Abstract
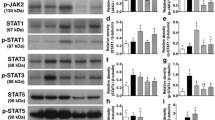
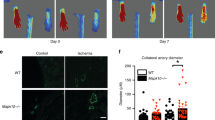
Increased circulating catecholamines have been linked with cardiovascular anomalies as well as with peripheral vascular diseases. Although the roles of epinephrine and norepinephrine have received considerable attention, the role of the other catecholamine, dopamine, has been less studied. Since dopamine is a potent endogenous inhibitor of angiogenesis and as angiogenesis is essential for ischemic healing, we therefore studied the role played by dopamine during ischemic healing using dopamine D2 receptor knockout (KOD2) mice. Although concentration of dopamine and its rate-limiting enzyme, tyrosine hydroxylase, was considerably high in the muscle tissues of wild-type and KOD2 mice with unilateral hind limb ischemia (HLI), recovery was significantly faster in the KOD2 mice compared to the wild-type controls, thereby indicating that peripheral dopamine might have a role in this healing process. In addition, we observed significant differences in post-ischemic angiogenesis between these two groups. Our study further revealed that elevated dopamine independently suppressed activation of local tissue-based renin-angiotensin system (RAS), a critical growth factor system stimulating angiogenesis in ischemia. Angiotensin II (ATII) and its receptor, angiotensin receptor type 1 (AT1R), are the key players in RAS-mediated angiogenesis. Dopamine acting through its D2 receptors in endothelial cells inhibited ATII-mediated angiogenesis by suppressing the expression of AT1R in these cells. This study thus for the first time demonstrates the role played by dopamine in prolonging post-ischemic recovery. Therefore, pharmacological intervention inhibiting the action of dopamine holds promise as future therapeutic strategy for the treatment of HLI and other peripheral arterial diseases.






Similar content being viewed by others
References
Hirsch AT, Duval S (2013) The global pandemic of peripheral artery disease. Lancet 9901:1312–1314
Baumgartner I (2015) Peripheral artery occlusive disease a major contributor to cardiovascular public health burden. Eur Heart J 15:894–896
Freedman SB, Isner JM (2002) Therapeutic angiogenesis for coronary artery disease. Ann Intern Med 136:54–71
Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, Moon T, Chronos NA (2002) Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105:788–793
Lekas M, Lekas P, Latter DA, Kutryk MB, Stewart DJ (2006) Growth factor-induced therapeutic neovascularization for ischaemic vascular disease: Time for a re-evaluation? Curr Opin Cardiol 21:376–384
Tongers J, Roncalli JG, Losordo DW (2008) Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation 118:9–16
Chung AS, Ferrara N (2011) Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 27:563–584
Collinson DJ, Donnelly R (2004) Therapeutic angiogenesis in peripheral arterial disease: Can biotechnology produce an effective collateral circulation? Eur J Vasc Endovasc Surg 1:9–23
Uchida C, Haas TL (2009) Evolving strategies in manipulating VEGF/VEGFR signaling for the promotion of angiogenesis in ischemic muscle. Curr Pharm Des 15:411–421
Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH (2007) Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res 101:948–956
Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, Dasgupta PS, Dvorak HF, Mukhopadhyay D (2001) The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med 7:569–574
Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S (2004) Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res 10:4349–4356
Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S (2004) Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol 287:H1554–H1560
Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S (2008) Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest 118:1380–1389
Shome S, Rana T, Ganguly S, Basu B, Chaki Choudhury S, Sarkar C, Chakroborty D, Dasgupta PS, Basu S (2011) Dopamine regulates angiogenesis in normal dermal wound tissues. PLoS ONE 6:e25215
Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE (2005) Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol 289:H947–H959
Bruno RM, Ghiadoni L, Seravalle G, Dell’oro R, Taddei S, Grassi G (2012) Sympathetic regulation of vascular function in health and disease. Front Physiol 3:284
Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S (2009) Catecholamines regulate tumor angiogenesis. Cancer Res 69:3727–3730
