Abstract
Purpose of Review
To review the etiology of inverse salt sensitivity of blood pressure (BP).
Recent Findings
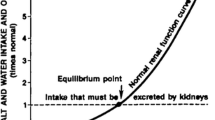
Both high and low sodium (Na+) intake can be associated with increased BP and cardiovascular morbidity and mortality. However, little is known regarding the mechanisms involved in the increase in BP in response to low Na+ intake, a condition termed inverse salt sensitivity of BP, which affects approximately 15% of the adult population. The renal proximal tubule is important in regulating up to 70% of renal Na+ transport. The renin-angiotensin and renal dopaminergic systems play both synergistic and opposing roles in the regulation of Na+ transport in this nephron segment. Clinical studies have demonstrated that individuals express a “personal salt index” (PSI) that marks whether they are salt-resistant, salt-sensitive, or inverse salt-sensitive. Inverse salt sensitivity results in part from genetic polymorphisms in various Na+ regulatory genes leading to a decrease in natriuretic activity and an increase in renal tubular Na+ reabsorption leading to an increase in BP.
Summary
This article reviews the potential mechanisms of a new pathophysiologic entity, inverse salt sensitivity of BP, which affects approximately 15% of the general adult population.








Similar content being viewed by others
Abbreviations
- Ang:
-
Angiotensin
- AT1R:
-
Angiotensin II type 1 receptor
- AT2R:
-
Angiotensin II type 2 receptor
- BP:
-
Blood pressure
- D2R:
-
Dopamine type 2 receptor
- DRD2 :
-
Dopamine type 2 receptor gene
- GRK4:
-
G protein-coupled receptor kinase type 4
- MAP:
-
Mean arterial pressure
- ISS:
-
Inverse salt-sensitive and inverse salt sensitivity
- SR:
-
Salt-resistant
- SS:
-
Salt-sensitive
- uRPTCs:
-
Urine renal proximal tubule cells
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484–594.
•• He FJ, Tan M, Ma Y, MacGregor GA. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632–47. This comprehensive review highlights the important role of elevated dietary salt in cardiovascular disease with or without elevated blood pressure.
•• Cappuccio FP, Beer M, Strazzullo P, Network ESA. Population dietary salt reduction and the risk of cardiovascular disease. A scientific statement from the European Salt Action Network. Nutr Metab Cardiovasc Dis. 2018;29:107–114. This paper discusses the fact that a low salt diet can lead to increased morbidity and mortality.
Elfassy T, Chamany S, Bartley K, Yi SS, Angell SY. Lower 24-h urinary sodium excretion is associated with hypertension control: the 2010 Heart Follow-Up Study. J Hum Hypertens. 2020;34:624–32.
Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, et al. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–66.
Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL, et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68:e7–46.
Rucker AJ, Rudemiller NP, Crowley SD. Salt, hypertension, and immunity. Annu Rev Physiol. 2018;80:283–307.
Lastra G, Dhuper S, Johnson MS, Sowers JR. Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. Nat Rev Cardiol. 2010;7:577–84.
Kurtz TW, DiCarlo SE, Pravenec M, Morris RC. The American Heart Association Scientific Statement on salt sensitivity of blood pressure: prompting consideration of alternative conceptual frameworks for the pathogenesis of salt sensitivity? J Hypertens. 2017;35:2214–25.
Skrabal F, Herholz H, Neumayr M, Hamberger L, Ledochowski M, Sporer H, Hortnagl H, Schwarz S, Schonitzer D. Salt sensitivity in humans is linked to enhanced sympathetic responsiveness and to enhanced proximal tubular reabsorption. Hypertension. 1984;6:152–8.
Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34:946–52.
Sigmund CD, Carey RM, Appel LJ, Arnett DK, Bosworth HB, Cushman WC, Galis ZS, Green Parker M, Hall JE, Harrison DG, et al. Report of the National Heart, Lung, and Blood Institute Working Group on hypertension: barriers to translation. Hypertension. 2020;75:902–17.
