Abstract
Purpose of Review
The regulatory steps necessary to bring new PET radiopharmaceuticals to the clinic will be reviewed. The US Food and Drug Administration (FDA) provides approval to manufacture and use diagnostic radiopharmaceuticals, including those for cardiovascular PET/CT. Medicare not only provides insurance reimbursement for imaging procedures for its beneficiaries but also sets an example for third-party insurers to cover these procedures.
Recent Findings
FDA provides extensive guidance for performing studies to obtain the safety and efficacy data needed to approve PET radiopharmaceuticals, and the pace of approval has recently increased. There also has been considerable progress in insurance coverage for PET by Medicare. Several promising agents for cardiovascular PET imaging are in the development pipeline. Challenges remain, however, including low levels of reimbursement and the application of appropriate use criteria for imaging procedures.
Summary
It is important for cardiologists to understand the regulatory steps involved in translating PET radiopharmaceuticals to the clinic. Recent progress in both FDA approvals and Medicare coverage should facilitate the clinical use of new PET agents for molecular imaging of the heart.
Similar content being viewed by others
Introduction
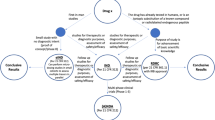
The past three decades have seen tremendous advances in the contribution of molecular imaging to clinical care [1]. Recently, more PET radiopharmaceuticals have become available, and PET/CT is transforming clinical care in oncology, cardiology, and neurology. The path for a new molecular imaging agent from discovery and preclinical studies to first use in humans and translation to the clinic can be challenging, however. This review will focus on the two key steps necessary for the translation of a PET molecular imaging agent to the clinic: (1) regulatory approval for a manufacturer to market a diagnostic radiopharmaceutical and (2) insurance coverage so that the provider of the imaging procedure is reimbursed. In the USA, marketing approval is granted by the US Food and Drug Administration (FDA). Insurance coverage is provided by the Centers for Medicare and Medicaid (CMS) for individuals 65 years and older (“Medicare”) and by a complex system of private and public insurance providers for the rest of the population.
Preclinical Studies of PET Radiopharmaceuticals
The first-in-human use of a PET radiopharmaceutical is preceded by a series of preclinical studies [2,3,4]. After identification of the proposed clinical need, candidate molecules to image the biological or pathological target of interest are studied in vitro. Experiments explore the expression of the target in tissue, the molecules’ binding to the target, and target versus non-target binding. This leads to identification of one or more lead agents and their translation to animal studies. These are typically performed in rodents, but often include larger animals and models of the human disease of interest. Studies include the agent’s imaging characteristics, target specificity, biodistribution and excretion over time, in vivo stability and metabolism, and estimated radiation dosimetry. Safety data are obtained, including studies for toxicology and pharmacologic effects. The synthesis of the radiopharmaceutical is refined and standardized. It should be manufactured in the same way as for the planned first-in-human studies, so that the preclinical results are translatable to humans. Current Good Manufacturing Practice (cGMP) methods must be used to ensure the agent’s quality and safety [5••, 6, 7••].
FDA Regulation of Diagnostic Radiopharmaceuticals
The administration of PET radiopharmaceuticals to humans is governed by the same general FDA regulations as for therapeutic drugs. “Investigational use” is the term for human studies that acquire the data needed by the FDA to approve a radiopharmaceutical for clinical use. Requests to perform these studies are submitted to the FDA via an Investigational New Drug (IND) application. Clinical use of a radiopharmaceutical subsequently occurs only after the FDA receives and approves a New Drug Application (NDA) from a manufacturer to market the agent for a specific indication. (Research use of PET radiopharmaceuticals in academic medical centers to study biochemical or physiological processes is also FDA-regulated.)
FDA guidance documents describe how investigations should be designed to obtain approval of PET radiopharmaceuticals. Investigational studies must show that the diagnostic radiopharmaceutical is both safe and effective [8, 9•]. Effectiveness is determined by the ability to provide useful clinical information related to the proposed indications for use. Two criteria must be met, accuracy and clinical value. Accuracy is defined in relation to a relevant truth standard such as histopathology or another accepted reference imaging test. It is demonstrated by performance characteristics in the clinical setting (e.g., sensitivity, specificity, positive and negative predictive value) as well as by reliability or reproducibility. Clinical usefulness is defined in the context of the agent’s intended use, e.g., helping to make an accurate diagnosis, helping to choose the right therapy, or providing accurate prognostic information. The FDA lists four general categories for the labeled indications of medical imaging agents [8]. Three of them are particularly relevant to PET: disease or pathology detection or assessment; functional, physiological, or biochemical assessment; and diagnostic or therapeutic patient management (Table 1). Note that the FDA does not require data showing the effect of the imaging procedure on patient health outcomes such as longevity or disability.
Investigational Studies of PET Radiopharmaceuticals
A sponsor’s investigational program for a new PET radiopharmaceutical is designed to demonstrate its safety and effectiveness. FDA approval to perform investigational studies is obtained via an IND; the FDA has published a specific PET guidance for this process [10]. The IND contains detailed information on how the agent is manufactured; data from preclinical toxicology, safety, pharmacology, and imaging studies in animals; and the clinical research protocol that describes the planned human studies. The process involves three phases of increasing complexity [11].
Phase 1 studies focus on safety and pharmacokinetics. They are typically performed in a small number of healthy subjects but may also include patients with the disease targeted by the agent. Physiologic monitoring includes EKG and pre- and post-administration blood tests. Pharmacokinetic studies are performed to image organ biodistribution, determine target engagement and routes of excretion, evaluate in vivo stability and metabolism from blood samples, and calculate radiation dosimetry.
