Abstract
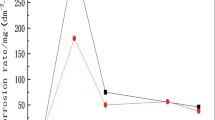
The ultra-high strength Cu-20Ni-20Mn alloy was prepared by vacuum melting and its mechanical property and corrosion behavior were investigated. After thermomechanical treatment, the alloy exhibited an ultra-high tensile strength of 1204 MPa and the applicable elongation of up to 6.2%. With the increasing exposure time in 3.5% NaCl solution, the corrosion current of the alloy decreased, while the polarization resistance and the charge-transfer resistance of the corrosion surface increased. The corrosion products formed on the surface of the alloy exposed for 1 d, and further corrosion made the corrosion product layer much dense, increasing the corrosion resistance and protecting the alloy from further corrosion.
摘要
通过真空熔炼制备了拥有超高强度的Cu-20Ni-20Mn 合金,并研究了其力学性能和腐蚀行为. 结果表明:经过一系列变形和热处理工艺后,该合金表现出1204 MPa 的超高拉伸强度,且伸长率高 达6.2%.随着合金在3.5%NaCl 溶液中暴露腐蚀时间的延长,合金样品的腐蚀电流降低,而腐蚀表面 的极化电阻和电荷转移电阻增加.腐蚀产物在暴露1 d 时已在合金表面上形成,并进一步腐蚀,使得 腐蚀产物层变得致密.腐蚀产物层增强了合金的耐腐蚀性能,保护合金免受进一步的腐蚀
Similar content being viewed by others
References
SHAIK M A, SYED K H, GOLLA B R. Electrochemical behavior of mechanically alloyed hard Cu-Al alloys in marine environment [J]. Corrosion Science, 2019, 153: 249–257. DOI: https://doi.org/10.1016/j.corsci.2019.03.043.
CHEN Shi-qiang, ZHANG Duan. Study of corrosion behavior of copper in 3.5wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria [J]. Corrosion Science, 2018, 136: 275–284. DOI: https://doi.org/10.1016/j.corsci.2018.03.017.
VIVEGNIS S, DELHALLE J, MEKHALIF Z, RENNER F U. Copper-zinc alloy electrodeposition mediated by triethanolamine as a complexing additive and chemical dealloying [J]. Electrochimica Acta, 2019, 319: 400–409. DOI: https://doi.org/10.1016/j.electacta.2019.07.007.
WANG Yan, ZHANG Lei, ZHAI Hong-fei, GAO Hong-xia, ZHOU Ke-chao. Tribological behavior of Cu-15Ni-8Sn/graphite under sea water, distilled water and dry-sliding conditions [J]. Journal of Central South University, 2019, 26(10): 2623–2633. DOI: https://doi.org/10.1007/s11771-019-4199-z.
HUANG Yuan-chun, LI Ming, MA Cun-qiang, XIAO Zheng-bing, LIU Yu. Flow behaviour constitutive model of CuCrZr alloy and 35CrMo steel based on dynamic recrystallization softening effect under elevated temperature [J]. Journal of Central South University, 2019, 26(6): 1550–1562. DOI: https://doi.org/10.1007/s11771-019-4111-x.
HU Sheng-bo, LIU Li, CUI Yu, LI Ying, WANG Fu-hui. Influence of hydrostatic pressure on the corrosion behavior of 90/10 copper-nickel alloy tube under alternating dry and wet condition [J]. Corrosion Science, 2019, 146: 202–212. DOI: https://doi.org/10.1016/j.corsci.2018.10.036.
JING Chuan, WANG Zhen-qiang, GONG Yu-long, HUANG Hai-jun, MA Yi-wen, XIE Huan-xin, LI Hong-ru, ZHANG Sheng-tao, GAO Fang. Photo and thermally stable branched corrosion inhibitors containing two benzotriazole groups for copper in 3.5wt% sodium chloride solution [J]. Corrosion Science, 2018, 138: 353–371. DOI: https://doi.org/10.1016/j.corsci.2018.04.027.
GAN Chun-lei, LIU Xue-feng, HUANG Hai-you, XIE Jian-xin. Effects of room-temperature deformation on mechanical properties, microstructure and texture of continuous columnar-grained BFe10-1-1 cupronickel alloy tubes [J]. Materials Science and Engineering A, 2013, 579: 202–208. DOI: https://doi.org/10.1016/j.msea.2013.05.029.
LI Jin, LI Jiu-yi, YUAN Wei-shuang, DU Yi-li. Biocorrosion characteristics of the copper alloys BFe30-1-1 and HSn70-1AB by SRB using atomic force microscopy and scanning electron microscopy [J]. International Biodeterio ration & Biodegradation, 2010, 64(5): 363–370. DOI: https://doi.org/10.1016/j.ibiod.2010.04.001.
