Abstract
There is increasing evidence that the intrarenal dopaminergic system plays an important role in the regulation of blood pressure, and defects in dopamine signaling appear to be involved in the development of hypertension. Recent experimental models have definitively demonstrated that abnormalities in intrarenal dopamine production or receptor signaling can predispose to salt-sensitive hypertension and a dysregulated renin-angiotensin system. In addition, studies in both experimental animal models and in humans with salt-sensitive hypertension implicate abnormalities in dopamine receptor regulation due to receptor desensitization resulting from increased G-protein receptor kinase 4 (GRK4) activity. Functional polymorphisms that predispose to increased basal GRK4 activity both decrease dopamine receptor activity and increase angiotensin II type 1 (AT1) receptor activity and are associated with essential hypertension in a number of different human cohorts.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2:637–50.
Zeng C, Jose PA. Dopamine receptors: important antihypertensive counterbalance against hypertensive factors. Hypertension. 57:11–7.
Quinones H, Collazo R, Moe OW. The dopamine precursor L-dihydroxyphenylalanine is transported by the amino acid transporters rBAT and LAT2 in renal cortex. Am J Physiol Renal Physiol. 2004;287:F74–80.
Pinho MJ, Serrao MP, Soares-da-Silva P. High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. Am J Physiol Renal. 2007;292:F1452–63.
Hayashi M, Yamaji Y, Kitajima W, Saruta T. Aromatic L-amino acid decarboxylase activity along the rat nephron. Am J Physiol Renal. 1990;258:F28–33.
Bertorello A, Hokfelt T, Goldstein M, et al. Proximal tubule Na+−K+−ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol Renal. 1988;254:F795–801.
Baines AD. Effects of salt intake and renal denervation on catecholamine catabolism and excretion. Kidney Int. 1982;21:316–22.
Bacic D, Capuano P, Baum M, et al. Activation of dopamine D1-like receptors induces acute internalization of the renal Na+/phosphate cotransporter NaPi-IIa in mouse kidney and OK cells. Am J Physiol Renal Physiol. 2005;288:F740–7.
Grider JS, Ott CE, Jackson BA. Dopamine D1 receptor-dependent inhibition of NaCl transport in the rat thick ascending limb: mechanism of action. Eur J Pharmacol. 2003;473:185–90.
Schafer JA. Abnormal regulation of ENaC: syndromes of salt retention and salt wasting by the collecting duct. Am J Physiol Renal Physiol. 2002;283:F221–35.
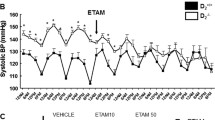
• Wang X, Luo Y, Escano CS, Yang Z, et al. Upregulation of renal sodium transporters in D5 dopamine receptor-deficient mice. Hypertension. 2010; 55:1431–1437. This study demonstrates the important role of normal dopamine signaling in the kidney to regulate expression of sodium transport mechanisms at numerous nephron segments.
• Zeng C, Jose PA. Dopamine receptors: important antihypertensive counterbalance against hypertensive factors. Hypertension. 2011;57:11–7. This is an up-to-date review of the role of dopamine receptors in the regulation of blood pressure.
Zeng C, Sanada H, Watanabe H, et al. Functional genomics of the dopaminergic system in hypertension. Physiol Genom. 2004;19:233–46.
Needleman P, Turk J, Jakschik BA, et al. Arachidonic acid metabolism. Ann Rev Biochem. 1986;55:69–102.
Horton R, Bughi S, Jost-Vu E, et al. Effect of dopamine on renal blood flow, prostaglandins, renin and electrolyte excretion in normal and hypertensive humans. Am J Hypertens. 1990;3:108S–11S.
Huo TL, Grenader A, Blandina P, et al. Prostaglandin E2 production in rat IMCD cells. II. Possible role for locally formed dopamine. Am J Physiol. 1991;261:F655–62.
