Abstract
Many melittophilous flowers display yellow and UV-absorbing floral guides that resemble the most common colour of pollen and anthers. The yellow coloured anthers and pollen and the similarly coloured flower guides are described as key features of a pollen and stamen mimicry system. In this study, we investigated the entire angiosperm flora of the Alps with regard to visually displayed pollen and floral guides. All species were checked for the presence of pollen- and stamen-imitating structures using colour photographs. Most flowering plants of the Alps display yellow pollen and at least 28% of the species display pollen- or stamen-imitating structures. The most frequent types of pollen and stamen imitations were (mostly yellow and UV-absorbing) colour patches on petals (65% of species displaying imitations), patterns of inflorescences (18%), stamen-like pistils (10%), and staminodes (6%), as well as three-dimensional structures such as convex lower lips and filamental hairs (<5%). Dichogamous and diclinous species display pollen- and stamen-imitating structures more often than non-dichogamous and non-diclinous species, respectively. The visual similarity between the androecium and other floral organs is attributed to mimicry, i.e. deception caused by the flower visitor’s inability to discriminate between model and mimic, sensory exploitation, and signal standardisation among floral morphs, flowering phases, and co-flowering species. We critically discuss deviant pollen and stamen mimicry concepts and evaluate the frequent evolution of pollen-imitating structures in view of the conflicting use of pollen for pollination in flowering plants and provision of pollen for offspring in bees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melittophilous flowers display a great diversity of visual signals to attract pollinators (Wester and Lunau 2017). For flower-visiting bees, floral colour patterns are of outstanding importance for long-distance perception and orientation at close range (Lunau et al. 1996). Whereas large-scale components of flowers and inflorescences serve as distant attractants, small-sized features function as floral guides directing flower visitors to distinct areas of the flower facilitating access to floral reward and/or pollen transfer (Lunau et al. 1996; Wilmsen et al. 2017). Several morphological structures contribute to the development of small-sized components of floral colour patterns such as pollen, anthers, stamens, receptacles, styles, staminodes, colour patches, or the arrangement of florets within an inflorescence (Lunau 2000, 2007). To understand signalling of flowers and inflorescences, it is helpful to assume that various structures might contribute to a uniform signalling colour pattern. By contrast, in many studies only colour markings of petals are regarded as floral colour patterns. In this study, the neutral term floral guide is preferred to nectar guide, pollen guide, or honey guide, which are terms that imply a specific purpose. Furthermore, the non-interpretive term imitation is used to indicate the similarity of structures, whereas the term mimicry is used to denominate structures thought or known to deceive flower visitors owing to their inability to discriminate between the mimic and a model signal (Dafni 1984; Roy and Widmer 1999; Johnson and Schiestl 2016; Lunau and Wester 2017).
Floral colour patterns consist of at least two different colours, one usually large-sized, peripheral colour component and one generally small-sized, central colour element. In the 1970s, two researchers highlighted—largely independently of one another—one common particular aspect of floral colour patterns, namely signalling structures visually resembling pollen and anthers in colour and shape. The zoologist Günther Osche focused on the evolution of these stamen-imitating floral guides (Osche 1979, 1983a). He regarded the colour of pollen as the very first floral colour signal in angiosperm evolution and a cue for flower visitors predating the evolution of conspicuous petals (Osche 1986). He also argued that primarily anemophilous flowering plants developed yellow and UV-absorbing pollen by embedding flavonoid pigments as protection against bacteria, fungi, and ultraviolet radiation. Flavonoids like quercetin thus represent a preadaptation and a reliable cue for the very first flower visitors at a time when flowers had neither petals nor other signalling structures (Osche 1983a). Osche (1979, 1983a, b, 1986) distinguished various forms of mimicry such as pollen, anther, stamen, and androecium mimicry, independently of the way of deception of the signal receivers.
The botanist Stefan Vogel discussed the deceptive nature of floral guides that possess the same colour as pollen and stamens (Vogel 1978) and categorised various types of deception (Vogel 1993). Contrary to Osche, Vogel did not agree that pollen- and stamen-mimicking floral guides might function as nectar guides and thus deceive nectar-seeking flower visitors. Consequently, Vogel distinguished between nectar guides and pollen guides. He only regarded orchids, in which the flower visitors are unable to eat or harvest pollen, and pollen-mimicking pistillate flowers of diclinous plants, which lack stamens, as absolute pollen deceptive flowers. Vogel also noticed that bees handle stamens and presumed stamen mimics differently, namely that bees search for pollen with characteristic movements exclusively at real stamens. We can confirm the observations of Vogel that bees do not show typical pollen collection behaviour at pollen- and stamen-imitating floral guides (Lunau, personal observation), but assume that Vogel observed experienced bees that might have altered their behavioural response to stamen mimics. Thus, it remains an open question whether naïve bees initially exhibit pollen-collecting behaviour at stamen mimics. These observations are supported by laboratory experiments demonstrating that bumblebees collect chemically inert pollen surrogates such as glass powder (Lunau et al. 2015), indicating that chemical stimuli of real pollen are dispensable for triggering this behaviour. Westerkamp (1996) discussed the different concepts of Osche and Vogel; for him, it is incomprehensible why flowers would display the same signal for the attraction of nectar foragers and pollen foragers. Both Vogel (1978, 1993) and Osche (1979, 1983a, b) regarded the pollen- and stamen-imitating signalling structures as a multi-faceted phenomenon. It includes the imitation of entire flowers, the imitation of pollen-bearing male flowers by female flowers in diclinous species, the imitation of pollen colour by poricidal anthers that conceal pollen, and the imitation of the flowers’ own pollen, which constitutes a partial deception. The feigning of a larger amount of pollen, the pretence of a continuous pollen offer in dichogamous flowers, and conspicuous pollen-imitating structures deflecting from less conspicuous real pollen represent additional variants of pollen imitation.
The works of Vogel (1975, 1978, 1993) and Osche (1979, 1983a, 1986), although both rich in the description of pollen- and stamen-imitating or -mimicking species, have inspired many researchers to describe additional pollen- and stamen-mimicking structures in various species of flowering plants. Some examples in the Orchidaceae family are Calypso bulbosa (Boyden 1982), Cephalanthera longifolia (Dafni and Ivri 1981), Thelymitra nuda (Bernhardt and Burns-Balogh 1986), Dendrobium unicum (Davies and Turner 2004), Eulophia spp. (Peter and Johnson 2013), and Phaius delavayi (Li et al. 2010). Imitations can also be found in the Scrophulariaceae Craterostigma plantaginea and Torenia polygonoides (Magin et al. 1989), diclinous begonias (Ågren and Schemske 1991; Schemske et al. 1996; Wyatt and Sazima 2011; Castillo et al. 2012), and Crocus flowers with stamen-imitating styles (Lunau et al. 2016). Additionally, there is an abundance of flowers with conspicuous staminodes (Hardy and Stevenson 2000; Hrycan and Davis 2005; Ushimaru et al. 2007; Walker-Larsen and Harder 2000) and various types of imitations in other flowering plants (Bernhardt et al. 1984; Simpson et al. 1986; Weber 1989; Harder and Barclay 1994; Leins and Erbar 1994; Barthlott 1995; Dobson et al. 1996; Peisl 1997; Lunau 2000, 2007; Sigrist and Sazima 2004; Sá-Otero et al. 2009). The phenomenon of pollen and stamen mimicry has also been discussed in reviews (Dafni 1984; Roy and Widmer 1999; Lunau 2000, 2007; Lunau and Wester 2017) and textbooks of pollination ecology (Weberling 1992; D’Arcy and Keating 1996; Lloyd and Barrett 1996; Willmer 2009; Leins and Erbar 2010; Schaefer and Ruxton 2011; Johnson and Schiestl 2016).