Phase 2 studies provide preliminary evidence of the agent’s efficacy for the proposed clinical indication. Therefore, patients with known disease and as well as normal subjects are included. Other goals are to obtain additional pharmacokinetic and safety data, optimize methods for image acquisition, and develop methods for image evaluation.
Phase 3 studies, considered “pivotal”, should confirm the principal clinical hypotheses developed in earlier investigations. They should demonstrate efficacy for the proposed indications(s) and continued safety. They also validate methods for imaging and image interpretation in the intended patient population. Phase 3 studies are often performed in several medical centers to support generalizability of the data. The number of subjects can vary but is typically smaller than for therapeutic drugs, e.g., 635 patients were enrolled in studies of 68 Ga-PSMA-11 [12] and only 68 patients for 18F-Fluorodopa [13]. A current phase 3 study of the myocardial perfusion agent 18F-Flurpiridaz has enrolled 730 participants [14].
New Drug Applications
The final step in translating a PET drug to the clinic is FDA approval of an NDA submitted by a manufacturer to market the agent for a label indication. The NDA must address safety, efficacy, and clinical utility in great detail. It includes information from preclinical, phase 1, 2, and pivotal phase 3 studies; details of the method of image interpretation and its validation; and extensive information on how the agent is manufactured. The FDA does have some flexibility for the design of phase 3 studies and the evidence required for NDA approval. The sponsor may be able to use information from published, high-quality clinical trials rather than new trials [8]; this was the case for 11C-choline used to image prostate cancer [15, 16•]. If the PET agent is intended to image an orphan disease, defined as having a prevalence of less than 200,000, the NDA process can be facilitated [16•]. In some cases, the truth standard for efficacy of an imaging agent can be expert clinical diagnosis and patient follow-up; this was the case for 18F-Fluorodopa [13]. FDA staff are available to sponsors for consultation on preparing INDs and NDAs. A PET NDA undergoes extensive review in FDA’s Division of Imaging and Radiation Medicine. The FDA may also seek advice from its Medical Imaging Drugs Advisory Committee, a group of imaging experts [17].
An abbreviated NDA (ANDA) can be submitted by a manufacturer to market a generic PET radiopharmaceutical that is bioequivalent to an already-approved agent, i.e., it has the identical active ingredient, dosage, route of administration, and indications for use [18]. The manufacturer does not have to submit preclinical animal studies or clinical trial data to show the drug’s safety and efficacy. The ANDA must demonstrate that the proposed product is bioequivalent and ensure the product’s identity, quality, strength, and purity. A new label indication, however, requires an NDA. ANDAs have facilitated the use of several generic PET drugs (i.e., those with no intellectual property rights): 18F-FDG, 18F-sodium fluoride, 11C-choline, and 13 N-ammonia. There are currently 29 ANDAs for 13 N-ammonia for myocardial perfusion, held by both commercial entities and medical centers [19].
Although industry submits most PET NDAs, some originate with academic medical centers (see Table 2) [20•, 21]. There are several reasons for an academic center to go through the NDA process. Many PET drugs have extensive studies demonstrating utility but lack intellectual property rights. As a result, commercial manufacturers may be reluctant to pursue them because the financial return is limited without patent exclusivity. The availability of a PET agent targeting a specific disease can support the academic center’s focus on that disease. Academic sponsors can benefit their patients by making these agents clinically available, and more broadly, can advance the field of molecular imaging, especially since other manufacturers can then submit ANDAs. Also, the center may be able to produce the agent at a lower cost than a commercial entity.
Radiopharmaceuticals approved by the FDA for both investigational studies and clinical use must be manufactured under rigorous CGMP standards [5••, 6, 7••, 20•, 22]. These standards ensure quality and safety, and go beyond simply testing the final product before its administration. CGMP involves all aspects of production, including personnel qualifications and training, supplies, equipment, facilities, and records. The FDA provides extensive guidance for CGMP production of PET drugs. Adherence to CGMP by a manufacturing facility is demonstrated by written documentation of its procedures and by extensive record keeping. After an NDA or ANDA is approved, the FDA inspects the sponsor’s manufacturing site, to ensure the radiopharmaceutical is produced in accordance with CGMP [23]. Subsequently, the site is inspected about every 2 years.
Exploratory and Expanded Access INDs
Exploratory INDs (eIND) facilitate early (“phase 0” or early phase 1) investigation of new drugs, including first-in-human use of new PET agents [24, 25]. The eIND uses a microdose approach [26]. A microdose is defined as less than 1/100th of the dose of the test substance that is calculated (based on animal data) to yield a pharmacologic effect, with a maximum dose of 100 μg. Because pharmacologic effects are not expected from PET drugs, preclinical pharmacology and toxicology studies can be reduced, substantially decreasing cost. eINDs can determine an agent’s binding properties to a neuroreceptor or oncologic target, whole-body biodistribution, in vivo metabolism, imaging characteristics, and radiation dosimetry. They can help select the most promising agent from similar candidates and can lead to an earlier, more cost-effective decision about whether a radiopharmaceutical will perform as intended.
Expanded access (EA) provides access to investigational PET drugs outside of traditional phase 1–3 studies [10, 27, 28]. The criteria for EA are that the patients have a serious or immediately life-threatening disease; there is no comparable or satisfactory alternative to diagnose or monitor the condition; the potential benefits justify the risk; and, the use will not interfere with or contribute to the clinical investigations to support marketing approval of the EA use. The regulatory submission can be either a clinical protocol added to an existing IND or a new IND.
