and phenotypic changes in human liver epithelial HepG2 cells which parallel the Leprdb/J mouse model of non-alcoholic fatty liver disease (NAFLD). PLoS One. 2019;14(12):e0225604. PMID: 31805072 PMC: PMC6894821.
Xie JX, et al. Na/K-ATPase/src complex mediates regulation of CD40 in renal parenchyma. Nephrol Dial Transplant. 2018;33(7):1138–1149. PMID: 29294050 PMC: PMC6031043.
Yan Y, et al. Protein carbonylation of an amino acid residue of the Na/K-ATPase alpha1 subunit determines Na/K-ATPase signaling and sodium transport in renal proximal tubular cells. J Am Heart Assoc. 2016;5(9). PMID: 27613772 PMC: PMC5079028.
Zhang S, et al. Renal fibrosis is significantly attenuated following targeted disruption of Cd40 in experimental renal ischemia. J Am Heart Assoc. 2020;9(7):e014072. PMID: 32200719 PMC: PMC7428653.
Buqaileh R, et al. Can cilia provide an entry gateway for SARS-CoV-2 to human ciliated cells. Physiol Genomics. 2021;53(6):249–258. PMID: 33855870 PMC: PMC8213509.
Kathem SH, et al. Capillary endothelia from two ADPKD patients are polyploidy. Ann Clin Cytol Pathol. 2016. 2(2). PMID: 28530000 PMC: PMC5436797.
Mohieldin AM, et al. Chemical-free technique to study the ultrastructure of primary cilium. Sci Rep. 2015;5:15982. PMID: 26521680 PMC: PMC4629161.
Mohieldin AM, et al. Proteomic identification reveals the role of ciliary extracellular-like vesicle in cardiovascular function. Adv Sci (Weinh). 2020;7(16):1903140. PMID: 32832346 PMC: PMC7435257.
Mohieldin AM, et al. Vascular endothelial primary cilia: mechanosensation and hypertension. Curr Hypertens Rev. 2016;12(1):57–67. PMID: 26122329.
Omran AJA, et al. Alcohol consumption impairs the ependymal cilia motility in the brain ventricles. Sci Rep. 2017;7(1):13652. PMID: 29057897 PMC: PMC5651853.
Saternos H, Ley S, AbouAlaiwi W. Primary cilia and calcium signaling interactions. Int J Mol Sci. 2020;21(19). PMID: 32993148 PMC: PMC7583801.
Saternos HC, AbouAlaiwi WA. Implications of dysfunction of mechanosensory cilia in polycystic kidney disease, in Polycystic Kidney Disease, X. Li, Editor. 2015: Brisbane (AU).
Saternos HC, AbouAlaiwi AW. Signaling interplay between primary cilia and nitric oxide: a mini review. Nitric Oxide. 2018;80:108–112. PMID: 30099097 PMC: PMC6186180.
Saternos HC, AbouAlaiwi WA. Commentary: alcohol consumption impairs the ependymal cilia motility in the brain ventricles. J Neurol Neuromedicine. 2019;4(2):20–21. PMID: 31912006 PMC: PMC6945116.
Saternos HC, et al. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics. 2018;50(1):1–9. PMID: 29093194.
Acknowledgements
The content of this article was made possible via interviews with past and present leaders in hypertension and cardiovascular research from the University of Toledo College of Medicine and Life Sciences. Authors are grateful to the interviewers and interviewees. The recordings of these interviews are available at https://drive.google.com/drive/folders/1E-Dmya8ViJTgHGjtRBbQgrCNMvUprFIX?usp=sharing.
Funding
B. J. received support from the University of Toledo and funding (R01HL14308) from the National Heart Lung and Blood Institute of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have existing conflicts of interest.
Informed Consent
Written consents were obtained from all individuals who participated in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and the Kidney
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gokula, V., Terrero, D. & Joe, B. Six Decades of History of Hypertension Research at the University of Toledo: Highlighting Pioneering Contributions in Biochemistry, Genetics, and Host-Microbiota Interactions. Curr Hypertens Rep 24, 669–685 (2022). https://doi.org/10.1007/s11906-022-01226-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-022-01226-0