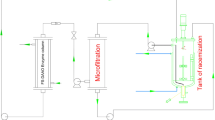
Abstract
World-wide production of l-lysine has rapidly increased in recent years. In the industrial scale production, it is cost effective to minimize waste as many waste materials are generated during downstream processing. Therefore, the conversion of crude lysine to a more valuable product reduces waste emission. In this study, 1,5-diaminopentane (DAP, trivial name: cadaverine) was produced by l-lysine decarboxylation using Hafnia alvei. The conditions of enzymatic reaction were determined. In particular, the addition of specific detergent (Brij 56) was significantly affected in the bioconversion system. Addition of hydrophobic organic solvent improved the mixing of the reactants. Finally, an industrial crude form of lysine served as a substrate. The DAP conversion by analytical, feed and industrial crude l-lysine was 93.9%, 90.3%, and 63.8%, respectively.
Similar content being viewed by others
References
R. Kelle, T. Hermann and B. Bathe, l-lysine production, Handbook of Corynebacterium glutamicum, CRC Press, Florida (2005).
J. Evans, Commercial amino acids, BCC Research: Market Research Reports, BIO007L (2017). https://doi.org/www.bccresearch.com
M. Elder, World markets for fermentation ingredients, BCC Research: Market Research Reports, FOD020E (2018). https://doi.org/www.bccresearch.com
L. Eggeling and M. Bott, Appl. Microbiol. Biotechnol., 99, 3387 (2015).
C. Wittmann and J. Becker, Microbiol. Monogr., 5, 39 (2007).
K. E. Uffmann and M. Binder, US Patent, 6,340,486 (2002).
J. Adkins, J. Jordan and D. R. Nielsen, Biotechnol. Bioeng., 110, 1726 (2015).
S. Jeong, Y. J. Yeon, E. G. Choi, S. Byun, D. H. Cho, I. K. Kim and Y. H. Kim, Korean J. Chem. Eng., 33, 1530 (2016).
C. G. Chae, Y. J. Kim, S. J. Lee, Y. H. Oh, J. E. Yang, J. C. Joo, K. H. Kang, Y.-A. Jang, H. Lee, A.-R. Park, B. K. Song, S.Y. Lee and S. J. Park, Biotechnol. Bioprocess Eng., 21, 169 (2016).
N, Li, H. Chou, L. Yu and Y. Xu, Biotechnol. Bioprocess Eng., 19, 965 (2014).
C. Wang, K. Zhang, C. Zhongjun, H. Cai and W. Honggui, Biotechnol. Bioprocess Eng., 20, 439 (2015).
T. Tateno, Y. Okada, Y. Tsuchidate, T. Tanaka, H. Fukuda and A. Kondo, Appl. Microbiol. Biotechnol., 82, 115 (2009).
F. Cassan, S. Maiale, O. Masciarelli, A. Vidal, V. Luna and O. Ruiz, Eur. J. Soil Biol., 45, 12 (2009).
J.-H. Kim, H.-M. Seo, G. Sathiyanarayanan, S. K. Bhatia, H.-S. Song, J. Kim, J.-M. Jeon, J.-J. Yoon, Y.-G. Kim, K. Park and Y.-H. Yang, J. Ind. Eng. Chem., 46, 44 (2017).
Y. Takatsuka, Y. Yamaguchi, M. Ono and Y. Kamio, J. Bacteriol., 182, 6732 (2000).
U. Kanjee, I. Gutsche, E. Alexopoulos, B. Zhao, M. El Bakkouri, M. Thibault, K. Liu, S. Ramachandran, J. Snider, E. F. Pai and W. A. Houry, EMBO J., 30, 931 (2011).
M. Abercrombie, In Vitro, 6, 128 (1970).
K. Han and O. Levenspiel, Biotechnol. Bioeng., 32, 430 (1987).
Z. Velioglu and R.O. Urek, Biotechnol. Bioprocess Eng., 21, 430 (2017).
M. Manaargadoo-Catin, A. Ali-Cherif, J. L. Pougnas and C. Perrin, Adv. Colloid Interface Sci., 228, 1 (2016).
S.K. Hait and S. P. Moulik, J. Surfactants Deterg., 4, 303 (2001).
D. Linke, Methods in Enzymol., 463, 603 (2009).
S. Luche, V. Santoni and T. Rabilloud, Proteomics, 3, 249 (2003).
S.B. Kim, H.Y. Yoo, J. S. Kim and S.W. Kim, Process Biochem., 49, 2203 (2014).
C. Laane, S. Boeren, K. Vos and C. Veeger, Biotechnol. Bioeng., 30, 81 (1987).
J. L. Gu, H. F. Tong and L.Y. Sun, Biotechnol. Bioprocess Eng., 22, 76 (2017).
T. Hermann, J. Biotechnol., 104, 155 (2003).
A.-T. Nguyen and W.-S. Kim, Korean J. Chem. Eng., 34, 2002 (2017).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, H., Yoo, H.Y., Ki, Y.H. et al. Improved reutilization of industrial crude lysine to 1,5-diaminopentane by enzymatic decarboxylation using various detergents and organic solvents. Korean J. Chem. Eng. 35, 1854–1859 (2018). https://doi.org/10.1007/s11814-018-0075-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-018-0075-z