PAN Qi-han. High-elastic Cu-20Ni-20Mn alloy [J]. The Chinese Journal of Nonferrous Metals, 1996, 6(4): 91–95. DOI: https://doi.org/10.3321/j.issn:1004-0609.1996.04.020. (in Chinese)
XIE Wei-bin, WANG Qiang-song, MI Xu-jun, XIE Guo-liang, LIU Dong-mei, GAO Xue-cheng, LI Yang. Microstructure evolution and properties of Cu-20Ni-20Mn alloy during aging process [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3247–3251. DOI: https://doi.org/10.1016/S1003-6326(15)63960-7.
LUO Fu-xin, PENG huai-chao, CHEN Hui-ming, XIAO Xiang-peng, XIE Wei-bin, WANG Fang, YANG Bin. Dislocation substructure-controlled softening of Cu-20Ni-20Mn alloy [J]. Materials Characterization, 2019, 147: 253–261. DOI: https://doi.org/10.1016/j.matchar.2018.11.013.
BADAWY W A, ISMAIL K M, FATHI A M. Effect of Ni content on the corrosion behavior of Cu-Ni alloys in neutral chloride solutions [J]. Electrochimica Acta, 2005, 50(18): 3603–3608. DOI: https://doi.org/10.1016/j.electacta.2004.12.030.
TIAN Wei, BI Li-ming. Effect of Zr on microstructure and properties of Cu-15Cr alloy [J]. Journal of Central South University, 2017, 24(12): 2757–2766. DOI: https://doi.org/10.1007/s11771-017-3689-0.
RAO B V A, KUMAR K C, HEBALKAR N Y. X-ray photoelectron spectroscopy depth-profiling analysis of surface films formed on Cu-Ni (90/10) alloy in seawater in the absence and presence of 1,2,3-benzotriazole [J]. Thin Solid Films, 2014, 556(4): 337–344. DOI: https://doi.org/10.1016/j.tsf.2014.02.054.
JIANG Ye-xin, LI Zhou, XIAO Zhu, XING Yan, ZHANG Yang, FANG Mei. Microstructure and properties of a Cu-Ni-Sn alloy treated by two-stage thermomechanical processing [J]. The Journal of The Minerals, Metals & Materials Society, 2019, 71(8): 2735–2741. DOI: https://doi.org/10.1007/s11837-019-03606-5.
SEMBOSHI S, TAKASUGI T. Fabrication of high-strength and high-conductivity Cu-Ti alloy wire by aging in a hydrogen atmosphere [J]. Journal of Alloys and Compounds, 2013, 580: 397–400. DOI: https://doi.org/10.1016/j.jallcom.2013.03.216.
WANG Bing-jie, ZHANG Yi, TIAN Bao-hong, AN Jun-chao, VOLINSKY A A, SUN Hui-li, LIU Yong, SONG Ke-xing. Effects of Ce addition on the Cu-Mg-Fe alloy hot deformation behavior [J]. Vacuum, 2018, 155: 594–603. DOI: https://doi.org/10.1016/j.vacuum.2018.06.006.
GONG Shen, LI Zhou, XIAO Zhu, ZHENG Feng. Microstructure and property of the composite laminate cladded by explosive welding of CuAlMn shape memory alloy and QBe2 alloy [J]. Materials & Design, 2009, 30(4): 1404–1408. DOI: https://doi.org/10.1016/j.matdes.2008.06.067
SHEN Lei-nuo, LI Zhou, DONG Qi-yi, XIAO Zhu, WANG Meng-ying, HE Peng-hui, LEI Qian. Dry wear behavior of ultra-high strength Cu-10Ni-3Al-0.8Si alloy [J]. Tribology International, 2015, 92: 544–552. DOI: https://doi.org/10.1016/j.triboint.2015.08.004.
HUANG Jia-zhen, XIAO Zhu, DAI Jie, LI Zhou, JIANG Hong-yun, WANG Wei, ZHANG Xiao-xuan. Microstructure and properties of a novel Cu-Ni-Co-Si-Mg alloy with super-high strength and conductivity [J]. Materials Science and Engineering A, 2019, 744: 754–763. DOI: https://doi.org/10.1016/j.msea.2018.12.075.
LEI Q, LI Z, DAI C, WANG J, CHEN X, XIE J M, YANG W. CHEN D L. Effect of aluminum on microstructure and property of Cu-Ni-Si alloys [J]. Materials Science and Engineering A, 2013, 572: 65–74. DOI: https://doi.org/10.1016/j.msea.2013.02.024.
LI Si, LI Zhou, XIAO Zhu, LI San-hua, SHEN Lei-nuo, DONG Qi-yi. Microstructure and property of Cu-2.7Ti-0.15Mg-0.1Ce-0.1Zr alloy treated with a combined aging process [J]. Materials Science and Engineering A, 2016, 650: 345–353. DOI: https://doi.org/10.1016/j.msea.2015.10.062.
YANG Guang, LI Zhou, YUAN Yuan, LEI Qian. Microstructure, mechanical properties and electrical conductivity of Cu-0.3Mg-0.05Ce alloy processed by equal channel angular pressing and subsequent annealing [J]. Journal of Alloys and Compounds, 2015, 640(15): 347–354. DOI: https://doi.org/10.1016/j.jallcom.2015.03.218.