Research with PET Radiopharmaceuticals
In addition to industry investigational programs, many academic centers conduct preclinical and clinical PET research to advance molecular imaging. The clinical studies are usually not designed to obtain FDA approval but are often proof-of-principle trials to show that a new agent can image a specific disease-related target, e.g., a tumor-related protein. In some cases, however, a novel agent developed in a medical center is taken up by industry for commercialization, e.g., 18F-fluciclovine to image prostate cancer [16•]. Often, the focus of the PET research program is to study physiology or pathophysiology. This is especially so in the clinical neurosciences, with numerous radiotracers to image brain neuroreceptor systems, metabolic pathways, neuroinflammation, and synaptic density.
There are several approaches for academic centers to conduct clinical PET research. Traditional phase 1 and 2 INDs can be used, as well as eINDs. Another mechanism is via the Radioactive Drug Research Committee (RDRC) [29, 30•], a local committee delegated by the FDA to approve basic science research in humans with radioactive drugs, including those for imaging. This includes obtaining information on the metabolism, kinetics, biodistribution, and radiation dosimetry of the drug, and also studying human physiology, biochemistry and disease pathophysiology. RDRC approval cannot be used for diagnostic or therapeutic purposes, or to determine the safety and efficacy of the radioactive drug; these goals require an IND. The radioactive drug must be known to be safe, and its injected mass dose cannot have pharmacologic effects; this precludes first-in-human studies. Radiolabeled molecules that can be studied include: (1) naturally occurring substances and FDA-approved drugs with a known safety profile, in which an atom has been substituted by its positron-emitting isotope, e.g., 11C-labelled amino acids, 11C-methylphenidate; (2) novel PET radiotracers previously studied under other mechanisms and with available safety and pharmacologic data. Other RDRC regulations concern committee membership, the number of research subjects in a study, and radiation dose limits.
Challenges for the Development of New PET Radiopharmaceuticals
The pathway from preclinical research to phase 1–3 IND studies to FDA approval of a PET radiopharmaceutical is well-defined. This process, however, can be challenging. For phase 1 and 2 trials, safety and toxicology requirements are high, even though these agents have no pharmacologic activity and will be used infrequently in individual patients. Phase 3 trials for agents that detect pathology or abnormal biochemistry typically need data from a “gold standard” of truth that can be difficult to obtain. The cost of clinical trials is relatively high compared to the potential return for agents that may be used only once per patient. For multicenter trials, several manufacturing sites across the country are required due to the short physical half-life of the PET radiolabel. Many potentially useful agents have no intellectual property rights, limiting future profitability and discouraging commercialization, e.g., 18F-FMISO to image tumor hypoxia [31]. In addition, barriers to insurance coverage may decrease industry’s enthusiasm to bring agents to market. Despite these challenges, several novel PET radiopharmaceuticals have recently been approved for oncologic and neurologic applications (Table 2).
New PET Radiopharmaceuticals for Cardiac Studies
There are established PET radiopharmaceuticals approved for myocardial blood flow (82Rb; 13 N-ammonia) and viability (18F-FDG), with the last approval in 2007 (Table 2). There are, however, two myocardial blood flow (MBF) agents currently in phase 3 studies. This is encouraging, since PET addresses some of the drawbacks of SPECT because of its ability to quantify regional and global MBF in absolute units of mL/min/g of tissue [32].
18F-flurpiridaz, has several advantages over current perfusion agents [14, 33,34,35]. The 110-min half-life of the 18F label permits distribution from commercial radiopharmacies; it does not require a local cyclotron as does 13 N-ammonia or a complex generator like 82Rb. Exercise and pharmacologic stress protocols are facilitated. In such protocols, residual 18F radioactivity from the rest injection can be subtracted from the stress scan. It has favorable in vivo behavior, including high myocardial extraction and retention to more accurately reflect MBF at high flows, and low lung and hepatic uptake which reduces background in the PET images.
15O-water has been widely used in clinical research, primarily to measure regional cerebral blood flow [36] but also to quantitate MBF [32, 33]. It is freely diffusible and has virtually complete extraction, so that calculated flow accurately reflects actual MBF. The short 2-min physical half-life facilitates pharmacological rest-stress protocols and shortens the procedure duration, but a disadvantage is the need for an on-site cyclotron. Recently, a phase 3 clinical trial of 15O-water has been initiated [37]. An automated system in the scan suite uses cyclotron-produced 15O-oxygen, hydrogen gas and heat to produce and inject 15O-water. One dose is injected at rest, the other during pharmacologic stress.
Clinical PET imaging of the heart has focused on myocardial perfusion and viability. However, there is much preclinical and translational research to develop PET agents for a wide variety of clinically-relevant targets. These include neuronal signaling, fibrosis, inflammation, amyloidosis, metabolic substrate utilization, and coronary artery plaques. These agents have great potential to address many clinical needs [35, 38••, 39,40,41].
Insurance Coverage of PET Radiopharmaceuticals
Insurance reimbursement is essential for the clinical use of PET. Not only does CMS provide health insurance for about one third of the US population, but also third-party insurers typically follow CMS’s lead in covering diagnostic procedures. Surprisingly, FDA approval does not automatically lead to CMS coverage. In fact, CMS has taken many years to cover several approved PET radiopharmaceuticals, with some still not covered. The critical distinction is that these two agencies use different approval criteria [42•]. FDA’s regulatory standard requires that radiopharmaceuticals provide useful clinical information related to the proposed indication for use; it does not require evidence of their effect on clinical management or patient health outcomes [43•, 44]. Data on clinical utility and safety are provided via the confidential NDA process. In contrast, CMS applies the criterion of “reasonable and necessary for the diagnosis or treatment of illness or injury,” with an increasing focus on improvement in health outcomes [45, 46, 47•]. CMS primarily uses information from the medical literature in its decision-making process. Of note, “reasonable and necessary” does not have a regulatory definition, and effect on health outcomes is not embodied in any regulation. Perhaps as a result, CMS PET coverage has changed over the years in a still-evolving process.