The response of flower visitors to pollen- and stamen-imitating signals (Fig. 1) has been studied for hoverflies, bumblebees, and western honey bees. Syrphid flies of the genus Eristalis respond to the yellow and UV-absorbing colour with a visually elicited proboscis reflex (Lunau 1988; Lunau and Wacht 1994), which is strongly inhibited by admixed ultraviolet or blue light (Lunau and Wacht 1997). These syrphid flies are also able to detect the amino acid proline as a common constituent of pollenkitt by taste receptors on the proboscis and on the tarsi (Wacht et al. 1996, 2000). Yellow dot guides were shown to prolong handling time of artificial flowers (Dinkel and Lunau 2001) and dot guides displaying a colour gradient from red to yellow direct hoverflies towards the yellow end of the colour gradient (Lunau et al. 2005). Besides wind, bees are regarded as the world’s most important pollination agents (Michener 2000), even though bees often have to be manipulated by plants to transfer pollen to the stigma of conspecific flowers. Bees also may visit flowers without touching the stigma or act as pollen thieves (Michener 2000). In fact, bees and flowers compete for the utilisation of pollen, since female bees collect pollen to provision their offspring with proteins (Westerkamp 1997). In this context, the pollen and stamen imitations of flowers might be interpreted as signalling structures to promote pollination by altering the flower handling by bees.
Floral guides and colour patterns manipulate flower visitors. a Rhododendron ponticum (Ericaceae) flower visited by a worker of Bombus pratorum (Apidae). The stamens are cryptically coloured, whereas yellow colour patches indicate the slit-shaped access towards the nectar. b Bombus hypnorum exhibiting antennal contact with the stigmatic lobes of a pistillate Begonia sp. (Begoniaceae) flower. c Saxifraga ferruginea (Saxifragaceae) flowers in the staminate (left) and pistillate flowering phase offer different amounts of pollen. d Saxifraga umbrosa flower displaying coloured dot guides from red to yellow indicating the direction to search for nectar in the centre of the flower and at the same time deflecting from the pollen offered by some anthers. e Apis mellifera (Apidae) drinking nectar from a flower of Myosotis palustris (Boraginaceae) after inserting its proboscis into the floral tubes marked by a three-dimensional, yellow ring. f Episyrphus balteatus (Syrphidae) hoverfly extending its proboscis towards the yellow ring of a Myosotis sp. flower
When approaching a flower, bumblebees target towards floral guides and make the initial physical contact with a flower with the tips of their antennae (Lunau 1990, 1991, 1992a, 1993; Lunau et al. 2006, 2009). The colour parameters of floral guides eliciting the antennal response are the colour contrast between floral guide and corolla, as well as the superior colour purity of the floral guide compared to the corolla (Lunau et al. 1996, 2006, 2009; Heuschen et al. 2005; Pohl and Lunau 2007; Pohl et al. 2008). Flower guides are also known to reduce nectar robbing (Leonard et al. 2013) and handling time if they are close to the site of reward, even in the case of inexperienced bees (Orban and Plowright 2014). However, black, white, and other coloured nectar guides that do not imitate stamens or pollen might have similar effects on the reduction of handling time (Dinkel and Lunau 2001; Leonard and Papaj 2011). These behavioural studies have resulted in a broad acceptance of floral guides being pollen and stamen imitations and acting as signalling structures of flowers (Dafni and Giurfa 1998, 1999; Dafni and Kevan 1996; Duffy and Johnson 2015).
A thorough investigation of how widely pollen-imitating signalling structures are distributed in flowering plants is missing. In this study, the flora of the Alps is investigated with regard to visual signalling of pollen and stamens, floral colour patterns imitating the colour pattern displayed by natural pollen, and floral guides contrasting against the corolla. The study ignores chemical cues of pollen (Dobson 1988; Dobson et al. 1996, 1999), since comparative data about pollen odour in alpine plants is rare and data about odour of pollen-imitating structures are missing completely. The case study comprises all species of flowering plants listed by the Flora alpina (Aeschimann et al. 2004), irrespective of pollination by wind, insects, or otherwise. The study aims at estimating the number of species contributing to the rather uniform colour pattern originating from yellow pollen and anthers as well as yellow pollen- and anther-imitating structures of flowers and inflorescences. The study further focuses on distinguishing the phenomena of true pollen and anther mimicry, signal standardisation, and sensory exploitation to denote floral colour patterns.
Materials and methods
All flowering plants of the Alps listed in the Flora alpina (Aeschimann et al. 2004), 4328 species in 42 orders and 136 families, were surveyed by means of the colour photographs included in the books and additional colour photographs from various reliable internet sources (see additional electronic sources). The presence of pollen- and stamen-imitating structures as well as the colour of pollen and pollen- or stamen-imitating structures was noted and evaluated for all species. Gymnospermae were excluded from this study. The classification of angiosperm taxa was conducted according to the Angiosperm Phylogeny Group (Chase and Reveal 2009). Additional information about the mode of pollination and floral reproductive morphology (dicliny, dichogamy, and heterostyly) was recorded and evaluated. The colours of pollen and anthers were classified into colour hue categories according to human colour vision. Reflectance in the ultraviolet waveband could not be taken into consideration; however, it has been shown that yellow pollen usually absorbs ultraviolet light (Lunau 1995). The presence of stamen-imitating structures was analysed concerning different insect pollinators and regarding plant reproductive morphology (dicliny and dichogamy). A Chi-square test of goodness of fit was conducted for the comparison of observed frequencies of imitations (i.e. in dichogamous species) to expected frequencies and a Chi-square test of independence (contingency test) was used to compare two sets of frequencies, i.e. the frequencies of stamen-imitating structures between dichogamous and non-dichogamous species.
Pollen and stamen imitations were categorised in yellow and UV-absorbing colour patches of flowers, inflorescences, staminodes, three-dimensional structures such as lower lips and protuberances, filamental hairs, and styles/stigmata coloured like stamens (Fig. 2). It was not possible to check the ultraviolet absorbance properties of yellow signalling structures for all species. Whenever possible, UV photographs and information about ultraviolet absorption were checked using the studies of Biedinger and Barthlott (1993), Burr and Barthlott (1993), and Burr et al. (1995), photographs published on the internet (Rørslett 2006), and by means of UV photographs (Lunau 1996, 2000, 2007, personal observation). Missing information about the ultraviolet reflectance of floral signalling structures is problematic, since it is crucial for the understanding of flower colours as perceived by bees (Daumer 1958). However, pollen- or anther-sized yellow signalling structures rarely reflect ultraviolet light (Lunau, personal observation).
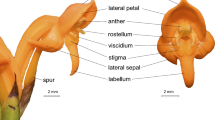
Diversity of pollen mimicry. a Orobanche gracilis (Orobanchaceae) flower displaying a bilobed, yellow stigma. b Colutea arborescens (Fabaceae) flower with hidden stamens and an almost invisible anther-shaped colour patch on the standard. c Colutea arborescens flower displaying an ultraviolet colour pattern. d Craterostigma plantagineum (Linderniaceae) flower with anther-like, three-dimensional colour patches on a knee of the filaments. e Verbascum phoeniceum (Scrophulariaceae) with yellowish filamental hairs and within-flower heteranthery. f Aesculus hippocastanum (Sapindaceae) flower with a yellow floral guide that undergoes colour change towards red. g Bougainvillea spectabilis (Nyctaginaceae) flowers resembling stamens and in combination with the violet bracts resemble a larger flower. h One of three floral morphs of Eichhornia crassipes (Pontederiaceae) with cryptic stamens and pollen and a conspicuous uniform yellow floral guide. i Commelina coelestis (Commelinaceae) with heteranthery including three yellow staminodes, one fodder stamen and two cryptic pollination stamens bent inwards to conceal the yellow pollen grains. j Iris × germanica (Iridaceae) with a beard of stamen-like protuberances on the downwards-bending perigone leaf. k Lagerstroemia indica (Lythraceae) with conspicuous yellow fodder stamens and cryptic pollination stamens
Results
More than one quarter of the 4328 angiosperms of the Alps (27.7%, 1198 species) possess pollen- and stamen-imitating structures of flowers or inflorescences. These comprised 32.4% of the total species of flowering plants that could be checked for the presence of these structures by means of colour photographs. Owing to insufficient photographs of the flowers, 629 species were not studied.