Raizada MK, Joe B, Bryan NS, Chang EB, Dewhirst FE, Borisy GG, Galis ZS, Henderson W, Jose PA, Ketchum CJ, et al. Report of the National Heart, Lung, and Blood Institute Working Group on the role of microbiota in blood pressure regulation: current status and future directions. Hypertension. 2017;HYPERTENSIONAHA.117.09699.
Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–32.
Alderman MH, Cohen HW. Dietary sodium intake and cardiovascular mortality: controversy resolved? Curr Hypertens Rep. 2012;14:193–201.
O’Donnell M, Mente A, Alderman MH, Brady AJB, Diaz R, Gupta R, López-Jaramillo P, Luft FC, Lüscher TF, Mancia G, et al. Salt and cardiovascular disease: insufficient evidence to recommend low sodium intake. Eur Heart J. 2020;41:3363–73.
Asayama K, Stolarz-Skrzypek K, Persu A, Staessen JA. Systematic review of health outcomes in relation to salt intake highlights the widening divide between guidelines and the evidence. Am J Hypertens. 2014;27:1138–42.
O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–23.
Strom BL, Anderson CA, Ix JH. Sodium reduction in populations: insights from the Institute of Medicine committee. JAMA. 2013;310:31–2.
Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–7.
Gildea JJ, Lahiff DT, Van Sciver RE, Weiss RS, Shah N, McGrath HE, Schoeffel CD, Jose PA, Carey RM, Felder RA. A linear relationship between the ex-vivo sodium mediated expression of two sodium regulatory pathways as a surrogate marker of salt sensitivity of blood pressure in exfoliated human renal proximal tubule cells: the virtual renal biopsy. Clin Chim Acta. 2013;421:236–42.
Overlack A, Ruppert M, Kolloch R, Göbel B, Kraft K, Diehl J, Schmitt W, Stumpe KO. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension. 1993;22:331–8.
Montasser ME, Douglas JA, Roy-Gagnon MH, Van Hout CV, Weir MR, Vogel R, Parsa A, Steinle NI, Snitker S, Brereton NH, Chang YP, Shuldiner AR, Mitchell BD. Determinants of blood pressure response to low-salt intake in a healthy adult population. Journal of Clinical Hypertension (Greenwich). 2011;13:795–800.
Overlack A, Ruppert M, Kolloch R, Kraft K, Stumpe KO. Age is a major determinant of the divergent blood pressure responses to varying salt intake in essential hypertension. Am J Hypertens. 1995;8(8):829–36.
Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–11.
Miller JZ, Weinberger MH, Daugherty SA, Fineberg NS, Christian JC, Grim CE. Heterogeneity of blood pressure response to dietary sodium restriction in normotensive adults. J Chronic Dis. 1987;40:245–50.
Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol. 2018;72:1278–93.
Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–85.
Graudal N, Jürgens G. Conflicting evidence on health effects associated with salt reduction calls for a redesign of the salt dietary guidelines. Prog Cardiovasc Dis. 2018;61:20–6.
• Alderman MH, Blumenfeld JD. Hypertension: evolving from standardized to individualized care. J Hypertens. 2020;38:1251–4. This paper provides the case for individualized recommendations on how patient treatment regimens should be individualized.
Thomas MC, Moran J, Forsblom C, Harjutsalo V, Thorn L, Ahola A, Wadén J, Tolonen N, Saraheimo M, Gordin D, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2011;34:861–6.
Longworth DL, Drayer JI, Weber MA, Laragh JH. Divergent blood pressure responses during short-term sodium restriction in hypertension. Clin Pharmacol Ther. 1980;27:544–6.
•• Castiglioni P, Parati G, Lazzeroni D, Bini M, Faini A, Brambilla L, Brambilla V, Coruzzi P. Hemodynamic and autonomic response to different salt intakes in normotensive individuals. J Am Heart Assoc. 2016;5: e003736. These studies clearly described the ISS phenotype as being distinct from salt resistant individuals.
Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens. 2013;22:65–76.
Willett WC, McCullough ML. Dietary pattern analysis for the evaluation of dietary guidelines. Asia Pac J Clin Nutr. 2008;17(Suppl 1):75–8.
Mitchell BD, McArdle PF, Shen H, Rampersaud E, Pollin TI, Bielak LF, Jaquish C, Douglas JA, Roy-Gagnon MH, Sack P, et al. The genetic response to short-term interventions affecting cardiovascular function: rationale and design of the Heredity and Phenotype Intervention (HAPI) Heart Study. Am Heart J. 2008;155:823–8.
Welling PA. Rare mutations in renal sodium and potassium transporter genes exhibit impaired transport function. Curr Opin Nephrol Hypertens. 2014;23(1):1–8.
Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–9.
Gildea JJ, Van Sciver R, McGrath HE, Weiss R, Shah I, Shan N, Felder RA. Renal proximal tubule cells isolated from human urine report the degree of salt sensitivity in test subjects. Hypertension. 2010;56: e61.
Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA. Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem. 2013;46:1131–4.
Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–73.
Tan PPS, Hall D, Chilian WM, Chia YC, Mohd Zain S, Lim HM, Kumar DN, Ching SM, Low TY, Md Noh MF, et al. Exosomal microRNAs in the development of essential hypertension and its potential as biomarkers. Am J Physiol Heart Circ Physiol. 2021;320:H1486-1497.
Manosroi W, Williams GH. Genetics of human primary hypertension: focus on hormonal mechanisms. Endocr Rev. 2019;40:825–56.
Rayner B, Ramesar R, Steyn K, Levitt N, Lombard C, Charlton K. G-protein-coupled receptor kinase 4 polymorphisms predict blood pressure response to dietary modification in Black patients with mild-to-moderate hypertension. J Hum Hypertens. 2012;26:334–9.
Jeong S, Kim JY, Cho Y, Koh SB, Kim N, Choi JR. Genetically, dietary sodium intake is causally associated with salt-sensitive hypertension risk in a community-based cohort study: a Mendelian randomization approach. Curr Hypertens Rep. 2020;22:45.
Lee M, Kim MK, Kim SM, Park H, Park CG, Park HK. Gender-based differences on the association between salt-sensitive genes and obesity in Korean children aged between 8 and 9 years. PLoS ONE. 2015;10: e0120111.
Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Watanabe T, Moore JH, Ritchie MD, Williams SM, Pezzullo JC, Sasaki M, et al. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–60.
Bengra C, Mifflin TE, Khripin Y, Manunta P, Williams SM, Jose PA, Felder RA. Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131–40.
Wang Z, Zeng C, Villar VA, Chen SY, Konkalmatt P, Wang X, Asico LD, Jones JE, Yang Y, Sanada H, Felder RA, Eisner GM, Weir MR, Armando I, Jose PA. Human GRK4γ142v variant promotes angiotensin II type I receptor-mediated hypertension via renal histone deacetylase type 1 inhibition. Hypertension. 2016;67:325–34.
Sanada H, Yoneda M, Yatabe J, Williams SM, Bartlett J, White MJ, Gordon LN, Felder RA, Eisner GM, Armando I, et al. Common variants of the G protein-coupled receptor type 4 are associated with human essential hypertension and predict the blood pressure response to angiotensin receptor blockade. Pharmacogenomics J. 2016;16:3–9.
Diao Z, Asico LD, Villar VAM, Zheng X, Cuevas S, Armando I, Jose PA, Wang X. Increased renal oxidative stress in salt-sensitive human GRK4γ486V transgenic mice. Free Radic Biol Med. 2017;106:80–90.
Armando I, Konkalmatt P, Felder RA, Jose PA. The renal dopaminergic system: novel diagnostic and therapeutic approaches in hypertension and kidney disease. Transl Res. 2015;165:505–11.