CMS Coverage of PET
There are two pathways for CMS coverage, local coverage determination (LCD) and national coverage determination (NCD) [48•]. Claims for medical products and services under Medicare Parts A and B are processed by twelve Medicare Administrative Contractors (MACs) in their geographic regions in the USA. When there is not an NCD, MACs determine coverage and utilization guidelines. Non-PET radiopharmaceuticals are handled via LCDs; PET agents, however, typically have been subject to NCDs. An NCD sets coverage policy nationally for a specific medical technology. It may be requested by stakeholders including Medicare beneficiaries, manufacturers, and professional associations, or be generated internally by CMS staff. The CMS Coverage and Analysis Group (CAG) determines whether to open an NCD. It uses an evidence-based process focused on the medical literature, which may be supplemented by an outside technology assessment or input from its Medicare Evidence Development and Coverage Advisory Committee (MEDCAC). CMS policy is not to consider cost when making an NCD. There are three possible outcomes of an NCD: (1) covered, because the evidence shows that the service is reasonable and necessary for the diagnosis or treatment of illness or injury; (2) not covered, when the evidence is to the contrary; or (3) coverage with evidence development (CED), when the evidence indicates the service might be reasonable and necessary, but is insufficient. Under CED, the cost of the procedure is covered for a CMS-approved clinical trial or patient registry to gather additional evidence.
Medicare has used NCDs and CED extensively for PET [48•], and its focus on health outcomes is particularly relevant. In 2000, CMS clarified that the primary factors to determine if a new technology is “reasonable and necessary” included not only whether it is safe, effective, and appropriate, but also whether it leads to improved health outcomes [46]. The application of this approach to PET was articulated by Louis Jacques, M.D., CAG Director, at a conference in 2012 [42•]. For a new PET radiopharmaceutical to be covered, the sponsor should “provide adequate evidence that the incremental information obtained by the new diagnostic technology compared to alternatives changes physician recommendations resulting in changes in therapy that improve clinically meaningful health outcomes.” More persuasive outcomes include longer life with improved function or arrested decline, better quality of life, and reduced need for burdensome tests and treatments. Less persuasive are longer life with declining function, better images, or improved physician confidence. It was recognized, however, that diagnostic tests per se do not have a direct therapeutic effect, so demonstrating improved health outcomes can be difficult. Rather, tests provide information to support management decisions that can be linked to improved health outcomes, e.g., avoiding unnecessary surgery, or informing drug therapy.
Evaluating diagnostic imaging based on outcomes rather than test accuracy was proposed in a prescient paper by Fryback and Thornbury in 1991 [49••], with a 6-step hierarchical approach: technical efficacy (e.g., image resolution); technical accuracy (e.g., sensitivity, specificity); change in physician’s diagnosis; resultant change in management; effect on outcomes; and societal benefit. These steps were recently updated for molecular imaging [4]. Randomized controlled trials could be used demonstrate the benefit of imaging, analogously to trials of therapeutic drugs. Patients could be managed for a disease with and without the new imaging technology, to see if it improves outcomes [50]. These studies are difficult to conduct, however, and have not been widely used [51, 52].
Of note, the first CMS-approved PET agent was 82Rb for myocardial perfusion, in 1995. Subsequent approvals over the next 10 years included 13NH3 for myocardial perfusion, and 18F-FDG for myocardial viability, differential diagnosis of dementia, pre-surgical localization of seizure foci, and some oncology indications [53]. This incremental progress came to a halt in 2005 when CMS published a sweeping non-coverage NCD for PET in its Medicare National Coverage Determinations Manual [53, 54]. The decision stated: “This manual Sect. 220.6 lists all Medicare-covered uses of PET scans. … a particular use of PET scans is not covered unless this manual specifically provides that such use is covered.” This meant that PET indications not already approved were automatically not covered, even if the radiopharmaceutical or indication had not yet been developed. Subsequently, there were two important NCDs granting CED for PET, the broad use of 18F-FDG in oncology and imaging brain amyloid plaques in cognitive decline.
National Oncologic PET Registry
In 2005, CMS approved CED to study the impact of FDG PET on patient management for most oncological indications not already covered. This led to the National Oncologic PET Registry (NOPR), a transformational study guided by several leaders in nuclear medicine and health care research [47•, 55, 56]. The goal was to assess the effect of PET on physicians’ intended management for a wide variety of uncovered cancers and indications, including initial diagnosis, staging, suspected recurrence, and therapy monitoring. Pre- and post-scan management plans were collected from referring physicians. Recruitment was tremendously successful, ultimately including about 288,000 scans and 1900 imaging facilities. After only 1 year, investigators found a 36.5% change in management plans in 23,000 patients.
Based on NOPR data, CMS issued NCDs in 2009 and 2013 [57]. These resulted in coverage for most cancers, one scan for initial treatment planning and up to three for subsequent treatment strategy after completion of initial therapy; additional scans are at the discretion of the local MAC. Some limitations of the NOPR design have been noted [47•, 56]. These include concern about overall data quality; use of change in intended rather than actual management; no data on patient health outcomes; and lack of a control group. These limitations, however, reflected a reasonable balance at the time between performing more-controlled studies versus the logistics of obtaining a large amount of relevant data in a timely fashion. In 2011, NOPR initiated another registry, for Na18F to identify bone metastasis; in a 2015 decision memo, however, CMS declined coverage of this radiopharmaceutical [58].