Besides wind, mostly insects were listed as pollinators of the studied species, including hymenopterans, dipterans, coleopterans, and lepidopterans in various combinations. Out of 2938 species labelled as entomophilous, 1068 species possess a pollen- or stamen-imitating structure. Stamen-imitating structures were significantly more frequent in flowering plants pollinated by Diptera and Coleoptera (Chi-square test of goodness of fit: Χ 2 = 4.83, p < 0.05). They were significantly less frequent in plants pollinated by Diptera and Hymenoptera (Χ 2 = 17.13, p < 0.0001), by Hymenoptera (Χ 2 = 198.82, p < 0.0001), by Diptera, Hymenoptera, and Coleoptera (Χ 2 = 60.81, p < 0.0001), by Diptera, Hymenoptera, and Lepidoptera (Χ 2 = 17.13, p < 0.001), and by Lepidoptera (Χ 2 = 31.02, p < 0.0001). The number of species with stamen-imitating structures did not differ significantly from the number of species without stamen imitations in flowering plants pollinated by Hymenoptera and Lepidoptera (Χ 2 = 0.04, p = 0.835), by Diptera (Χ 2 = 3.26, p = 0.071), and by other Insecta (Χ 2 = 3.77, p = 0.052) (Fig. 3a).
Proportion of plant species displaying pollen or stamen mimicry regarding different insect pollinators and plant reproductive morphology. The numbers in the columns indicate the number of species. Results of the Chi-square test of goodness of fit are shown above the columns with p > 0.05 as n.s. (not significant), *p < 0.05, **p < 0.01, and ***p < 0.001. a Percentage of flowering plant species with and without pollen or stamen imitation for different groups of pollinators including Diptera (D), Hymenoptera (H), Coleoptera (C), Lepidoptera (L), and Insecta (I). b Percentage of diclinous and dichogamous flowering plant species with and without pollen or stamen imitation
Out of 2938 entomophilous species, 1244 species are dichogamous and 41.5% of them feature stamen imitations. Dichogamous species display stamen-imitating structures significantly more often than non-dichogamous species (Chi-square test of independence: Χ 2 = 22.58, p < 0.001). The percentage of species displaying stamen-imitating structures is higher in protogynous species (57.9% out of 240 species; Chi-square test of goodness of fit: Χ 2 = 6.02, p < 0.05) than in protandrous species (37.6% out of 1004 species; Χ 2 = 62.25, p < 0.001); this difference is highly significant (Chi-square test of independence: Χ 2 = 33.10, p < 0.001) (Fig. 3b).
Out of the 534 species that are both entomophilous and diclinous, 52.1% possess stamen-imitating structures. Diclinous species display stamen imitations significantly more often than non-diclinous species (Chi-square test of independence: Χ 2 = 67.66, p < 0.001) and monoecious species display stamen imitations significantly more often than dioecious species (Χ 2 = 47.65, p < 0.001). In monoecious and gynomonoecious plants, the number of species displaying stamen-imitating structures is significantly higher than the number of plants without imitations (Chi-square test of goodness of fit; monoecious: Χ 2 = 31.12, p < 0.001; gynomonoecious: Χ 2 = 49.28, p < 0.001). In contrast, dioecious, gynodioecious, and andromonoecious species possess stamen imitations significantly less often (dioecious: Χ 2 = 16.90, p < 0.001; gynodioecious: Χ 2 = 14.22, p < 0.001; andromonoecious: Χ 2 = 42.12, p < 0.001).
In some species-rich plant families, the frequency of pollen and stamen imitations is above average, particularly in the Asteraceae, Boraginaceae, Scrophulariaceae, Violaceae, Ranunculaceae, Saxifragaceae, and Iridaceae (Table 1), whereas in others pollen and stamen imitations are less frequent, for example in the wind-pollinated families Juncaceae, Cyperaceae, Poaceae, and in the Apiaceae with many small-sized flowers forming umbels. Interestingly, some plant families with extraordinary 3D-morphology such as Fabaceae and Lamiaceae include only few species with pollen and stamen imitations. In the Orchidaceae, many species with sexual deception have evolved exceptional attraction strategies that dispense with pollen and stamen imitation (Table 1).
44% of the alpine angiosperms have yellow pollen, followed by 8% with white, 4% with violet, and 3% with rose pollen. Flowering plants with brown, orange, red, black, green, and blue pollen are rare and constitute less than 3% (Table 2). Thus, 70.9% of the species that could be checked for their pollen colour had yellow or orange pollen. Pollen colour could not be determined for 36% of species due to a lack of photographs in which the pollen colour was visible. Out of the 1198 species possessing stamen-imitating structures, 67% have yellow pollen and 57% have yellow anthers, whereas only 44% of the 2501 species without stamen-imitating structures have yellow pollen and 31% have yellow anthers (Table 2).
The most frequent types of pollen and stamen mimicry were yellow and UV-absorbing colour patches of flowers (65%) and of inflorescences (18%), stamen-like pistils (10%), and staminodes (6%); three-dimensional structures such as lower lips and protuberances as well as filamental hairs were rarely found (<4%). The predominant colour of pollen- and stamen-imitating structures was yellow or orange, which was found in 70% of the species (Table 2).
Discussion
The signal uniformity of flowering plants of the Alps is enormous, since 44% of the species display yellow pollen and 28% of the species display pollen-imitating signalling structures (Fig. 4). This confirms the finding of previous studies stating that yellow is the predominant pollen colour (Osche 1979; Lunau 1995). The overlap between species possessing yellow pollen and species displaying yellow pollen-imitating signalling structures is large and amounts to 545 species (28.4% out of all species with yellow pollen).
Diversity of pollen mimicry in alpine flowering plants. a Flowers of eyebright, Euphrasia rostkoviana (Orobanchaceae), with yellow floral guide. b Snow-rose, Rhododendron ferrugineum (Ericaceae), displaying stamens with yellow anthers and whitish pollen. c Flower of grass-of-Parnassus, Parnassia palustris (Celastraceae), with cryptic white stamens and pollen, and conspicuous yellow staminodes. d Caltha palustris (Ranunculaceae) flower with yellow stamens and pollen. e Flower of marsh-marigold, Caltha palustris, with an enhanced floral guide providing an ultraviolet bull’s eye. f Flowers of forget-me-not, Myosotis arvensis (Boraginaceae), with three-dimensional yellow inner ring undergoing colour change towards white. g Flowers of common cow-wheat, Melampyrum pratense (Orobanchaceae), with arched, deep yellow lower lip. h Inflorescences of the alpine aster, Aster alpinus (Asteraceae), with yellow disc florets and violet ray florets. i Alpine toadflax, Linaria alpina (Plantaginaceae), displaying a gullet blossom with an orange-yellow, three-dimensional mask. j Dark mullein, Verbascum nigrum (Scrophulariaceae), with orange pollen and contrasting violet filamental hairs
The evaluation of the extent of this mimicry system is complex, since it comprises model species, mimic species, and signal receivers. One might consider all flowering plants that visually display yellow, UV-absorbing pollen as model species. Flowers in which pollen is invisible but advertised in yellow and UV-absorbing anthers, e.g. the flowers of the Solanaceae with poricidal anthers, might be deemed either model or mimic. Flowers displaying yellow, UV-absorbing floral guides that offer any kind of reward might also be considered either model species or mimic species. Consequently, only nectarless orchids that offer pollen in the form of uneatable and uncollectable pollinaria are pollen- and stamen-mimicking species—with few exceptions (Sanguinetti et al. 2012). It is noteworthy that some orchids offer pollen surrogates (Beck von Mannagetta and Lerchenau 1912, 1914; Davies et al. 2013).
Recipients of pollen-imitating signals are pollinators that collect pollen, e.g. female bees and masarid wasps (Mueller 1996), or eat pollen on flowers, e.g. many flies—particularly hoverflies—some flower-visiting beetles, and few micropterygid butterflies (Faegri and van der Pijl 1966). Marking the beginning of pollination ecology, Sprengel (1793) described that yellow flower markings (e.g. the yellow centre of Myosotis flowers) often guide insects to floral rewards and he inferred that all flower colours serve as orientation help for insects. Thus, the first mentioning of flowers not being colourful to please humans, but to attract insects included an anther imitation (Osche 1983a).