Harris RC, Zhang MZ. Dopamine, the kidney, and hypertension. Curr Hypertens Rep. 2012;14:138–43.
Felder RA, Jose PA, Xu P, Gildea JJ. The renal sodium bicarbonate cotransporter NBCe2: is it a major contributor to sodium and pH homeostasis? Curr Hypertens Rep. 2016;18:71.
Padia SH, Kemp BA, Howell NL, Fournie-Zaluski MC, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension. 2008;51:460–5.
Röhnert P, Schmidt W, Emmerlich P, Goihl A, Wrenger S, Bank U, Nordhoff K, Täger M, Ansorge S, Reinhold D, et al. Dipeptidyl peptidase IV, aminopeptidase N and DPIV/APN-like proteases in cerebral ischemia. J Neuroinflammation. 2012;9:44.
Kotlo K, Shukla S, Tawar U, Skidgel RA, Danziger RS. Aminopeptidase N reduces basolateral Na+ -K+ -ATPase in proximal tubule cells. Am J Physiol Renal Physiol. 2007;293:F1047-1053.
Padia SH, Howell NL, Kemp BA, Fournie-Zaluski MC, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition restores defective angiontesin II type 2-mediated natriuresis in spontaneously hypertensive rats. Hypertension. 2010;55:474–80.
Hussain T, Abdul-Wahab R, Kotak DK, Lokhandwala MF. Bromocriptine regulates angiotensin II response on sodium pump in proximal tubules. Hypertension. 1998;32:1054–9.
Sarkar C, Ganju RK, Pompili VJ, Chakroborty D. Enhanced peripheral dopamine impairs post-ischemic healing by suppressing angiotensin receptor type 1 expression in endothelial cells and inhibiting angiogenesis. Angiogenesis. 2017;20:97–107.
Garrido-Gil P, Dominguez-Meijide A, Moratalla R, Guerra MJ, Labandeira-Garcia JL. Aging-related dysregulation in enteric dopamine and angiotensin system interactions: implications for gastrointestinal dysfunction in the elderly. Oncotarget. 2018;9:10834–46.
Gao DQ, Canessa LM, Mouradian MM, Jose PA. Expression of the D2 subfamily of dopamine receptor genes in kidney. Am J Physiol. 1994;266:F646-650.
Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Rosón MI, Rodríguez Fermepin M, Fernández BE. Renal dopaminergic system: pathophysiological implications and clinical perspectives. World J Nephrol. 2015;4:196–212.
Feng Y, Lu Y. Immunomodulatory effects of dopamine in inflammatory diseases. Front Immunol. 2021;12: 663102.
Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, D Asico L, Yu P, Grandy DK, Felder RA, Armando I, et al. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med. 2012;53:437–446.
Yang Y, Cuevas S, Yang S, Villar VA, Escano C, Asico L, Yu P, Jiang X, Weinman EJ, Armando I, et al. Sestrin2 decreases renal oxidative stress, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of reactive oxygen species production. Hypertension. 2014;64:825–32.
Jiang X, Konkalmatt P, Yang Y, Gildea J, Jones JE, Cuevas S, Felder RA, Jose PA, Armando I. Single-nucleotide polymorphisms of the dopamine D2 receptor increase inflammation and fibrosis in human renal proximal tubule cells. Hypertension. 2014;63:e74-80.
Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner G, et al. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS ONE. 2012;7: e38745.
Konkalmatt PR, Asico LD, Zhang Y, Yang Y, Drachenberg C, Zheng X, Han F, Jose PA, Armando I. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight. 2016;1: e85888.
Gildea J, Daley M, Xu P, Shiermeyer K, Yue W, Carey R, Jose P, Felder R. Differential redox signaling in urine derived renal proximal tubule cells isolated from inverse salt sensitive vs salt resistant clinical study participants. Hypertension. 2020;76:AP009.