Yao B, Harris RC. MZ: Intrarenal dopamine attenuates deoxycorticosterone acetate/high salt-induced blood pressure elevation in part through activation of a medullary cyclooxygenase 2 pathway. Hypertension. 2009;54:1077–83.
Harris RC. An update on cyclooxygenase-2 expression and metabolites in the kidney. Curr Opin Nephrol Hypertens. 2008;17:64–9.
Kurtz A, Della Bruna R, Pratz J, et al. Rat juxtaglomerular cells are endowed with DA-1 dopamine receptors mediating renin release. J Cardiovasc Pharm. 1988;12:658–63.
Antonipillai I, Broers MI, Lang D. Evidence that specific dopamine-1 receptor activation is involved in dopamine-induced renin release. Hypertension. 1989;13:463–8.
Yamaguchi I, Yao L, Sanada H, et al. Dopamine D1A receptors and renin release in rat juxtaglomerular cells. Hypertension. 1997;29:962–8.
Zhang MZ, Yao B, Fang X, et al. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–70.
Zhang MZ, Yao B, Harris RC. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol. 2005;288:F840–5.
Chen CJ, Apparsundaram S, Lokhandwala MF. Intrarenally produced angiotensin II opposes the natriuretic action of the dopamine-1 receptor agonist fenoldopam in rats. J Pharmacol Exp Ther. 1991;256:486–91.
Gesek FA, Schoolwerth AC. Hormone responses of proximal Na(+)-H+ exchanger in spontaneously hypertensive rats. Am J Physiol. 1991;261:F526–36.
Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest. 1996;97:2745–52.
Aperia A, Holtback U, et al. Activation/deactivation of renal Na+, K(+)-ATPase: a final common pathway for regulation of natriuresis. FASEB J. 1994;8:436–9.
Zeng C, Liu Y, Wang Z, et al. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500.
Zeng C, Yang Z, Wang Z, et al. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–10.
Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to aging. Curr Opin Nephrol Hypertens. 2011;20:84–8.
•• Zhang MZ, Yao B, Wang S, et al. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011;121:2845–54. This paper describes a mouse model with deficiency of local renal dopamine deficiciency.
Salomone LJ, Howell NL, McGrath HE, et al. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–61.
Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–30.
Yasunari K, Kohno M, Kano H, et al. Dopamine as a novel antioxidative agent for rat vascular smooth muscle cells through dopamine D(1)-like receptors. Circulation. 2000;101:2302–8.
Yang Z, Asico LD, Yu P, et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104.
Armando I, Wang X, Villar VA, et al. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–8.
Yang Z, Asico LD, Yu P, et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104.
Helkamaa T, Mannisto PT, Rauhala P, et al. Resistance to salt-induced hypertension in catechol-O-methyltransferase-gene-disrupted mice. J Hypertens. 2003;21:2365–74.
Sidhu A, Kumar U, Uh M, Patel S. Diminished expression of renal dopamine D1A receptors in the kidney inner medulla of the spontaneously hypertensive rat. J Hypertens. 1998;16:601–8.
•• Asico L, Zhang X, Jiang J, et al. lack of renal dopamine D5 receptors promotes hypertension. JASN. 2011;22:82–9. This study used kidneys transplanted from D5−/− mice into wild-type mice to demonstrate the importance of intrarenal dopaminergic signaling in blood pressure control.
Shikuma R, Yoshimura M, Kambara S, et al. Dopaminergic modulation of salt sensitivity in patients with essential hypertension. Life Sci. 1986;38:915–21.
Jose PA, Soares-da-Silva P, Eisner GM, et al. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta. 2010;1802:1259–67.
Zeng C, Wang D, Asico LD, et al. Aberrant D1 and D3 dopamine receptor transregulation in hypertension. Hypertension. 2004;43:654–60.
Jose PA, Eisner GM, Felder RA. Dopaminergic defect in hypertension. Pediatr Nephrol. 1993;7:859–64.