The evolution of pollen and stamen mimicry provides insight into the exceptional role of the original pollen colour as the primary floral colour signal (Osche 1979, 1983a, b, 1986). Primarily wind-pollinated flowering plants do not possess conspicuous flowers; in fact, all bulky floral structures that might impede pollen transfer are absent. The flowers are thus inconspicuous except for the pollen, which is yellow due to flavonoid pigments in the pollen exine (Thompson et al. 1972; Rieseberg and Schilling 1985). The pollen sac walls possess these protective pigments as well. Pollenkitt is mostly absent in wind-pollinated plants. It is known that flower visitation of insects predated the origin of angiosperms (Pellmyr 1992) and that pollen herbivores also ate pollen of primarily wind-pollinated plants (Stanley and Linskens 1974; Dobson 1988). Since primarily wind-pollinated flowers are unisexual, one might suggest that pollen herbivores only visited staminate flowers and might have been attracted by the colour of pollen and anthers (Labandeira et al. 1994; Labandeira 1997), whereas pollination droplet drinkers might have specialised to visit pistillate flowers (Kato and Inoue 1994). It has been suggested that early angiosperms possibly evolved in dry and/or windless habitats and benefitted from the evolution of hermaphroditic flowers (Friedman and Barrett 2009), because then pollen herbivores and pollination droplet drinkers were not solely antagonistic flower visitors, but could transfer pollen from stamens to stigmas of conspecific flowers. For these early flower visitors, flowers made an appearance by means of their conspicuous pollen grains and anthers. The signalling apparatus of present-day flowers might have thus evolved to enhance or contrast against the visual pollen signal.
Hermaphroditic flowers face the problem of self-pollination and have evolved various mechanisms to reduce self-pollination, such as heterostyly, dichogamy, and secondary dicliny. These floral morphs and flowering phases offer different amounts of pollen rewards and thus benefit from displaying uniform pollen-imitating signals instead. Furthermore, flowers pollinated by pollen-eating or pollen-collecting insects might benefit from shielding their pollen against harvesting and offering alternative rewards. This justifies regarding yellow, UV-absorbing pollen and pollen-bearing anthers as models, similarly coloured signalling structures as mimics and the pollen-eating or -collecting flower visitors as signal receivers of a species-rich mimicry system (Lunau and Wester 2017).
Vogel’s consequent line of argument (Vogel 1993), in which he accepts pollen- and anther-mimicking structures only for pollen-rewarding flowers and flower visitors searching for pollen, is strictly based on the mimicry concept that requires the deception of a signal receiver that cannot (fully) discriminate between the signals of model and mimic. This arguable point of view ignores, however, that flower visitors are accustomed to finding flowers that have been emptied by previous visits by other flower visitors. Moreover, pollen-eating hoverflies as well as pollen-collecting bees exploit floral nectar and pollen resources on the same foraging bout and even on the same flowers and thus the assignment of a flower visit to one particular resource is difficult (Konzmann and Lunau 2014; Lunau and Ren personal observations).
The phenomenon of pollen, anther, stamen, and androecium mimicry comprises true mimicry, sensory bias, sensory exploitation, and signal standardisation. In the following paragraphs, definitions of these phenomena are presented and discussed by means of common and well-known exemplary species.
True mimicry
Mimicry, in the narrower sense, is the deception of a signal receiver that is unable to discriminate between a model and a mimic. True mimicry thus involves three protagonists, which are model, mimic, and signal receiver. In pollen mimicry systems, perceptible properties of pollen grains represent the model, similar properties of other structures represent the mimic, and flower-visiting insects are the signal receivers orienting by means of pollen cues and deceived by responding to pollen-mimicking signals. Here, pollen, anther, stamen, and androecium mimicry are treated as aspects of one single phenomenon. For example, in the unisexual flowers of Cucurbita pepo, pistillate flowers deceive pollen-collecting insects. The visual resemblance between androecium and style is striking, although the staminate and pistillate flowers may considerably vary in diameter (Costich and Meagher 2001). In the genus Begonia, dicliny is combined with the absence of nectar; in this case, only the staminate flowers offer pollen, whereas the pistillate flowers are deceptive flowers (Schemske and Ågren 1995; Schemske et al. 1996; Wyatt and Sazima 2011). Pollination by deceit is common among plants with unisexual flowers (Willson and Ågren 1989).
The issue of mimicry in floral guides is a complex phenomenon and includes several controversial aspects. An essential aspect of mimicry is the deception of a signal receiver that cannot fully discriminate between model and mimic. By definition, mimicry systems include a model as protagonist; mimicry without model is thus self-contradictory. However, in many pollen and stamen mimicry case studies the specific model for a given pollen- or stamen-mimicking signalling structure is not easily identified (Osche 1983a). The correct approach is to consider the experience of individual flower visitors, i.e. the sequence of visited flowers or flowering plants. One might alternatively argue that the response to pollen- and stamen-mimicking signals is innate, which means that there is no specific model flower but rather an innate search image or fixed response to a key stimulus. In this argumentation, the model is in the eye of the beholder.
When looking for the model of an assumed pollen and stamen mimic, the most obvious are conspecific flowers. Automimicry is defined as mimicry within one species. In the context of pollen and stamen mimicry, automimicry means that model and mimic are found on the same flower or on different flowers of the same species. If pollen and stamen mimicry is regarded as an evolutionary process in which the display of real pollen and stamens has been replaced by the display of fake pollen and stamens, one might expect a transitional phylogenetic stage in which both real stamens and mimic stamens equally contributed to the visual stimulus. Only few species represent this transitional stage, e.g. Commelina spp. (Hrycan and Davis 2005). Many species that visually display real pollen and stamens and pollen- and stamen-imitating signals possess cryptic or inconspicuous real stamens and visually conspicuous stamen-mimicking structures. However, there are many species in which yellow and UV-absorbing floral guides enhance the colour signal of the androecium. Ranunculus ficaria displays yellow and UV-absorbing pollen and stamens in front of a similarly coloured floral guide of the bull’s eye type (Silberglied 1979; Medel et al. 2003; Koski and Ashman 2014).
Regarding automimicry, there are heterantherous species in which some stamens serve as feeding stamens and others as pollination stamens (Pacini and Bellani 1986). In some Commelina species, there are three different types of stamens in one flower, which are three conspicuous yellow and UV-absorbing staminodes without pollen, one stamen presenting and offering yellow and UV-absorbing pollen, and two pollination stamens which expose the inconspicuous side of the anther towards the pollinators, while the yellow pollen is invisible to approaching flower visitors (Faden 1992; Hrycan and Davis 2005; Ushimaru et al. 2007).
Floral mimicry
In pollination ecology, floral mimicry is categorised as Batesian and Müllerian mimicry (Dafni 1984; Roy and Widmer 1999; Johnson and Schiestl 2016), with the former being based on deception, i.e. rewarding and non-rewarding species displaying similar signals, and the latter on adaptive resemblance in signalling between rewarding species (Johnson and Schiestl 2016), i.e. rewarding species displaying similar signals and thus signalling honestly. According to Wickler (1965), Dafni (1984), and Lunau (2011), Müllerian mimicry does not fit the definition of mimicry, requiring a signal copy by which a signal receiver is deceived. Müllerian mimicry, including the classical example of two nectar-producing species with similar flower colour patterns, is better termed signal standardisation. However, the differences between Batesian and Müllerian mimicry are weak, since different flower visitors might respond differently to differences in nectar composition and concentration. When it comes to floral guides, the issue is even more complex, because mimic pollen signals are displayed to advertise nectar as well as pollen. Johnson and Schiestl (2016) discussed the semantics of Müllerian mimicry and reason that floral Müllerian mimicry systems are compiled by the consecutive addition of new species, rather than coevolution of species.