Tilan J, Kitlinska J (2010) Sympathetic neurotransmitters and tumor angiogenesis—link between stress and cancer progression. J Oncol 2010:539706
Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW (2007) Sympathetic activity in major depressive disorder: Identifying those at increased cardiac risk? J Hypertens 25:2117–2124
Kaye DM, Lambert GW, Lefkovits J, Morris M, Jennings G, Esler MD (1994) Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol 23:570–578
Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD (1995) Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 26:1257–1263
Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE (2009) Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135:302–307
Basu S, Sarkar C, Chakroborty D, Nagy J, Mitra RB, Dasgupta PS, Mukhopadhyay D (2004) Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res 64:5551–5555
Sasaki K, Murohara T, Ikeda H, Sugaya T, Shimada T, Shintani S, Imaizumi T (2002) Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest 109:603–611
Gul R, Ramdas M, Mandavia CH, Sowers JR, Pulakat L (2012) RAS-mediated adaptive mechanisms in cardiovascular tissues: confounding factors of RAS blockade therapy and alternative approaches. Cardiorenal Med 2:268–280
Tufan H, Zaki BM, Tecder-Unal M, Erdem SR, Take G (2007) Angiotensin II captopril cotreatment augments angiogenesis in abdominal skin flap in rats. Ann Plast Surg 58:441–448
Everson-Rose SA, Roetker NS, Lutsey PL, Kershaw KN, Longstreth WT, Sacco RL, Diez Roux AV, Alonso A (2014) Chronic stress, depressive symptoms, anger, hostility, and risk of stroke and transient ischemic attack in the multi-ethnic study of atherosclerosis. Stroke 45:2318–2323
Pieterse C, Schutte R, Schutte AE (2016) Leptin relates to prolonged cardiovascular recovery after acute stress in Africans: the SABPA study. Nutr Metab Cardiovasc Dis 26:45–52
Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC (2005) Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 352:539–548
Cas LD, Metra M, Nodari S, Nardi M, Giubbini R, Visioli O (1993) Stress and ischemic heart disease. Cardiologia 38:415–425
Stone PH, Krantz DS, McMahon RP, Goldberg AD, Becker LC, Chaitman BR, Taylor HA, Cohen JD, Freedland KE, Bertolet BD, Coughlan C, Pepine CJ, Kaufmann PG, Sheps DS (1999) Relationship among mental stress-induced ischemia and ischemia during daily life and during exercise: the psychophysiologic investigations of myocardial ischemia (PIMI) study. J Am Coll Cardiol 33:1476–1484
Adler CM, Elman I, Weisenfeld N, Kestler L, Pickar D, Breier A (2000) Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacology 22:545–550
Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ (1989) Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52:1655–1658
Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S (1991) Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res 554:217–222
Tamarat R, Silvestre JS, Huijberts M, Benessiano J, Ebrahimian TG, Duriez M, Wautier MP, Wautier JL, Lévy BI (2003) Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. Proc Natl Acad Sci USA 100:8555–8560
Hendgen-Cotta UB, Luedike P, Totzeck M, Kropp M, Schicho A, Stock P, Rammos C, Niessen M, Heiss C, Lundberg JO, Weitzberg E, Kelm M, Rassaf T (2012) Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation 126:1983–1992
Ferrara N (2001) Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280:1358–1366
Toko H, Zou Y, Minamino T, Masaya M, Harada M, Nagai T, Sugaya T, Terasaki F, Kitaura Y, Komuro I (2004) Angiotensin II type 1a receptor is involved in cell infiltration, cytokine production, and neovascularization in infarcted myocardium. Arterioscler Thromb Vasc Biol 24:664–670
Sheikh-Hamad D, Wang YP, Jo OD, Yanagawa N (1993) Dopamine antagonizes the actions of angiotensin II in renal brush-border membrane. Am J Physiol 264:F737–F743
Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, Jose PA (2006) Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor–deficient mice. Hypertension 47:288–295
Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL (2009) The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem 109:656–669
Chakroborty D, Sarkar C, Yu H, Wang J, Liu Z, Dasgupta PS, Basu S (2011) Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci USA 108:20730–20735
Rossi NF (1998) Dopaminergic control of angiotensin II-induced vasopressin secretion in vitro. Am J Physiol 275:E687–E693