ZHANG Rong-wei, SUN Jun-wei, LI Sheng-yan, ZHANG Ying-hui, ZHU Zhi-yun. Effect of manganese on electrochemical properties of copper-nickel alloys [J]. Nonferrous Metal Science and Engineering, 2018, 9(4): 60–65. DOI: https://doi.org/10.13264/j.cnki.ysjskx.2018.04.010. (in Chinese)
XIAO Zhu, LI Zhou, ZHU An-yin, ZHAO Yu-yuan, CHEN Jing-lin, ZHU Yun-tian. Surface characterization and corrosion behavior of a novel gold-imitation copper alloy with high tarnish resistance in salt spray environment [J]. Corrosion Science, 2013, 76: 42–51. DOI: https://doi.org/10.1016/j.corsci.2013.05.026.
ZHANG Yang, XIAO Zhu, ZHAO Yu-yuan, LI Zhou, XING Yan, ZHOU Ke-chao. Effect of thermo-mechanical treatments on corrosion behavior of Cu-15Ni-8Sn alloy in 3.5 wt% NaCl solution [J]. Materials Chemistry and Physics, 2017, 199: 54–66. DOI: https://doi.org/10.1016/j.matchemphys.2017.06.041.
ZHANG Xiao-hui, PEHKONEN S O, KOCHERGINSKY N, ELLIS G A. Copper corrosion in mildly alkaline water with the disinfectant monochloramine [J]. Corrosion Science, 2002, 44: 2507–2528. DOI: https://doi.org/10.1016/S0010-938X(02)00021-5.
MARTIN F J, CHEEK G T, O’GRADY W E, NATISHAN P M. Impedance studies of the passive film on aluminium [J]. Corrosion Science, 2005, 47: 3187–3201. DOI: https://doi.org/10.1016/j.corsci.2005.05.058.
YUAN S J, PEHKONEN S O. Surface characterization and corrosion behavior of 70/30 Cu-Ni alloy in pristine and sulfide-containing simulated seawater [J]. Corrosion Science, 2007, 49: 1276–1304. DOI: https://doi.org/10.1016/j.corsci.2006.07.003.
HENDERSON J D, EBRAHIMI N, DEHNAVI V, GUO M, SHOESMITH D W, NOEL J J. The role of internal cathodic support during the crevice corrosion of Ni-Cr-Mo alloys [J]. Electrochimica Acta, 2018, 283: 1600–1608. DOI: https://doi.org/10.1016/j.electacta.2018.07.048.
WALKOWICZ M, OSUCH P, SMYRAK B, KNYCH T, RUDNIK E, CIENIEK L, ROZANSKA A, CHMIELARCZYK A, ROMANISZYN D, BULANDA M. Impact of oxidation of copper and its alloys in laboratory-simulated conditions on their antimicrobial efficiency [J]. Corrosion Science, 2018, 140: 321–332. DOI: https://doi.org/10.1016/j.corsci.2018.05.033.
SCHUSSLER A, EXNER H E. The corrosion of nickel-aluminium bronzes in seawater I. Protective layer formation and the passivation mechanism [J]. Corrosion Science, 1993, 34: 1793–1802. DOI: https://doi.org/10.1016/0010-938x(93)90017-b.
ZHOU Hong-ming, HU Xue-yi, LI Jian. Corrosion behaviors and mechanism of electroless Ni-Cu-P/n-TiN composite coating [J]. Journal of Central South University, 2018, 25(6): 1350–1357. DOI: https://doi.org/10.1007/s11771-018-3831-7.
FAJARDO S, LLORENTE I, JIMENEZ J A, BASTIDAS J M, BASTIDAS D M. Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution [J]. Corrosion Science, 2019, 154: 246–253. DOI: https://doi.org/10.1016/j.corsci.2019.04.026.
LIU Xia, YUAN Yi-zhi, WU Zhu-ying, TIAN Gao-deng, ZHENG Yu-gui. Synergistic corrosion inhibition behavior of rare-earth cerium ions and serine on carbon steel in 3% NaCl solutions [J]. Journal of Central South University, 2018, 25(8): 1914–1919. DOI: https://doi.org/10.1007/s11771-018-3881-x.
CHEN Jing-lin, LI Zhou, Zhao Yu-yuan. Corrosion characteristic of Ce Al brass in comparison with As Al brass [J]. Materials and Design, 2009, 30: 1743–1747. DOI: https://doi.org/10.1016/j.matdes.2008.07.041.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2017YFB0306105) supported by the National Key R&D Program of China; Projects(51601227, 51701241) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Tang, Sk., Li, Z., Gong, S. et al. Mechanical property and corrosion behavior of aged Cu-20Ni-20Mn alloy with ultra-high strength. J. Cent. South Univ. 27, 1158–1167 (2020). https://doi.org/10.1007/s11771-020-4356-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-020-4356-4