Evolution of pollen, anther, stamen, and androecium mimicry
The convergent evolution of pollen, anther, stamen, and androecium mimicry was facilitated by the preadapted yellow colour of pollen (Lunau 2002, 2004). The yellow and UV-absorbing flavonoid pigments in the exine of pollen grains in primarily wind-pollinated plants—for example Gymnospermae and Gnetaceae—originally had a protective function (Osche 1983a). In early insect-pollinated flowers, pollen and anthers additionally adopted a signalling function (Osche 1986). Since pollen-eating and pollen-collecting insects, e.g. hoverflies and bees, evolved innate responses to cues of pollen and anthers, flowers did not need to advertise pollen using real pollen and anthers, but could replace pollen and anther cues by pollen- and anther-mimicking signals (Lunau 2007; Papiorek et al. 2016). In many evolutionary lines in which angiosperms might have benefitted from saving real pollen, pollen- and anther-mimicking structures have evolved. This holds for plants with diclinous flowers, dichogamous flowers, heterostylous flowers, heterantherous flowers, and flowers that conceal their pollen in the floral tube, keel, and other structures, or display camouflaged or otherwise less attractive pollen. Simple pollen- and anther-mimicking structures like staminodes, floral dot guides, and stigmas seemingly have evolved frequently and independently in many plant families (Osche 1983a; Walker-Larsen and Harder 2000; Lunau 2007; Table 1). A more specific type of anther mimicry displayed by bilabiate flowers, the yellow and UV-absorbing bulged lower lip closing the floral tube, has evidently evolved independently several times. Bilabiate flowers bearing anther-mimicking structures on the lower lip are known in the unrelated plant families Scrophulariaceae (Nemesia), Lentibulariaceae (Utricularia and Genlisea), Phrymaceae (Mimulus), Orobanchaceae (Melampyrum), and several genera of Plantaginaceae (Stevens 2001 onwards; Glover et al. 2015). At least some genera of the Plantaginaceae displaying bilabiate flowers, i.e. Antirrhinum, Kickxia, Linaria, Cymbalaria, Asarina, Chaenorhinum, Gambelia, and Misopates, have evolved this feature independently as is apparent in the phylogeny of the Antirrhineae (Ogutcen and Vamosi 2016). Another example of convergent evolution of pollen- and anther-mimicking structures is the genus Iris (Iridaceae), in which many species display seemingly non-homologous pollen- and anther-mimicking structures. These features include a simple yellow and UV-absorbing patch in Iris pseudacorus, a comb in Iris cristata, a bilobed ridge in Iris reticulata, a lacerated protuberance in Iris japonica, and a beard-like structure in Iris x germanica (Fig. 2j). It seems that many branches within the genus Iris have formed before the onset of selective pressure to display pollen- and anther-mimicking structures shaped the floral evolution.
Sensory bias
Flowers are ‘sensory billboards’ emitting stimuli that are modulated by the sensory system or cognitive process of the flower visitors. The variability of flower stimuli causes variation in the relative salience of these stimuli for flower visitors and selects their appropriate behavioural responses. One mechanism by which the adaptive outcomes are promoted is through sensory biases (Raine and Chittka 2007) causing the flower visitors to respond more strongly to those signals that appear pertinent to them. The spectral sensitivity of the flower visitors’ photoreceptors (Peitsch et al. 1992; Lunau and Maier 1995) and the preference of colour stimuli causing distinct excitation patterns of photoreceptors have led to an intrinsic preference of yellow and UV-absorbing floral guides in hoverflies (Lunau and Wacht 1994, 1997) and spectrally pure colours in bumblebees (Lunau 1992b; Lunau et al. 1996). Most stamen-imitating floral guides display a spectrally pure and yellow, UV-absorbing colour and thus are attractive for both hoverflies and bumblebees. There are, however, some notable exceptions like the flowers of Digitalis purpurea displaying dark purple floral guides that have been interpreted as stamen mimics (Osche 1979).
Sensory exploitation
The sensory exploitation hypothesis explains secondary sexual characters as adaptations to exploit the mate’s response that evolved in another context (Ryan 1990). Regarding pollen and stamen mimicry, sensory exploitation is defined as the display of pollen- and stamen-imitating signals as nectar guides. In laboratory experiments, naïve bumblebees (Bombus terrestris) and hoverflies (Eristalis tenax) innately respond to visual signals of pollen and anthers displayed by artificial flowers (Lunau and Wacht 1994; Lunau et al. 1996; Lunau 2014). In training experiments, the bumblebees and hoverflies readily accept sugar water as a reward despite their guidance by pollen- and anther-imitating signals. In Rhododendron ponticum, yellow, anther-imitating, large-area colour patches are displayed on the upward facing petal next to the nectary, which makes the stamens less conspicuous for flower visitors. Some anther-imitating structures even offer reward in the form of production of nectar droplets or secondary pollen presentation (Fig. 5).
Pollen and nectar mimicry and reward. a Eranthis hyemalis (Ranunculaceae) flowers visited by Eristalis tenax (Syrphidae) hoverflies feeding on nectar offered by stamen-like staminodes. b Cleome monophylla (Cleomaceae) displaying nectar-imitating as well as pollen-imitating structures. c Euphrasia rostkoviana (Orobanchaceae) with secondary pollen presentation on the anther-mimicking colour patch. d Swertia bimaculata (Gentianaceae) offers nectar on anther-mimicking colour patches. e Tinantia erecta (Commelinaceae) displaying heteranthery and some filaments with visually and tactile stimulating structures. f Verbascum densiflorum (Scrophulariaceae) with filamental hairs intensifying visual and tactile stimulation on the three feeding stamens
Signal standardisation
Bees can learn features of the flowers they visit and associate floral signals with the reward. In many angiosperms, the visual signals of pollen and stamens in general are not constant within species. Diclinous species possess staminate and pistillate morphs with only the former offering pollen. Heterostylous species possess two or three morphs that differ in the length of their stamens and the position of their anthers, respectively; these differences among morphs are correlated with differences in the amount of pollen, the size of pollen grains, and handling by pollen-collecting bees (Wolfe and Barrett 1987; Ashman 2000). Dichogamous species possess a pistillate and a staminate flowering phase and offer more pollen in the staminate flowering phase. Successful cross-pollination requires the transport of pollen grains between flowers of different flowering phases rather than transport between flowers of the same flowering phase. To avoid bees learning the differences between the morphs or flowering phases in an effort to optimise pollen transfer, many species display pollen- or stamen-imitating structures and have inconspicuous or camouflaged pollen (Pohl et al. 2008). The blue flowers of all three morphs of the tristylous Eichhornia paniculata possess cryptic pollen and stamens and display a yellow floral guide. In monomorphic island populations, the flowers have no yellow floral guide (Barrett 1985). The cryptic dioecy in Actinidia polygama and some Solanum species including pistillate flowers offering pseudo pollen fit into this scheme as well (Knapp et al. 1998; Kawagoe and Suzuki 2004). Nevertheless, pollen- and stamen-imitating structures that improve flower signal standardisation within and between species might be assigned to mimicry or sensory exploitation. Similarly, filamental hairs simulate a distinct amount of pollen independent of the amount of real pollen (Fig. 5)
Bees are known to generalise when switching to new food plants (Gack 1981; Gumbert 2000). Due to the abundance of yellow and UV-absorbing pollen, yellow anthers, and yellow floral guides, bees are able to orient at flowers visited for the first time by means of these standardised signals. In the context of pollen and stamen mimicry, it is important that in the spring time many flowers display their pollen, whereas in the summer time more flowers display pollen- and stamen-imitating floral guides while pollen and anthers are hidden in the corolla (Lunau and Ren, personal observation). Flowers and inflorescences display similar colour patterns caused by different morphological structures including pollen, anthers, colour patterns, protuberances, and disc florets (Fig. 2).
According to Osche (1979, 1983a, b, 1986), a strong selective pressure to replace signalling stamens with stamen-mimicking structures is caused by the instability of the signalling properties of real stamens due to wilting, anthesis, and pollen depletion. Osche (1979) as well as Vogel (1993) regards the stiff anthers of Saintpaulia ionantha and some flowers of the Solanaceae family as stamen mimics, because flower visitors are unable to detect whether they contain pollen or not (Burkart et al. 2014), causing new opportunities for deception. In dioecious Solanum species, the pistillate flowers offer less sterile pollen than the staminate flowers (Anderson 1979; Knapp et al. 1998). Nectar mimicry is a rare phenomenon as compared to pollen mimicry (Vogel 1993) probably because pollen-imitating signals guide bees towards nectaries (Fig. 5).
Within species, stamens and pollen are ephemeral and variable floral signals. The variation in this signal is caused by wilting of the stamens and by the condition of the anthers (closed, open, or depleted of pollen). This is due to the age of the flower, but also owing to the flowering phase of dichogamous flowers. Moreover, many flowering plants possess floral morphs because of within-flower heteranthery, e.g. feeding stamens and pollination stamens (Luo et al. 2008; Vallejo-Marín et al. 2009). Diclinous species possess flowers with and without stamens, and heterostylous species possess stamens of different lengths or position within the flower (Barrett 2012). Between-species signal standardisation is advantageous for flower visitors that switch from one food plant to another using innate and learnt, generalised cues to orient at the new flowers.