Hu C, Dandapat A, Mehta JL (2007) Angiotensin II induces capillary formation from endothelial cells via the LOX-1 dependent redox-sensitive pathway. Hypertension 50:952–957
Ma J, Liu W, Yan X, Wang Q, Zhao Q, Xue Y, Ren H, Wu L, Cheng Y, Li S, Miao L, Yao L, Zhang J (2012) Inhibition of endothelial cell proliferation and tumor angiogenesis by up-regulating NDRG2 expression in breast cancer cells. PLoS ONE 7:e32368
Lu K, Chakroborty D, Sarkar C, Lu T, Xie Z, Liu Z, Basu S (2012) Triphala and its active constituent chebulinic acid are natural inhibitors of vascular endothelial growth factor-a mediated angiogenesis. PLoS ONE 7:e43934
Arnold SA, Rivera LB, Carbon JG, Toombs JE, Chang CL, Bradshaw AD, Brekken RA (2012) Losartan slows pancreatic tumor progression and extends survival of SPARC-null mice by abrogating aberrant TGFβ activation. PLoS ONE 7:e31384
Ramkhelawon B, Vilar J, Rivas D, Mees B, de Crom R, Tedgui A, Lehoux S (2009) Shear stress regulates angiotensin type 1 receptor expression in endothelial cells. Circ Res 105:869–875
Ichiki T, Usui M, Kato M, Funakoshi Y, Ito K, Egashira K, Takeshita A (1998) Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension 31:342–348
Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C (2012) The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 10:4–18
Sakkoula E, Pipili-Synetos E, Maragoudakis ME (1997) Involvement of nitric oxide in the inhibition of angiogenesis by interleukin-2. Br J Pharmacol 122:793–795
Sarkar R, Webb RC, Stanley JC (1995) Nitric oxide inhibition of endothelial cell mitogenesis and proliferation. Surgery 118:274–279
Cartwright JE, Johnstone AP, Whitley GS (2000) Endogenously produced nitric oxide inhibits endothelial cell growth as demonstrated using novel antisense cell lines. Br J Pharmacol 131:131–137
Pyne-Geithman GJ, Caudell DN, Cooper M, Clark JF, Shutter LA (2009) Dopamine D2-receptor-mediated increase in vascular and endothelial NOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in vitro. Neurocrit Care 10:225–231
Granado N, Ares-Santos S, Oliva I, O’Shea E, Martin ED, Colado MI, Moratalla R (2011) Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis 42:391–403
Nouet S, Nahmias C (2000) Signal transduction from the angiotensin II AT2 receptor. Trends Endocrinol Metab 11:1–6
Ager EI, Neo J, Christophi C (2008) The renin-angiotensin system and malignancy. Carcinogenesis 29:1675–1684
Cooke JP, Losordo DW (2002) Nitric oxide and angiogenesis. Circulation 105:2133–2135
Babaei S, Stewart DJ (2002) Overexpression of endothelial NO synthase induces angiogenesis in a co-culture model. Cardiovasc Res 55:190–200
Kon K, Fujii S, Kosaka H, Fujiwara T (2003) Nitric oxide synthase inhibition by N(G)-nitro-l-arginine methyl ester retards vascular sprouting in angiogenesis. Microvasc Res 65:2–8
Silvestre JS, Mallat Z, Tedgui A, Lévy BI (2008) Post-ischaemic neovascularization and inflammation. Cardiovasc Res 78:242–249
Jaipersad AS, Lip GY, Silverman S, Shantsila E (2014) The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol 63:1–11
Pardanaud L, Pibouin-Fragner L, Dubrac A, Mathivet T, English I, Brunet I, Simons M, Eichmann A (2016) Sympathetic innervation promotes arterial fate by enhancing endothelial ERK activity. Circ Res 119(5):607–620
Erami C, Zhang H, Tanoue A, Tsujimoto G, Thomas SA, Faber JE (2005) Adrenergic catecholamine trophic activity contributes to flow-mediated arterial remodeling. Am J Physiol Heart Circ Physiol 289(2):H744–H753
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Acknowledgements
The authors thank Dr. Sujit Basu, Department of Pathology and Division of Medical Oncology, Department of Internal Medicine, Ohio State University, for careful reading, insightful comments and suggestions.
Funding
This work is supported by AHA Grant No. 10BGIA4230012 to DC. CS is partly supported by NIH R01 DK098045 and RKG is partly supported by NIH R01 Grants CA109527 and CA153490.
Author contributions
D. C. and C. S. designed research; D. C. and C. S., performed experiments; D. C., C. S., R. K. G. and V. J. P. analyzed data; D. C. and C. S. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarkar, C., Ganju, R.K., Pompili, V.J. et al. Enhanced peripheral dopamine impairs post-ischemic healing by suppressing angiotensin receptor type 1 expression in endothelial cells and inhibiting angiogenesis. Angiogenesis 20, 97–107 (2017). https://doi.org/10.1007/s10456-016-9531-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-016-9531-8