PET Imaging of Brain Amyloid
Another major NCD came in 2013 for PET imaging of brain amyloid in patients with cognitive impairment being evaluated for Alzheimer’s disease (AD). This is an important use of PET because the clinical diagnosis of AD is only about 70% accurate [59]. Rather than granting full coverage, however, CMS approved only one scan per patient in CED studies that could include short-term outcomes related to changes in management as well as longer-term dementia outcomes [60, 61]. This led to the Imaging Dementia—Evidence for Amyloid Scanning (“IDEAS”) Study [62•]. The primary hypothesis was that in diagnostically uncertain cases, knowledge of PET amyloid status would lead to significant changes in patient management that improved medical outcomes. Eleven thousand four hundred nine patients with mild cognitive impairment or dementia were scanned at 343 imaging centers.
The first aim was to test whether amyloid PET resulted in a 30% or greater change between intended and actual patient management in AD drug therapy, other drug therapy, or counseling about safety and future planning. (Note the difference from NOPR, which looked at intended management.) The results were dramatic; there was a change in diagnosis (AD vs. non-AD) in 35.6% of patients and a management change in 61.5%. The results of the second aim, to assess the impact on hospital admissions and ER visits over one year, were disappointing. Scanned patients were 4.5% less likely to be hospitalized, less than the 10% pre-specified endpoint, with no effect on ER visits. Amyloid PET is still under CED. A second IDEAS study was approved in 2020 that focuses on underrepresented minority populations [63].
More Recent CMS PET Approvals
In 2012, an industry group asked CMS to reconsider its 2005 non-coverage NCD for new PET radiopharmaceuticals [42•]. After reviewing the extensive published guidelines for oncological PET, CMS withdrew its non-coverage for oncology, allowing coverage decisions to be made by MACs [64••]. It found the more limited guidelines for cardiac PET to be less convincing, and the non-coverage language remained. CMS also did not change its non-coverage for amyloid PET. In 2021, however, in a surprising but welcome decision, CMS removed its exclusionary language for non-oncologic PET, including cardiologic and neurologic uses, with coverage to be decided by MACs [65]. Radiopharmaceuticals for MBF and viability that are currently FDA-approved are already covered by CMS, but this decision should incentivize industry to bring new cardiac PET agents to the clinic. This decision should also lead to coverage of 18F-fluortaucipir and 18F-fluorodopa for brain imaging (see Table 2). Paradoxically, brain amyloid agents remain under CED, with one scan per patient per lifetime. CMS is currently reconsidering this policy, however, in the context of amyloid PET in CED studies of anti-amyloid monoclonal antibodies to treat AD [66, 67]. In another major decision in 2021, CMS extended coverage for 18F-FDG to infection and inflammation imaging, revoking a 2008 non-coverage NCD. This will support the use of 18F-FDG for several cardiovascular indications, including cardiac device-related infection, endocarditis, large-vessel vasculitis, and sarcoid [68, 69•].
Appropriate Use Criteria
In response to federal legislation in 2014, CMS developed a program requiring reference to appropriate use criteria (AUC) when advanced diagnostic imaging services are ordered [70]. AUCs link a specific clinical condition or presentation to specific services, indicating which service is appropriate. AUC are typically developed by national medical specialty societies following an evidence-based methodology that uses published studies. Advanced imaging services include PET and other nuclear medicine procedures, CT, and MRI. A physician ordering these services must consult an online Clinical Decision Support Mechanism which determines whether the order adheres to the relevant AUC. Implementation of the AUC program will be complicated, and it has been delayed due to the COVID-19 public health emergency. The Society of Nuclear Medicine and Molecular Imaging (SNMMI) has developed several AUCs relevant to PET, including for myocardial perfusion imaging [71•], neuroendocrine tumors, prostate cancer, and staging and response assessment of malignant disease [72].
Medicare Reimbursement Mechanism for Radiopharmaceuticals
In addition to whether Medicare covers a PET radiopharmaceutical, a key issue is the related level of reimbursement. Under the Hospital Outpatient Prospective Payment System (HOPPS), Medicare uses a “pass-through” approach for the first 2 to 3 years of coverage, with reimbursement approximating the cost of the agent. Subsequently, however, the cost is “bundled” into the reimbursement for the overall PET procedure, with the radiopharmaceutical essentially being treated as a supply. Therefore, reimbursement is the same whether the scan uses low-cost 18F-FDG or a higher-cost newer agent, and it may not even cover the cost of the agent. This discourages providers from performing these scans and limits patient access. This may also discourage industry from developing new radiopharmaceuticals. In response, SNMMI and industry groups are supporting legislation to correct this anomaly. The Facilitating Innovative Nuclear Diagnostics (FIND) Act would direct Medicare to pay separately for diagnostic radiopharmaceuticals with a per day cost that exceeds $500 [73]. The bill was introduced in July 2021; its fate is uncertain.
Conclusions
There have been tremendous advances in preclinical and translational studies of PET radiopharmaceuticals for cardiovascular, neurological, and oncological applications. To bring new PET agents to the clinic, however, regulatory approval and insurance reimbursement are required. The steps for regulatory approval of diagnostic radiopharmaceuticals are clearly set out by the FDA, with the criteria focusing on safety and clinical efficacy. The pace of FDA approval has recently increased, with many new PET agents approved for neurology and oncology applications. Insurance coverage of PET by Medicare has been more challenging, although recent decisions have substantially broadened coverage.