Why can flowers benefit from displaying pollen-mimicking structures instead of real pollen? The ultimate answer relies on the biology of bees that not only passively transport pollen grains between flowers, but also collect large amounts of pollen to provision their offspring (Michener 2000) and store pollen grains in pollen transport organs which reduces the probability of pollination (Michener et al. 1978; Michener 1999, 2000). Moreover, Michener and Grimaldi (1988) provided evidence that, throughout their evolution, flowering plants had to cope with the most effective way of pollen collection by bees with corbiculae, pollen baskets in which pollen grains are compacted with admixed nectar. Thus, the effective pollen collection in bees facilitates adaptations of flowering plants to hide the pollen (Lunau 2007) or protect pollen against the collection by corbiculate bees (Lunau et al. 2015). The high frequency of pollen- and stamen-imitating and -mimicking structures in flowering plants of various plant families reflects the crucial role of bees in shaping floral characteristics of angiosperms.
References
Aeschimann D, Lauber K, Moser DM, Theurillat JP (2004) Flora alpina. Haupt, Bern/Belin, Paris/Zanichelli, Bologna
Ågren J, Schemske DW (1991) Pollination by deceit in a neotropical monoecious herb, Begonia involucrata. Biotropica 23:235–241
Anderson GJ (1979) Dioecious Solanum species of hermaphroditic origin is an example of a broad convergence. Nature 282:836–838
Ashman TL (2000) Pollinator selectivity and its implications for the evolution of dioecy and sexual dimorphism. Ecology 81:2577–2591
Barrett SCH (1985) Floral trimorphism and monomorphism in continental and island populations of Eichhornia paniculata (Spreng.) Solms. (Pontederiaceae). Biol J Linn Soc 25:41–60
Barrett SCH (2012) Evolution and function of heterostyly. Springer, Berlin
Barthlott W (1995) Mimikry Nachahmung und Täuschung im Pflanzenreich. Biol unserer Zeit 25:74–82
Beck von M, Lerchenau G (1912) Die Futterschuppen der Blüten von Vanilla planifolia Andr. Sitzungsber kaiserl Akad Wiss, Mathem-naturwiss Kl:509–521
Beck von M, Lerchenau G (1914) Die Pollennachahmung in den Blüten der Orchideengattung Eria. Sitzungsber kaiserl Akad Wiss, Math-naturwiss Kl:1033–1046
Bernhardt P, Burns-Balogh P (1986) Floral mimesis in Thelymitra nuda (Orchidaceae). Plant Syst Evol 151:187–202
Bernhardt P, Kenrick J, Knox RB (1984) Pollination biology and the breeding system of Acacia retinodes (Leguminosae: Mimosoideae). Ann Missouri Bot Gard 71:17–29
Biedinger N, Barthlott W (1993) Untersuchungen zur Ultraviolettreflexion von Angiospermenblüten. I Monocotyledonae. Trop Subtrop Pflanzenwelt 86:1–122
Boyden TC (1982) The pollination biology of Calypso bulbosa var. americana (Orchidaceae): initial deception of bumblebee visitors. Oecologia 55:178–184
Burkart A, Schlindwein C, Lunau K (2014) Assessment of pollen reward and pollen availability in Solanum stramoniifolium and Solanum paniculatum for buzz-pollinating carpenter bees. Plant Biol 16:503–507
Burr B, Barthlott W (1993) Untersuchungen zur Ultraviolettreflexion von Angiospermenblüten II. Magnoliidae, Ranunculidae, Hamamelididae, Caryophyllidae, Rosidae. Trop Subtrop Pflanzenwelt 87:1–193
Burr B, Rosen D, Barthlott W (1995) Untersuchungen zur Ultraviolettreflexion von Angiospermenblüten III. Dilleniidae und Asteridae s.l. Trop Subtrop Pflanzenwelt 93:1–185
Castillo RA, Caballero H, Boege K, Fornoni J, Domínguez CA (2012) How to cheat when you cannot lie? deceit pollination in Begonia gracilis. Oecologia 169:773–782
Chase MW, Reveal JL (2009) A phylogenetic classification of the land plants to accompany APG III. Bot J Linn Soc 161:122–127
Costich DE, Meagher TR (2001) Impacts of floral gender and whole-plant gender on floral evolution in Ecballium elaterium (Cucurbitaceae). Biol J Linn Soc 74:475–487
Dafni A (1984) Mimicry and deception in pollination. Annu Rev Ecol Syst 15:259–278
Dafni A, Giurfa M (1998) Nectar guides and insect pattern recognition—a reconsideration. Anais do Encontro sobre Abelhas 3:55–66
Dafni A, Giurfa M (1999) The functional ecology of floral guides in relation to insect behaviour and vision. In: Wasser SP (ed) Evolutionary theory and processes: modern perspectives, papers in honour of eviatar nevo. Kluwer Academic Publishers, Dordrecht, pp 363–383
Dafni A, Ivri Y (1981) The flower biology of Cephalanthera longifolia (Orchidaceae) pollen imitation and facultative floral mimicry. Plant Syst Evol 137:229–240
Dafni A, Kevan PG (1996) Floral symmetry and nectar guides: ontogenetic constraints from floral development, colour pattern rules and functional significance. Bot J Linn Soc 120:371–377
D’Arcy WG, Keating RC (eds) (1996) The anther—form, function and phylogeny. Cambridge University Press, Cambridge
Daumer K (1958) Blumenfarben wie sie die Bienen sehen. Z vergl Physiol 41:49–110
Davies KL, Turner MP (2004) Pseudopollen in Dendrobium unicum Seidenf. (Orchidaceae): reward or deception? Ann Bot 94:129–132
Davies KL, Stpiczyńska M, Kamińska M (2013) Dual deceit in pseudopollen-producing Maxillaria s.s. (Orchidaceae: Maxillariinae). Bot J Linn Soc 173:744–763
de Sá-Otero MP, Armesto-Baztan S, Díaz-Losada E (2009) Analysis of protein content in pollen loads produced in north-west Spain. Grana 48:290–296
Dinkel T, Lunau K (2001) How drone flies (Eristalis tenax L., Syrphidae, Diptera) use floral guides to locate food sources. J Insect Physiol 47:1111–1118
Dobson HEM (1988) Survey of pollen and pollenkitt lipids—chemical cues to flower visitors? Amer J Bot 75:170–182
Dobson HEM, Groth I, Bergström G (1996) Pollen advertisement: chemical contrasts between whole-flower and pollen odors. Amer J Bot 83:877–885
Dobson HEM, Danielson EM, Van Wesep ID (1999) Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae). Plant Species Biol 14:153–166
Duffy KJ, Johnson SD (2015) Staminal hairs enhance fecundity in the pollen-rewarding self-incompatible lily Bulbine abyssinica. Bot J Linn Soc 177:481–490
Faden RB (1992) Floral attraction and floral hairs in the Commelinaceae. Ann Missouri Bot Gard 79:46–52
Faegri K, van der Pijl L (1966) The principles of pollination ecology. Pergamon Press, Toronto
Friedman J, Barrett SCH (2009) Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann Bot 103:1515–1527
Gack C (1981) Zur bedeutung von Staubgefäßattrappen als signale für die bestäuber. experimente mit hummeln (Bombus terrestris). Zool Jb Syst 108:229–246
Glover BJ, Airoldi CA, Brockington SF, Fernández-Mazuecos M, Martínez-Pérez C, Mellers G, Moyroud E, Taylor L (2015) How have advances in comparative floral development influenced our understanding of floral evolution? Int J Plant Sci 176:307–323
Gumbert A (2000) Color choices by bumblebees (Bombus terrestris): innate preferences and generalization after learning. Behav Ecol Sociobiol 48:36–43
Harder LD, Barclay RMR (1994) The functional significance of poricidal anthers and buzz pollination: controlled pollen removal from Dodecatheon. Funct Ecol 8:509–517
Hardy CR, Stevenson DW (2000) Development of the flower, gametophytes, and floral vasculature in Cochliostema odoratissimum (Commelinaceae). Bot J Linn Soc 134:131–157