Felder RA, Sanada H, Xu J, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–7.
Premont RT, Macrae AD, Aparicio SA, et al. The GRK4 subfamily of G protein-coupled receptor kinases. Alternative splicing, gene organization, and sequence conservation. J Biol Chem. 1999;274:29381–9.
Nishi A, Eklof AC, Bertorello AM, et al. Dopamine regulation of renal Na+, K(+)-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension. 1993;21:767–71.
Hussain T, Lokhandwala MF. Renal dopamine DA1 receptor coupling with G(S) and G(q/11) proteins in spontaneously hypertensive rats. Am J Physiol Renal. 1997;272:F339–46.
Debska-Slizien A, Ho P, Drangova R, et al. Endogenous dopamine regulates phosphate reabsorption but not NaK-ATPase in spontaneously hypertensive rat kidneys. JASN. 1994;5:1125–32.
Sanada H, Jose PA, Hazen-Martin D, et al. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036–42.
Gildea JJ, Shah I, Weiss R, et al. HK-2 human renal proximal tubule cells as a model for G protein-coupled receptor kinase type 4-mediated dopamine 1 receptor uncoupling. Hypertension. 2010;56:505–11.
Villar VA, Jones JE, Armando I, et al. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor, J. Biol Chem. 2009;284:21425–34.
Sanada H, Yatabe J, Midorikawa S, et al. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension. 2006;47:1131–9.
Allayee H, de Bruin TW, Michelle Dominguez K, et al. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension. 2001;38:773–8.
Chen W, Li S, Srinivasan SR, et al. Autosomal genome scan for loci linked to blood pressure levels and trends since childhood: the Bogalusa Heart Study. Hypertension. 2005;45:954–9.
Lohmueller KE, Wong LJ, Mauney MM, et al. Patterns of genetic variation in the hypertension candidate gene GRK4: ethnic variation and haplotype structure. Ann Hum Genet. 2006;70:27–41.
Bengra C, Mifflin TE, Khripin Y, et al. Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131–40.
Speirs HJ, Katyk K, Kumar NN, et al. Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–6.
Zhu H, Lu Y, Wang X, et al. The G protein-coupled receptor kinase 4 gene affects blood pressure in young normotensive twins. Am J Hypertens. 2006;19:61–6.
Sanada H, Yatabe J, Midorikawa S, et al. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–60.
Williams SM, Ritchie MD, Phillips 3rd JA, et al. Multilocus analysis of hypertension: a hierarchical approach. Hum Hered. 2004;57:28–38.
Bhatnagar V, O’Connor DT, Brophy VH, et al. G-protein-coupled receptor kinase 4 polymorphisms and blood pressure response to metoprolol among African Americans: sex-specificity and interactions. Am J Hypertens. 2009;22:332–8.
Martinez Cantarin MP, Ertel A, Deloach S, et al. Variants in genes involved in functional pathways associated with hypertension in African Americans. Clin Transl Sci. 2010;3:279–86.
Rana BK, Insel PA, Payne SH, et al. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106.
Staessen JA, Kuznetsova T, Zhang H, et al. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008;51:1643–50.
Harrison M, Maresso K, Broeckel U. Genetic determinants of hypertension: an update. Curr Hypertens Rep. 2008;10:488–95.
Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–76.
Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–87.
Adeyemo A, Gerry N, Chen G, et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genetics. 2009;5:e1000564.
Wang Y, O’Connell JR, McArdle PF, et al. Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA. 2009;106:226–31.
Cho YS, Go MJ, Kim YJ, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–34.
Acknowledgments
These studies were supported in part by grants from the National Institutes of Health (DK62794, DK51265) and funds from the Veterans Administration.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harris, R.C., Zhang, MZ. Dopamine, the Kidney, and Hypertension. Curr Hypertens Rep 14, 138–143 (2012). https://doi.org/10.1007/s11906-012-0253-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-012-0253-z