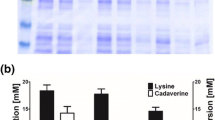
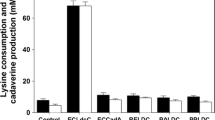
Abstract
Cadaverine, as a biogenic amine, is an important platform chemical for the production of industrial polymers, such as polyamides, polyurethanes, and nylon. Previous efforts focused on the bio-based production of cadaverine using two lysine decarboxylases of Escherichia coli CadA and LdcC. In this study, we report the biotransformation of cadaverine using a lysine decarboxylase from Klebsiella oxytoca. Codon optimization of the gene encoding this enzyme was carried on for the heterologous expression in E. coli, which led to a system that converted more than 24% lysine-HCl to cadaverine compared to the same system expressing CadA. The system was further optimized by using three different inducible promoters to control the expression of lysine decarboxylase gene of K. oxytoca in E. coli. The final optimized system converted lysine-HCl to cadaverine at a conversion rate of 0.133%/min/g. When the optimized system described above is coupled to an industrial process, the combined process has the potential to produce cadaverine with high conversion efficiency (46%) from sugar.
Similar content being viewed by others
References
Thielen, M. (2010) Bio-polyamides for automotive applications. Bioplastics Magazine 1: 10–11.
Brieger, L. (1885) Weitere Untersuchungen uber Ptomaine. August. Hirschwald, Berlin.
Sabo, D. L., E. A. Boeker, B. Byers, H. Waron, and E. H. Fisher (1974) Purification and physical properties of inducible Escherichia coli lysine decarboxylase. Biochem. 13: 662–670.
Kikuchi, Y., H. Kojima, T. Tanaka, Y. Takatsuka, and Y. Kamio (1997) Characterization of a second lysine decarboxylase isolated from Escherichia coli. J. Bacteriol. 179: 4486–4492.
Lemonnier, M. and D. Lane (1998) Expression of the second lysine decarboxylase gene of Escherichia coli. Microbiol. 144: 751–760.
Neely, M. N., C. L. Dell, and E. R. Olson (1994) Roles of LysP and CadC in mediating the lysine requirement for acid induction of the Escherichia coli cad Operon. J. Bacteriol. 176: 3278–3285.
Küper, C. and K. Jung (2005) CadC-mediated activation of the cadBA Promoter in Escherichia coli. J. Mol. Microb. Biotech. 10: 26–39.
Krithika, G., J. Arunachalam, H. Priyanka, and K. Indulekha (2010) The two forms of lysine decarboxylase; Kinetics and effect of expression in relation to acid tolerance response in E. coli. J. Exp. Sci. 1: 10–21.
Mimitsuka, T., H. Sawai, M. Hatsu, and K. Yamada (2007) Metabolic engineering of Corynebacterium glutamicum for cadaverine fermentation. Biosci. Biotech. Bioch. 71: 2130–2135.
Tateno, T., Y. Okada, T. Tsuchidate, T. Tanaka, H. Fukuda, and A. Kondo (2009) Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing a-amylase and lysine decarboxylase. Appl. Microbiol. Biot. 82: 115–121.
Kelle, R., B. Laufer, C. Brunzema, D. Weuster-Botz, R. Krämer, and C. Wandrey(1996) Reaction engineering analysis of L-lysine transport by Corynebacterium glutamicum. Biotechnol. Bioeng. 51: 40–50.
Kalinowski, J., J. Cremer, B. Bachmann, L. Eggeling, H. Sahm, and A. Puhler (1991) Genetic and biochemical analysis of the aspartokinase from Corynebacterium glutamicum. Mol. Microbiol. 5: 1197–1204.
Kind, S., W. K. Jeong, H. Schro der, and C. Wittmann (2010) Systems-wide metabolic pathway engineering in Corynebacterium glutamicum for bio-based production of diaminopentane. Metab. Eng. 12: 341–351.
Kind, S., W. K. Jeong, H. Schröder, O. Zelder, and C. Wittmann (2010) Identification and elimination of the competing N-acetyldiaminopentane pathway for improved production of diaminopentane by Corynebacterium glutamicum. Appl. Environ. Microb. 76: 5175–5180.
Qian, Z., X. Xia, and S. Y. Lee (2011) Metabolic engineering of Escherichia coli for the production of cadaverine: A five carbon diamine. Biotech. Bioeng. 108: 93–103.
Na, D., S. M. Yoo, H. Chung, H. Park, J. H. Park, and S. Y. Lee (2013) Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat. Biotechnol. 31: 170–174.
Ozgul, F. and Y. Ozgul (2005) Formation of biogenic amines by Gram-negative rods isolated from fresh, spoiled, VP-packed and MAP-packed herring (Clupea harengus). Eur. Food Re. Technol. 221: 575–581.
Leuchtenberger, W., K. Huthmacher, and K. Drauz (2005) Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biot. 69: 1–8.
Hoover, D. M. and J. Lubkowski (2002). DNAWorks: An automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acid. Res. 30: e43.
Li, W., I. S. Ng, B. Fang, J. Yu, and G. Zhang (2011) Codon optimization of 1,3-propanediol oxidoreductase expression in Escherichia coli and enzymatic properties. Electron. J. Biotechn. 14: 4.
Lee, S., B. Kim, M. Oh, Y. Kim, and J. Lee (2013) Enhanced activity of meso-secondary alcohol dehydrogenase from Klebsiella species by codon optimization. Bioproc. Biosyst. Eng. 36: 2005–1010.
Alper, H., C. Fischer, E. Nevoigt, and G. Stephanopoulos (2005) Tuning genetic control through promoter engineering. Proc. Nat. Acad. Sci. USA. 102: 12678–12683.
Kim, S. W. and J. D. Keasling (2001) Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol. Bioeng. 72: 408–415.
Thakker, C., J. Zhu, K. Y. San, and G. Bennett (2011) Heterologous pyc gene expression under various natural and engineered promoters in Escherichia coli for improved succinate production. J. Biotechnol. 155: 236–243.
Amann, E., J. Brosius, and M. Ptashne (1983) Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene 25: 167–178.
Soisson, S. M., B. MacDougall-Shackleton, R. Schleif, and C. Wolberger (1997) Structural basis for ligand-regulated oligomerization of AraC. Sci. 276: 421–425.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, N., Chou, H., Yu, L. et al. Cadaverine production by heterologous expression of Klebsiella oxytoca lysine decarboxylase. Biotechnol Bioproc E 19, 965–972 (2014). https://doi.org/10.1007/s12257-014-0352-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-014-0352-6