Cao G, Della Penna SL, Kouyoumdzian NM, Choi MR, Gorzalczany S, Fernández BE, Toblli JE, Rosón MI. Immunohistochemical expression of intrarenal renin angiotensin system components in response to tempol in rats fed a high salt diet. World J Nephrol. 2017;6:29–40.
Samuel P, Ali Q, Sabuhi R, Wu Y, Hussain T. High Na intake increases renal angiotensin II levels and reduces expression of the ACE2-AT(2)R-MasR axis in obese Zucker rats. Am J Physiol Renal Physiol. 2012;303:F412-419.
Gonzalez M, Lobos L, Castillo F, Galleguillos L, Lopez NC, Michea L. High-salt diet inhibits expression of angiotensin type 2 receptor in resistance arteries. Hypertension. 2005;45:853–9.
Dopona EPB, Rocha VF, Furukawa LNS, Oliveira IB, Heimann JC. Myocardial hypertrophy induced by high salt consumption is prevented by angiotensin II AT2 receptor agonist. Nutr Metab Cardiovasc Dis. 2019;29:301–5.
Gildea JJ, Wang X, Shah N, Tran H, Spinosa M, Van Sciver R, Sasaki M, Yatabe J, Carey RM, Jose PA, et al. Dopamine and angiotensin type 2 receptors cooperatively inhibit sodium transport in human renal proximal tubule cells. Hypertension. 2012;60:396–403.
Yang S, Han Y, Zheng S, Kou X, Asico LD, Huang H, Gao Z, Jose PA, Zeng C. Enhanced natriuresis and diuresis in Wistar rats caused by the costimulation of renal dopamine D3 and angiotensin II type 2 receptors. Am J Hypertens. 2015;28:1267–76.
Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1660-1669.
Jiang X, Chen W, Liu X, Wang Z, Liu Y, Felder RA, Gildea JJ, Jose PA, Qin C, Yang Z. The synergistic roles of cholecystokinin B and dopamine D5 receptors on the regulation of renal sodium excretion. PLoS ONE. 2016;11: e0146641.
Jiang X, Zhang Y, Yang Y, Yang J, Asico LD, Chen W, Felder RA, Armando I, Jose PA, Yang Z. Gastrin stimulates renal dopamine production by increasing the renal tubular uptake of l-DOPA. Am J Physiol Endocrinol Metab. 2017;312:E1–10.
Svitok P, Molcan L, Vesela A, Kruzliak P, Moravcik R, Zeman M. Increased salt intake during early ontogenesis lead to development of arterial hypertension in salt-resistant Wistar rats. Clin Exp Hypertens. 2015;37:142–7.
Walsh KR, Kuwabara JT, Shim JW, Wainford RD. Norepinephrine-evoked salt-sensitive hypertension requires impaired renal sodium chloride cotransporter activity in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R115-124.
Sharma AM, Schorr U, Oelkers W, Distler A. Effects of sodium salts on plasma renin activity and norepinephrine response to orthostasis in salt-sensitive normotensive subjects. Am J Hypertens. 1993;6:780–5.
Wainford RD, Carmichael CY, Pascale CL, Kuwabara JT. Gαi2-protein-mediated signal transduction: central nervous system molecular mechanism countering the development of sodium-dependent hypertension. Hypertension. 2015;65:178–86.
Stein CM, Nelson R, Brown M, He H, Wood M, Wood AJ. Dietary sodium intake modulates systemic but not forearm norepinephrine release. Clin Pharmacol Ther. 1995;58:425–33.
Stupin A, Drenjančević I, Šušnjara P, Debeljak Ž, Kolobarić N, Jukić I, Mihaljević Z, Martinović G, Selthofer-Relatić K. Is there association between altered adrenergic system activity and microvascular endothelial dysfunction induced by a 7-day high salt intake in young healthy individuals. Nutrients. 2021;13:1731.
Friberg P, Meredith I, Jennings G, Lambert G, Fazio V, Esler M. Evidence for increased renal norepinephrine overflow during sodium restriction in humans. Hypertension. 1990;16:121–30.
Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022.
Yang L, Sandberg M, Can A, Pihakashi-Maunsback K, McDonough A. Effects of dietary salt on renal Na+ transporters’ subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol. 2008;295:F1003–16.
Natarajan AR, Eisner GM, Armando I, Browning S, Pezzullo JC, Rhee L, Dajani M, Carey RM, Jose PA. The Renin-Angiotensin and Renal Dopaminergic Systems Interact in Normotensive Humans. J Am Soc Nephrol. 2016;27:265–79.
Masilamani S, Wang X, Kim GH, Brooks H, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol. 2002;283:F648-657.
Frindt G, Palmer LG. Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol. 2009;297:F1249-1255.
Udwan K, Abed A, Roth I, Dizin E, Maillard M, Bettoni C, Loffing J, Wagner CA, Edwards A, Feraille E. Dietary sodium induces a redistribution of the tubular metabolic workload. J Physiol. 2017;595:6905–22.
Fenton RA, Poulsen SB, de la Mora CS, Soleimani M, Dominguez Rieg JA, Rieg T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int. 2017;92:397–414.
Lahjouji K, Aouameur R, Bissonnette P, Coady MJ, Bichet DG, Lapointe JY. Expression and functionality of the Na+/myo-inositol cotransporter SMIT2 in rabbit kidney. Biochim Biophys Acta. 2007;1768:1154–9.
Groenen PM, Klootwijk R, Schijvenaars MM, Straatman H, Mariman EC, Franke B, Steegers-Theunissen RP. Spina bifida and genetic factors related to myo-inositol, glucose, and zinc. Mol Genet Metab. 2004;82:154–61.
Packer M. Activation and inhibition of sodium-hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation. 2017;136:1548–59.
Yang LE, Leong PK, Ye S, Campese VM, McDonough AA. Responses of proximal tubule sodium transporters to acute injury-induced hypertension. Am J Physiol Renal Physiol. 2003;284:F313-322.
Crajoinas RO, Polidoro JZ, Carneiro de Morais CP, Castelo-Branco RC, Girardi AC. Angiotensin II counteracts the effects of cAMP/PKA on NHE3 activity and phosphorylation in proximal tubule cells. Am J Physiol Cell Physiol. 2016;311:C768-C776.
Titze J, Rakova N, Kopp C, Dahlmann A, Jantsch J, Luft FC. Balancing wobbles in the body sodium. Nephrol Dial Transplant. 2016;31(7):1078–81.
Kirabo A. A new paradigm of sodium regulation in inflammation and hypertension. Am J Physiol Regul Integr Comp Physiol. 2017;313(6):R706–10.
Viknesh Selvarajah V, Mäki-Petäjä K, Pedro L, Bruggraber S, Keith Burling K, Goodhart A, Brown M, McEniery C, Wilkinson I. Novel mechanism for buffering dietary salt in humans: efects of salt loading on skin sodium, vascular endothelial growth factor C, and blood pressure. Hypertension. 2017;70(5):930–937.
Wang J, Deng Y, Zou X, Luo H, Jose PA, Fu C, Yang J, Zeng C. Long-term low salt diet increases blood pressure by activation of the renin-angiotensin and sympathetic nervous systems. Clin Exp Hypertens. 2019;41:739–46.
Webb DJ, Clark SA, Brown WB, Fraser R, Lever AF, Murray GD, Robertson JI. Dietary sodium deprivation raises blood pressure in the rat but does not produce irreversible hyperaldosteronism. J Hypertens. 1987;5:525–31.
Ott CE, Welch WJ, Lorenz JN, Whitescarver SA, Kotchen TA. Effect of salt deprivation on blood pressure in rats. Am J Physiol. 1989;256:H1426-1431.
Drüeke TB, Muntzel M. Heterogeneity of blood pressure responses to salt restriction and salt appetite in rats. Klin Wochenschr. 1991;69(Suppl 25):73–8.