Heuschen B, Gumbert A, Lunau K (2005) A generalised mimicry system involving angiosperm flower colour, pollen and bumblebee innate colour preferences. Plant Syst Evol 252:121–137
Hrycan WC, Davis AR (2005) Comparative structure and pollen production of the stamens and pollinator-deceptive staminodes of Commelina coelestis and C. dianthifolia Commelinaceae). Ann Bot 95:1113–1130
Johnson SD, Schiestl FP (2016) Floral mimicry. Oxford University Press, New York
Kato M, Inoue T (1994) Origin of insect pollination. Nature 368:195
Kawagoe T, Suzuki N (2004) Cryptic dioecy in Actinidia polygama: a test of the pollinator attraction hypothesis. Can J Bot 82:214–218
Knapp S, Persson V, Blackmore S (1998) Pollen morphology and functional dioecy in Solanum (Solanaceae). Plant Syst Evol 210:113–139
Konzmann S, Lunau K (2014) Divergent rules for pollen and nectar foraging bumblebees—a laboratory study with artificial flowers offering diluted nectar substitute and pollen surrogate. PLoS ONE 9(3):e91900
Koski MH, Ashman T-L (2014) Dissecting pollinator responses to a ubiquitous ultraviolet floral pattern in the wild. Funct Ecol 28:868–877
Labandeira CC (1997) Permian pollen eating. Science 277:1421–1423
Labandeira CC, Dilcher DL, Davis DR, Wagner DL (1994) Ninety-seven million years of angiosperm-insect association: paleobiological insights into the meaning of coevolution. Proc Natl Acad Sci USA 91:12278–12282
Leins P, Erbar C (1994) Flowers in Magnoliidae and the origin of flowers in other subclasses of the angiosperms. II. The relationships between flowers of Magnoliidae, Dilleniidae and Caryophyllidae. Plant Syst Evol 8:209–218
Leins P, Erbar C (2010) Flower and fruit. morphology, ontogeny, phylogeny, function and ecology. Schweizerbart Science Publishers, Stuttgart
Leonard AS, Papaj DR (2011) ‘X’marks the spot: the possible benefits of nectar guides to bees and plants. Funct Ecol 25:1293–1301
Leonard AS, Brent J, Papaj DR, Dornhaus A (2013) Floral nectar guide patterns discourage nectar robbing by bumble bees. PLoS ONE 8(2):e55914
Li P, Zheng GL, Dafni A, Luo YB (2010) Reproductive biology of an alpine orchid Phaius delavayi. Plant Syst Evol 286:167–173
Lloyd DG, Barrett SCH (1996) Floral biology: studies on floral evolution in animal-pollinated plants. Chapman & Hall, New York
Lunau K (1988) Angeborenes und erlerntes Verhalten beim Blütenbesuch von Schwebfliegen—Attrappenversuche mit Eristalis pertinax (Scopoli) (Diptera, Syrphidae). Zool Jb Physiol 92:487–499
Lunau K (1990) Colour saturation triggers innate reactions to flower signals: flower dummy experiments with bumblebees. J Comp Physiol A 166:827–834
Lunau K (1991) Innate flower recognition in bumblebees (Bombus terrestris, B. lucorum; Apidae)—optical signals from stamens as landing reaction releasers. Ethology 88:203–214
Lunau K (1992a) Innate recognition of flowers by bumble bees—orientation of antennae to visual stamen signals. Can J Zool 70:2139–2144
Lunau K (1992b) A new interpretation of flower guide colouration: absorption of ultraviolet light enhances colour saturation. Plant Syst Evol 183:51–65
Lunau K (1993) Interspecific diversity and uniformity of flower colour patterns as cues for learned discrimination and innate detection of flowers. Experientia 49:1002–1010
Lunau K (1995) Notes on the colour of pollen. Plant Syst Evol 198:235–252
Lunau K (1996) Signalling function of floral colour patterns for insect flower visitors. Zool Anz 235:11–30
Lunau K (2000) The ecology and evolution of visual pollen signals. Plant Syst Evol 222:89–111
Lunau K (2002) The evolution of flowering plants, flower visitors and interactions between them—a look at flower biology with G. von Wahlert. Bonn Zool Monogr 50:109–146
Lunau K (2004) Adaptive radiation and coevolution—pollination biology case studies. Org Divers Evol 4:207–224
Lunau K (2007) Stamens and mimic stamens as components of floral colour patterns. Bot Jahrb Syst 127:13–41
Lunau K (2011) Warnen, tarnen, täuschen. mimikry und nachahmung bei pflanze, tier und mensch. Wissenschaftliche Buchgesellschaft, Darmstadt
Lunau K (2014) Visual ecology of flies with particular reference to colour vision and colour preferences. J Comp Physiol A 200:497–512
Lunau K, Maier EJ (1995) Innate colour preferences of flower visitors. J Comp Physiol A 177:1–19
Lunau K, Wacht S (1994) Optical releasers of innate proboscis extension of the hoverfly Eristalis tenax L. (Syrphidae, Diptera). J Comp Physiol A 174:574–579
Lunau K, Wacht S (1997) Angeborene Blütenerkennung bei der Schwebfliege Eristalis tenax L. Mitt Dtsch Ges allg angew Ent 11:481–484
Lunau K, Wester P (2017) Mimicry and deception in pollination. Adv Bot Res 82:259–279
Lunau K, Wacht S, Chittka L (1996) Colour choices of naive bumble bees and their implications for colour perception. J Comp Physiol A 178:477–489
Lunau K, Hofmann N, Valentin S (2005) Response of Eristalis tenax towards floral dot guides with colour transition from red to yellow. Entomol Gener 27:249–256
Lunau K, Heuschen B, Fieselmann G, van de Loo A (2006) Visual targeting of components of floral colour patterns in flower-naïve bumblebees (Bombus terrestris; Apidae). Naturwissenschaften 93:325–328
Lunau K, Unseld K, Wolter F (2009) Visual detection of diminutive floral guides in the bumblebee Bombus terrestris and in the honeybee Apis mellifera. J Comp Physiol A 195:1121–1130
Lunau K, Piorek V, Krohn O, Pacini E (2015) Just spines—mechanical defence of malvaceous pollen against collection by corbiculate bees. Apidologie 46:144–149
Lunau K, Konzmann S, Bossems J, Harpke D (2016) A Matter of contrast: yellow flower colour constrains style length in Crocus species. PLoS ONE 11(4):e0154728
Luo ZL, Zhang DX, Renner SS (2008) Why two kinds of stamens in buzz-pollinated flowers? Experimental support for Darwin’s division-of-labour hypothesis. Funct Ecol 22:794–800
Magin N, Claßen R, Gack C (1989) The morphology of false anthers in Craterostigma plantaginea and Torenia polygonoides (Scrophulariaceae). Can J Bot 67:1931–1937
Medel R, Botto-Mahan C, Kalin-Arroyo M (2003) Pollinator mediated selection on the nectar guide phenotype in the andean monkeyflower, Mimulus luteus. Ecology 84:1721–1732
Michener CD (1999) The corbiculae of bees. Apidologie 30:67–74
Michener CD (2000) The bees of the world. Johns Hopkins University Press, Baltimore
Michener CD, Grimaldi DA (1988) The oldest fossil bee: apoid history, evolutionary stasis, and antiquity of social behavior. Proc Natl Acad Sci USA 85:6424–6426
Michener CD, Winston ML, Jander R (1978) Pollen manipulation and related activities and structures in bees of the family Apidae. Univ Kans Sci Bull 5:575–601
Mueller A (1996) Convergent evolution of morphological specializations in Central European bee and honey wasp species as an adaptation to the uptake of pollen from nototribic flowers (Hymenoptera, Apoidea and Masaridae). Biol J Linn Soc 57:235–252
Ogutcen E, Vamosi JC (2016) A phylogenetic study of the tribe Antirrhineae: genome duplications and long-distance dispersals from the Old World to the New World. Am J Bot 103:1071–1081
Orbán LL, Plowright CMS (2014) Getting to the start line: how bumblebees and honeybees are visually guided towards their first floral contact. Insectes Soc 61:325–336
Osche G (1979) Zur evolution optischer signale bei blütenpflanzen. Biol uns Zeit 9:161–170