For many years, cardiac PET has focused on myocardial perfusion and viability; these studies have substantially advanced the care of patients with coronary artery disease. There is considerable ongoing research to develop new PET radiopharmaceuticals to probe a wide variety of cardiovascular targets. The recent progress in regulatory approval for PET should facilitate translation of these agents to the clinic.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ross BD, Gambhir SS, editors. Molecular imaging: principles and practice. 2nd ed.: Academic Press; 2021.
Haberkorn U, Mier W, Kopka K, Herold-Mende C, Altmann A, Babich J. Identification of ligands and translation to clinical applications. J Nucl Med. 2017;58(Suppl 2):27s–33s. https://doi.org/10.2967/jnumed.116.186791.
Griffiths GL, Vasquez C, Escorcia F, Clanton J, Lindenberg L, Mena E, et al. Translating a radiolabeled imaging agent to the clinic. Adv Drug Deliv Rev. 2022;181: 114086. https://doi.org/10.1016/j.addr.2021.114086.
Graham MM, Weber WA. Evaluation of the efficacy of targeted imaging agents. J Nucl Med. 2016;57(4):653–9. https://doi.org/10.2967/jnumed.115.169235.
•• Schwarz SW, Dick D, VanBrocklin HF, Hoffman JM. Regulatory requirements for PET drug production. J Nucl Med. 2014;55(7):1132–7. https://doi.org/10.2967/jnumed.113.132472. (Review of the FDA requirements for the manufacture and marketing approval of PET radiopharmaceuticals.)
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pet-drugs-current-good-manufacturing-practice-cgmp-small-entity-compliance-guide. PET Drugs — Current Good Manufacturing Practice (CGMP) small entity compliance guide 2011. Accessed February 28 2022.
•• https://www.gmp-compliance.org/files/guidemgr/UCM070306.pdf. PET Drugs — Current Good Manufacturing Practice (CGMP) 2009. Accessed March 1 2022. (Detailed description of the components involved in the CGMP production of PET radiopharmaceuticals.)
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/developing-medical-imaging-drug-and-biological-products-part-2-clinical-indications. Developing medical imaging drug and biological products part 2: clinical indications 2004. Accessed March 2 2022.
• Gorovets A, Marzella L, Rieves D, Yang L. Efficacy considerations for U.S. Food and Drug Administration approval of diagnostic radiopharmaceuticals. J Nucl Med. 2013;54(8):1479–84. doi: https://doi.org/10.2967/jnumed.112.117804 (A review of how the FDA assesses efficacy of diagnostic radiopharmaceuticals.)
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/investigational-new-drug-applications-positron-emission-tomography-pet-drugs. Investigational new drug applications for positron emission tomography (PET) drugs 2012. Accessed February 28 2022.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/developing-medical-imaging-drug-and-biological-products-part-3-design-analysis-and-interpretation. Developing medical imaging drug and biological products part 3: design, analysis, and interpretation of clinical studies 2004. Accessed February 28 2022.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212642s000lbl.pdf. Gallium Ga 68 PSMA-11 Injection, for intravenous use 2020. Accessed March 2 2022.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200655s000lbl.pdf. FLUORODOPA F 18 Injection, for intravenous use 2019. Accessed March 2 2022.
https://www.clinicaltrials.gov/ct2/show/NCT03354273. An international study to evaluate diagnostic efficacy of flurpiridaz (18F) injection PET MPI in the detection of coronary artery disease (CAD). Accessed July 11 2022.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203155s000lbl.pdf. Choline C 11 Injection, for intravenous use 2012. Accessed March 3 2022.
• Schwarz SW, Clarke B. Perspective on how the FDA should review diagnostic radiopharmaceuticals. J Nucl Med. 2018;59(6):865–7. https://doi.org/10.2967/jnumed.117.204446. (Reviews the history of the regulation of PET radiopharmaceuticals by the FDA.)
https://www.fda.gov/advisory-committees/human-drug-advisory-committees/medical-imaging-drugs-advisory-committee. Medical Imaging Drugs Advisory Committee. Accessed Jan 10 2022.
https://www.fda.gov/files/drugs/published/PET-Drug-Applications---Content-and-Format-for-NDAs-and-ANDAs.pdf. PET drug applications — Content and format for NDAs and ANDAs 2011. Accessed March 3 2022.
https://www.accessdata.fda.gov/scripts/cder/daf/. Drugs@FDA: FDA-approved drugs. Accessed March 3 2022.
• Carlucci G, Ippisch R, Slavik R, Mishoe A, Blecha J, Zhu S. (68)Ga-PSMA-11 NDA approval: a novel and successful academic partnership. J Nucl Med. 2021;62(2):149–55. https://doi.org/10.2967/jnumed.120.260455. (Summary of the documentation describing the manufacture of a PET drug that is required by the FDA.)
Sunderland JJ. The academic NDA: Justification, process, and lessons learned. J Nucl Med. 2020;61(4):480–7. https://doi.org/10.2967/jnumed.119.238287.
Zhu S, Mosessian S, Kroeger K, Sadeghi S, Slavik R, Kinloch S, et al. Transforming an academic radiochemistry facility for positron emission tomography drug cGMP compliance. Mol Imaging Biol. 2020;22(2):256–64. https://doi.org/10.1007/s11307-019-01395-6.
Bunning S, Ignace C, Mattmuller S, Schwarz SW, Scott PJH, Zigler S, et al. Proceedings: PET Drugs - a workshop on inspections management and regulatory considerations. J Nucl Med. 2022;63(7):1117–23. https://doi.org/10.2967/jnumed.121.263443.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/exploratory-ind-studies. Exploratory IND studies 2006. Accessed February 28 2022.