Vari RC, Freeman RH, Davis JO, Sweet WD. Role of renal nerves in rats with low-sodium, one-kidney hypertension. Am J Physiol. 1986;250:H189-194.
Rakova N, Jüttner K, Dahlmann A, Schröder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013;17:125–31.
Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell NRC, Li Q, Lackland DT, Leung AA, Anderson CAM, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368: m315.
Galan-Rodriguez B, Martin E, Brouillet E, Déglon N, Betuing S, Caboche J. Coupling of D2R Short but not D2R long receptor isoform to the Rho/ROCK signaling pathway renders striatal neurons vulnerable to mutant huntingtin. Eur J Neurosci. 2017;45:198–206.
Glier MB, Green TJ, Devlin AM. Methyl nutrients, DNA methylation, and cardiovascular disease. Mol Nutr Food Res. 2014;58:172–82.
Randunu RS, Bertolo RF. The effects of maternal and postnatal dietary methyl nutrients on epigenetic changes that lead to non-communicable diseases in adulthood. Int J Mol Sci. 2020;21.
Wang J, Yin N, Deng Y, Wei Y, Huang Y, Pu X, Li L, Zheng Y, Guo J, Yu J, et al. Ascorbic acid protects against hypertension through downregulation of ACE1 gene expression mediated by histone deacetylation in prenatal inflammation-induced offspring. Sci Rep. 2016;6:39469.
Lamothe J, Khurana S, Tharmalingam S, Williamson C, Byrne CJ, Lees SJ, Khaper N, Kumar A, Tai TC. Oxidative stress mediates the fetal programming of hypertension by glucocorticoids. Antioxidants (Basel). 2021;10:531.
Feng W, Dell’Italia LJ, Sanders PW. Novel paradigms of salt and hypertension. J Am Soc Nephrol. 2017;28:1362–9.
Shabaka A, Cases-Corona C, Fernandez-Juarez G. Therapeutic insights in chronic kidney disease progression. Front Med (Lausanne). 2021;8: 645187.
Kawarazaki W, Fujita T. Kidney and epigenetic mechanisms of salt-sensitive hypertension. Nat Rev Nephrol. 2021;17:350–63.
Cuevas S, Yang Y, Konkalmatt P, Asico LD, Feranil J, Jones J, Villar VA, Armando I, Jose PA. Role of nuclear factor erythroid 2-related factor 2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1. Hypertension. 2015;65:1251–7.
Qaddumi WN, Jose PA. The role of the renal dopaminergic system and oxidative stress in the pathogenesis of hypertension. Biomedicines. 2021;9:139.
Armando I, Asico LD, Wang X, Jones JE, Serrão MP, Cuevas S, Grandy DK, Soares-da-Silva P, Jose PA. Antihypertensive effect of etamicastat in dopamine D2 receptor-deficient mice. Hypertens Res. 2018;41:489–98.
Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–8.
Cuevas S, Villar VAM, Jose PA. Genetic polymorphisms associated with reactive oxygen species and blood pressure regulation. Pharmacogenomics J. 2019;19:315–36.
Banday AA, Lokhandwala MF. Transcriptional regulation of renal dopamine D1 receptor function during oxidative stress. Hypertension. 2015;65:1064–72.
Yang S, Yang Y, Yu P, Yang J, Jiang X, Villar VA, Sibley DR, Jose PA, Zeng C. Dopamine D1 and D5 receptors differentially regulate oxidative stress through paraoxonase 2 in kidney cells. Free Radic Res. 2015;49:397–410.
Xia XG, Schmidt N, Teismann P, Ferger B, Schulz JB. Dopamine mediates striatal malonate toxicity via dopamine transporter-dependent generation of reactive oxygen species and D2 but not D1 receptor activation. J Neurochem. 2001;79:63–70.