Osche G (1983a) Optische signale in der coevolution von pflanze und tier. Ber Deutsch Bot Ges 96:1–27
Osche G (1983b) Zur evolution optischer signale bei pflanze tier und mensch. Ernst-Haeckel-Vorlesung an der Friedrich-Schiller-Universität Jena, Jena, pp 4–35
Osche G (1986) Vom “Erscheinungsbild” der Blütenpflanzen. Zur Evolution optischer Signale. Mannheimer Forum 86(87):63–123
Pacini E, Bellani LM (1986) Lagerstroemia indica L. pollen: form and function. In: Blackmore S, Ferguson IK (eds) Pollen and spores, form and function. Academic Press, London, pp 347–357
Papiorek S, Junker RR, Alves-dos-Santos I, Melo GAR, Amaral-Neto LP, Sazima M, Wolowski M, Freitas L, Lunau K (2016) Bees, birds and yellow flowers: pollinator-dependent convergent evolution of UV-patterns. Plant Biol 18:46–55
Peisl P (1997) Die Signalfunktionen von Blüten. Bot Helv 107:3–28
Peitsch D, Fietz A, Hertel H, de Souza H, Ventura DF, Menzel R (1992) The spectral input system of hymenopteran insects and their receptor-based colour vision. J Comp Physiol A 170:23–40
Pellmyr O (1992) Evolution of insect pollination and angiosperm diversification. TREE 7:46–48
Peter CI, Johnson SD (2013) Generalized food deception: colour signals and efficient pollen transfer in bee-pollinated species of Eulophia (Orchidaceae). Bot J Linn Soc 171:713–729
Pohl M, Lunau K (2007) Modification of the innate antennal reaction at floral guides in experienced bumblebees, Bombus terrestris (Hymenoptera: Apidae). Entomol Gener 29:111–123
Pohl M, Watolla T, Lunau K (2008) Anther-mimicking floral guides exploit a conflict between innate and learning in bumblebees (Bombus terrestris). Behav Ecol Sociobiol 63:295–302
Raine NE, Chittka L (2007) The adaptive significance of sensory bias in a foraging context: floral colour preferences in the bumblebee Bombus terrestris. PLoS ONE 2(6):e556
Rieseberg LH, Schilling EE (1985) Floral flavonoids and ultraviolet patterns in Viguiera (Compositae). Am J Bot 72:999–1004
Rørslett B (2006) Flowers in ultraviolet arranged by plant family. http://www.naturfotograf.com/UV_flowers_list.html
Roy BA, Widmer A (1999) Floral mimicry: a fascinating yet poorly understood phenomenon. Trends Plant Sci 4:325–330
Ryan MJ (1990) Sensory systems, sexual selection, and sensory exploitation. Oxford Surveys Evol Biol 7:157–195
Sanguinetti A, Buzatto CR, Pedron M, Davies KL, de Abreu Ferreira PM, Maldonado S, Singer RB (2012) Floral features, pollination biology and breeding system of Chloraea membranacea Lindl. (Orchidaceae: Chloraeinae). Ann Bot 110:1607–1621
Schaefer HM, Ruxton G (2011) Plant-animal communication. Oxford University Press, Oxford
Schemske DW, Ågren J (1995) Deceit pollination and selection on female flower size in Begonia involucrata: an experimental approach. Evolution 49:207–214
Schemske DW, Ågren J, Le Corff J (1996) Deceit pollination in the moneocic, neotropical herb Begonia oaxacana (Begoniaceae). In: Lloyd DG, Barrett SCH (eds) Floral biology: studies on floral evolution in animal-pollinated plants. Chapman & Hall, New York, pp 292–318
Sigrist MR, Sazima M (2004) Pollination and reproductive biology of twelve species of Neotropical Malpighiaceae: stigma morphology and its implications for the breeding system. Ann Bot 94:33–41
Silberglied RE (1979) Communication in the ultraviolet. Ann Rev Ecol Syst 10:373–398
Simpson B, Neff J, Dieringer G (1986) Reproductive biology of Tinantia anomala (Commelinaceae). Bull Torrey Bot Club 113:149–158
Sprengel CK (1793) Das entdeckte geheimnis der natur im bau und in der befruchtung der blumen. Vieweg, Berlin
Stanley RG, Linskens HF (1974) Pollen. Biology biochemistry management. Springer, Berlin
Stevens PF (2001 onwards) Angiosperm Phylogeny Website. Version 12, July 2012: http://www.mobot.org/MOBOT/research/APweb/
Thompson WR, Meinwald J, Aneshansley D, Eisner T (1972) Flavonols: responsible for ultraviolet absorption in nectar guide of flower. Science 177:528–530
Ushimaru A, Watanabe T, Nakata K (2007) Colored floral organs influence pollinator behavior and pollen transfer in Commelina communis (Commelinaceae). Am J Bot 94:249–258
Vallejo-Marín M, Manson JS, Thomson JD, Barrett SC (2009) Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. J Evol Biol 22:828–839
Vogel S (1975) Mutualismus und Parasitismus in der Nutzung von Pollenträgern. Verh Dtsch Zool Ges 68:102–110
Vogel S (1978) Evolutionary shifts from reward to deception in pollen flowers. In: Richards AJ (ed) The Pollination of Flowers by Insects. Linnean Society Symposium Series 6:89–96; Academic Press, London
Vogel S (1993) Betrug bei Pflanzen: Die Täuschblumen. Abh Math-Naturwiss Kl Akad Wiss Mainz:1–48
Wacht S, Lunau K, Hansen K (1996) Optical and chemical stimuli control pollen feeding in the hoverfly Eristalis tenax L. (Syrphidae; Diptera). Entomol Exp Appl 80:50–53
Wacht S, Lunau K, Hansen K (2000) Chemosensory control of pollen ingestion in the hoverfly Eristalis tenax L. by labellar taste hairs. J Comp Physiol A 186:193–203
Walker-Larsen J, Harder LD (2000) The evolution of staminodes in angiosperms: patterns of stamen reduction, loss, and functional re-invention. Amer J Bot 87:1367–1384
Weber A (1989) Didymocarpus geitleri, a remarkable new species of Gesneriaceae with deceptive pollen flowers. Plant Syst Evol 165:95–100
Weberling F (1992) Morphology of flowers and inflorescences. Cambridge University Press, Cambridge
Wester P, Lunau K (2017) Plant-pollinator communication. Adv Bot Res 82:225–257
Westerkamp C (1996) Pollen in bee-flower relations. Some considerations on melittophily. Bot Acta 109:325–332
Westerkamp C (1997) Flowers and bees are competitors—not partners. Towards a new understanding of complexity in specialized bee flowers. In: Richards KW (ed) Pollination: from theory to practise. Proc 7th internatl Symp Pollin Acta Hort 437, pp 71–74
Wickler W (1965) Mimicry and the evolution of animal communication. Nature 208:519–521
Willmer P (2009) Pollination and floral ecology. Princeton University Press, Princeton and Oxford
Willson MF, Ågren J (1989) Differential floral rewards and pollination by deceit in unisexual flowers. Oikos 55:23–29
Wilmsen S, Gottlieb R, Junker RR, Lunau K (2017) Bumblebees require visual pollen stimuli to initiate and multimodal stimuli to complete a full behavioral sequence in close-range flower orientation. Ecol Evol 7:1384–1393
Wolfe LM, Barrett SCH (1987) Pollinator foraging behaviour and pollen collection on the floral morphs of tristylous Pontederia cordata L. Oecologia 74:347–351
Wyatt GE, Sazima M (2011) Pollination and reproductive biology of thirteen species of Begonia in the Serra do Mar State Park, São Paulo, Brazil. J Poll Ecol 6:95–107
Acknowledgements
The authors wish to thank all researchers who discussed the topic of stamen mimicry with us, particularly Amots Dafni, Robert Junker, and Petra Wester.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Isabel Alves dos Santos and Isabel Machado.
In honour of Charles Michener and Stefan Vogel.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lunau, K., Konzmann, S., Winter, L. et al. Pollen and stamen mimicry: the alpine flora as a case study. Arthropod-Plant Interactions 11, 427–447 (2017). https://doi.org/10.1007/s11829-017-9525-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-017-9525-5