Schwarz SW, Oyama R. The role of exploratory investigational new drugs for translating radiopharmaceuticals into first-in-human studies. J Nucl Med. 2015;56(4):497–500. https://doi.org/10.2967/jnumed.114.146472.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/microdose-radiopharmaceutical-diagnostic-drugs-nonclinical-study-recommendations. Microdose radiopharmaceutical diagnostic drugs: nonclinical study recommendations 2018. Accessed February 28 2022.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expanded-access-investigational-drugs-treatment-use-questions-and-answers. Expanded access to investigational drugs for treatment use - questions and answers 2016. Accessed February 28 2022.
Hung JC. Bringing new PET drugs to clinical practice - a regulatory perspective. Theranostics. 2013;3(11):885–93. https://doi.org/10.7150/thno.5513•OverviewofFDAregulationofPETradiopharmaceuticals.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/radioactive-drug-research-committee-human-research-without-investigational-new-drug-application. The Radioactive Drug Research Committee: human research without an investigational new drug application 2010. Accessed February 23 2022.
• Jackson IM, Lee SJ, Sowa AR, Rodnick ME, Bruton L, Clark M, et al. Use of 55 PET radiotracers under approval of a Radioactive Drug Research Committee (RDRC). EJNMMI Radiopharm Chem. 2020;5(1):24. doi: https://doi.org/10.1186/s41181-020-00110-z (Describes the RDRC process and the activities of an RDRC in an academic medical center.)
Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer. 2015;112(2):238–50. https://doi.org/10.1038/bjc.2014.610.
Murthy VL, Bateman TM, Beanlands RS, Berman DS, Borges-Neto S, Chareonthaitawee P, et al. Clinical quantification of myocardial blood flow using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Med. 2018;59(2):273–93. https://doi.org/10.2967/jnumed.117.201368.
Liga R, Neglia D. Emerging F-18-Labelled PET myocardial perfusion tracers. Curr Cardiol Rep. 2020;22(10):116. https://doi.org/10.1007/s11886-020-01368-0.
Moody JB, Poitrasson-Rivière A, Hagio T, Buckley C, Weinberg RL, Corbett JR, et al. Added value of myocardial blood flow using (18)F-flurpiridaz PET to diagnose coronary artery disease: The flurpiridaz 301 trial. J Nucl Cardiol. 2021;28(5):2313–29. https://doi.org/10.1007/s12350-020-02034-2.
Stendahl JC, Kwan JM, Pucar D, Sadeghi MM. Radiotracers to address unmet clinical needs in cardiovascular imaging, part 1: technical considerations and perfusion and neuronal imaging. J Nucl Med. 2022;63(5):649–58. https://doi.org/10.2967/jnumed.121.263506.
Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983;24(9):790–8.
https://clinicaltrials.gov/ct2/show/NCT05134012?term=rapid+water+flow&draw=2&rank=1. Radiolabeled perfusion to identify coronary artery disease using water to evaluate responses of myocardial flow (RAPID-WATER). Accessed April 3 2022.
•• Farber G, Boczar KE, Wiefels CC, Zelt JGE, Guler EC, deKemp RA, et al. The future of cardiac molecular imaging. Semin Nucl Med. 2020;50(4):367–85. https://doi.org/10.1053/j.semnuclmed.2020.02.005. (Comprehensive review of various targets for molecular imaging of the heart.)
Thackeray JT. Molecular imaging using cardiac PET/CT: opportunities to harmonize diagnosis and therapy. Curr Cardiol Rep. 2021;23(8):96. https://doi.org/10.1007/s11886-021-01526-y.
Werner RA, Thackeray JT, Diekmann J, Weiberg D, Bauersachs J, Bengel FM. The changing face of nuclear cardiology: Guiding cardiovascular care toward molecular medicine. J Nucl Med. 2020;61(7):951–61. https://doi.org/10.2967/jnumed.119.240440.
Stendahl JC, Kwan JM, Pucar D, Sadeghi MM. Radiotracers to address unmet clinical needs in cardiovascular imaging, part 2: inflammation, fibrosis, thrombosis, calcification, and amyloidosis imaging. J Nucl Med. 2022;63(7):986–94. https://doi.org/10.2967/jnumed.121.263507.
Hillman BJ, Frank RA, Abraham BC. The Medical Imaging & Technology Alliance conference on research endpoints appropriate for Medicare coverage of new PET radiopharmaceuticals. J Nucl Med. 2013;54(9):1675–9. https://doi.org/10.2967/jnumed.113.127886ProvidesasummaryofMedicare’sexpectationsforapprovalofaPETradiopharmaceutical.
• Marzella L, Czernin J, Hope T. Perspectives on radiopharmaceutical agents from the FDA: A conversation between Lou Marzella, Johannes Czernin, and Thomas Hope. J Nucl Med. 2021;62(7):881–3. https://doi.org/10.2967/jnumed.121.262450. (A discussion of the FDA approval process for PET radiopharmaceuticals with an FDA division director.)
Yang L, Rieves D, Ganley C. Brain amyloid imaging–FDA approval of florbetapir F18 injection. N Engl J Med. 2012;367(10):885–7. https://doi.org/10.1056/NEJMp1208061.
Jensen TS, Jacques LB. Medicare coverage: engaging on evidence. Regen Med. 2011;6(6 Suppl):99–101. https://doi.org/10.2217/rme.11.60.
Neumann PJ, Tunis SR. Medicare and medical technology–the growing demand for relevant outcomes. N Engl J Med. 2010;362(5):377–9. https://doi.org/10.1056/NEJMp0912062.
Siegel BA. 2014 Cassen Lecture: What have we learned from the National Oncologic PET Registry? J Nucl Med. 2014;55(12):9n–15n (A detailed review of the National Oncologic PET Registry by one of its leaders).