Gerö D, Módis K, Nagy N, Szoleczky P, Tóth ZD, Dormán G, Szabó C. Oxidant-induced cardiomyocyte injury: identification of the cytoprotective effect of a dopamine 1 receptor agonist using a cell-based high-throughput assay. Int J Mol Med. 2007;20:749–61.
Gildea JJ. Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr Opin Nephrol Hypertens. 2009;18:28–32.
Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, et al. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension. 2006;47:288–95.
Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–9.
Gildea JJ, Wang X, Jose PA, Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension. 2008;51:360–6.
Khella HW, Bakhet M, Lichner Z, Romaschin AD, Jewett MA, Yousef GM. MicroRNAs in Kidney Disease: an emerging understanding. Am J Kidney Dis. 2013;61:798–808.
Gildea J, Xu P, Schiermeyer K, Yue W, Carey R, Jose P, Felder R. The etiology of inverse salt sensitivity of blood pressure: Mirna-485-5p binds to the dopamine type 2 receptor (D2R) SNP Rs6276 and decreases D2R expression. Hypertension. 2020;76:A2.
Wu M, Liang G, Duan H, Yang X, Qin G, Sang N. Synergistic effects of sulfur dioxide and polycyclic aromatic hydrocarbons on pulmonary pro-fibrosis via mir-30c-1-3p/transforming growth factor β type II receptor axis. Chemosphere. 2019;219:268–76.
Zhang Y, Jiang X, Qin C, Cuevas S, Jose PA, Armando I. Dopamine D2 receptors’ effects on renal inflammation are mediated by regulation of PP2A function. Am J Physiol Renal Physiol. 2016;310:F128-134.
Rukavina Mikusic NL, Silva MG, Mazzitelli LR, Santos RAS, Gómez KA, Grecco HE, Gironacci MM. Interaction between the angiotensin-(1–7) Mas receptor and the dopamine D2 receptor: implications in inflammation. Hypertension. 2021;77:1659–69.
Han X, Li B, Ye X, Mulatibieke T, Wu J, Dai J, Wu D, Ni J, Zhang R, Xue J, Wan R, Wang X, Hu G. Dopamine D 2 receptor signalling controls inflammation in acute pancreatitis via a PP2A-dependent Akt/NF-κB signalling pathway. Br J Pharmacol. 2017;174:4751–70.
Wang X, Li F, Jose PA, Ecelbarger CM. Reduction of renal dopamine receptor expression in obese Zucker rats: role of sex and angiotensin II. Am J Physiol Renal Physiol. 2010;299(5):F1164–70.
Acknowledgements
We thank Drs. Brackston Mitchell and Huichun Xu at the Division of Endocrinology, Diabetes and Nutrition, Department of Medicine University of Maryland School of Medicine, and Dr. Gordon Williams at Brigham and Woman’s Hospital, Harvard Medical School, Division of Endocrinology, Diabetes and Hypertension, for sharing their salt sensitivity data that appear in Fig. 2. This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) P01 HL074940; the Principal Investigator is Dr. Felder. Dr. Carey is Principal Investigator of R01 HL128189 and Project Director for Program Project Grant, P01 HL074940. Dr. Jose is Principal Investigator of R01 DK039308 and R01 DK119652 and Project Director of P01 HL074940.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors, Robin A. Felder, John J. Gildea, Peng Xu, Wei Yue, Ines Armando, Robert M. Carey, and Pedro A. Jose, declare no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines). All authors have read and approved the submission of the manuscript; the manuscript, figures, and tables have not been published and are not being considered for publication elsewhere, except for abstracts presented to the American Heart Association Hypertension conference in the 2018–2021 Scientific Sessions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and the Kidney
Rights and permissions
About this article
Cite this article
Felder, R.A., Gildea, J.J., Xu, P. et al. Inverse Salt Sensitivity of Blood Pressure: Mechanisms and Potential Relevance for Prevention of Cardiovascular Disease. Curr Hypertens Rep 24, 361–374 (2022). https://doi.org/10.1007/s11906-022-01201-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-022-01201-9