• Gambhir SS, Shankar LK, Rosenthal E, Warram JM, Ghesani M, Hope TA, et al. Proceedings: Pathways for successful translation of new imaging agents and modalities-phase III studies. J Nucl Med. 2019;60(6):736–44. https://doi.org/10.2967/jnumed.118.219824. (A review of Medicare’s coverage process and decisions for imaging.)
•• Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making. 1991;11(2):88–94. https://doi.org/10.1177/0272989x9101100203. (A classic paper describing how to evaluate diagnostic imaging based on efficacy and patient outcomes.)
Lu B, Gatsonis C. Efficiency of study designs in diagnostic randomized clinical trials. Stat Med. 2013;32(9):1451–66. https://doi.org/10.1002/sim.5655.
Siepe B, Hoilund-Carlsen PF, Gerke O, Weber WA, Motschall E, Vach W. The move from accuracy studies to randomized trials in PET: current status and future directions. J Nucl Med. 2014;55(8):1228–34. https://doi.org/10.2967/jnumed.113.127076.
Dahabreh IJ, Gatsonis C. A flexible, multifaceted approach is needed in health technology assessment of PET. J Nucl Med. 2014;55(8):1225–7. https://doi.org/10.2967/jnumed.114.142331.
https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=211&ncdver=3. National coverage determination (NCD) PET scans 2005. Accessed March 8 2022.
https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=288&ncdver=3. FDG PET for dementia and neurodegenerative diseases. 2009. Acessed May 20 2022.
Hillner BE, Liu D, Coleman RE, Shields AF, Gareen IF, Hanna L, et al. The National Oncologic PET Registry (NOPR): design and analysis plan. J Nucl Med. 2007;48(11):1901–8. https://doi.org/10.2967/jnumed.107.043687.
Tunis S, Whicher D. The National Oncologic PET Registry: lessons learned for coverage with evidence development. J Am Coll Radiol. 2009;6(5):360–5. https://doi.org/10.1016/j.jacr.2009.01.016.
https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=263. Positron emission tomography (FDG) for solid tumors 2013. Accessed April 17 2022.
https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=336&ncdver=2. Positron emission tomography (NaF-18) to identify bone metastasis of cancer 2021. Accessed March 8 2022.
Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71(4):266–73. https://doi.org/10.1097/NEN.0b013e31824b211b.
https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=265. Beta amyloid positron emission tomography in dementia and neurodegenerative disease. Accessed March 8 2022.
Herscovitch P. Regulatory approval and insurance reimbursement: the final steps in clinical translation of amyloid brain imaging. Clinical and Translational Imaging. 2015;3(1):75–7. https://doi.org/10.1007/s40336-015-0101-7.
• Rabinovici GD, Gatsonis C, Apgar C, Chaudhary K, Gareen I, Hanna L, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286–94. https://doi.org/10.1001/jama.2019.2000. (Results of a large coverage with evidence development study sponsored by Medicare to assess the clinical impact of PET imaging of brain amyloid.)
https://www.ideas-study.org/. New IDEAS. Accessed March 8, 2022.
•• https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=261. Positron emission tomography 2013. Accessed March 9 2022. (Medicare’s decision memo to approve PET for oncologic applications, with a discussion of how the decision was made.)
https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=211&ncdver=3. National coverage determination (NCD) positron emission tomography (PET) scans 2022. Accessed March 9 2022.
https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305. Monoclonal antibodies directed against amyloid for the treatment of Alzheimer’s Disease 2022. Accessed May 20 2022.
https://www.cms.gov/medicare-coverage-database/view/ncacal-tracking-sheet.aspx?ncaid=308. Beta amyloid positron emission tomography in dementia and neurodegenerative disease 2022. Accessed June 30 2022.
Dilsizian V, Budde RPJ, Chen W, Mankad SV, Lindner JR, Nieman K. Best practices for imaging cardiac device-related infections and endocarditis: A JACC:Cardiovascular Imaging expert panel statement. JACC: Cardiovasc Imaging. 2021. https://doi.org/10.1016/j.jcmg.2021.09.029.
• Wahl RL, Dilsizian V, Palestro CJ. At last, (18)F-FDG for inflammation and infection! J Nucl Med. 2021;62(8):1048–9. https://doi.org/10.2967/jnumed.121.262446. (Overview of Medicare’s approval of F-18 FDG for PET/CT imaging of inflammation and infection.)
https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-B/part-414/subpart-B/section-414.94. Appropriate use criteria for advanced diagnostic imaging services. Accessed April 29 2022.
• Schindler TH, Bateman TM, Berman DS, Chareonthaitawee P, De Blanche LE, Dilsizian V, et al. Appropriate use criteria for PET myocardial perfusion imaging. J Nucl Med. 2020;61(8):1221–65. https://doi.org/10.2967/jnumed.120.246280. (Describes appropriate use criteria methodology and its application to PET myocardial perfusion imaging.)
https://www.snmmi.org/ClinicalPractice/content.aspx?ItemNumber=15666. Appropriate use criteria (AUC). Accessed March 10, 2022.
https://www.snmmi.org/NewsPublications/NewsDetail.aspx?ItemNumber=37601. The FIND Act gets a Senate companion bill. Accessed March 10 2022.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH Clinical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author reports the following: Member, FDA Medical Imaging Drugs Advisory Committee.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology (V Dilsizian, Section Editor)
Rights and permissions
About this article
Cite this article
Herscovitch, P. Regulatory Agencies and PET/CT Imaging in the Clinic. Curr Cardiol Rep 24, 1361–1371 (2022). https://doi.org/10.1007/s11886-022-01749-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01749-7