Abstract
Purpose
We reviewed existing personalized, web-based, interactive decision-making tools available to guide breast cancer treatment and survivorship care decisions in clinical settings.
Methods
The study was conducted using the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR). We searched PubMed and related databases for interactive web-based decision-making tools developed to support breast cancer treatment and survivorship care from 2013 to 2023. Information on each tool’s purpose, target population, data sources, individual and contextual characteristics, outcomes, validation, and usability testing were extracted. We completed a quality assessment for each tool using the International Patient Decision Aid Standard (IPDAS) instrument.
Results
We found 54 tools providing personalized breast cancer outcomes (e.g., recurrence) and treatment recommendations (e.g., chemotherapy) based on individual clinical (e.g., stage), genomic (e.g., 21-gene-recurrence score), behavioral (e.g., smoking), and contextual (e.g., insurance) characteristics. Forty-five tools were validated, and nine had undergone usability testing. However, validation and usability testing included mostly White, educated, and/or insured individuals. The average quality assessment score of the tools was 16 (range: 6–46; potential maximum: 63).
Conclusions
There was wide variation in the characteristics, quality, validity, and usability of the tools. Future studies should consider diverse populations for tool development and testing.
Implications for cancer survivors
There are tools available to support personalized breast cancer treatment and survivorship care decisions in clinical settings. It is important for both cancer survivors and physicians to carefully consider the quality, validity, and usability of these tools before using them to guide care decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast oncologists and surgeons have long recognized that breast cancer care should be refined by individual patient needs, preferences, and values, as patients may respond to treatment differently based on a variety of factors. Over the last three decades, personalized care has gained traction with the emergence of genomic medicine [1], ‘big data’ [2], digital health [3, 4], and advanced treatment for breast cancer [5, 6]. In this context, several web-based, interactive decision-making tools have been introduced to clinical practice to support personalized breast cancer care [7,8,9,10,11]. These breast cancer-specific tools were designed to provide tailored outcomes and care recommendations considering individual demographic (e.g., age) [12], genomic (e.g., 21-gene recurrence score) [13], clinical (e.g., tumor size) [14], behavioral (e.g., smoking) [15], and contextual (e.g., insurance status) [16] characteristics together with patient needs, preferences, and values [17]. For example, the ‘BreastCHOICE’ tool is a personalized decision-making tool used to estimate the risk of surgical complications in early-stage breast cancer patients considering breast reconstruction based on their individual height, weight, past medical history, smoking status, and personal preferences/values [15].
Overall, studies have shown that personalized decision-making tools could increase knowledge, reduce negative emotions, such as anxiety and fear, associated with treatment, and improve overall quality of life among breast cancer patients and survivors [7, 18,19,20]. Furthermore, breast cancer decision-making tools that include contextual factors, such as treatment costs, insurance status, and access to treatment facilities, could potentially help address root causes of disparities in clinical settings [21,22,23,24]. For example, decision-making tools for medical situations, including chest pain, diabetes, Graves’ disease, depression, osteoporosis, and cardiovascular risk prevention, have shown that tools that raise cost as an issue could increase the occurrence of conversations related to the costs of drugs, insurance, and health care between patients and their physicians [25].
Recently, the U.S. Food and Drug Administration (FDA) issued a guidance to regulate decision-making tools as medical devices, increasing the focus on using high-quality tools to support clinical care in the U.S. [26]. However, there are several barriers to integrating high-quality personalized decision-making tools into current clinical care [19]. For instance, physicians and patients have reported a lack of understanding of existing tools, limited knowledge on how these tools can be used to support clinical care, and as a result, low motivation to use decision-making tools to guide clinical care [27,28,29]. Studies have also found that both patients and physicians have limited knowledge on the validity, usability, and quality of existing tools to assess their performance in real-world practice settings [30,31,32,33,34].
While breast cancer decision-making tools exist, there is limited information about their quality, validity, usability, feasibility, and acceptability. We aimed to fill this knowledge gap by critically reviewing the characteristics of existing English-language, interactive, web-based personalized decision-making tools available to support breast cancer care. The overarching goal of our review was to present evidence on the existing decision-making tools for breast cancer treatment and survivorship to support the integration of these tools into clinical practice.
Methods
This scoping review followed the methodological framework initially proposed by Arksey and O’Malley, Levac and colleagues, and the Joanna Briggs Institute [35,36,37]. This framework includes six stages to guide scoping review processes: (1) specifying the research question, (2) identifying relevant literature, (3) selecting studies, (4) data mapping, (5) summarizing, synthesizing, and reporting the results, and (6) including expert consultation. Our review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist (Supplemental Table 1) [38]. The study was registered in Open Science Framework [39]. Since the study included a review of published articles and study-level results, institutional review board approval or exemption was not required.
Data sources and search strategy
We conducted a search of published literature to identify articles that discussed personalized, interactive, dynamic, web-based decision-making tools designed to support breast cancer treatment and survivorship decisions for physicians and individuals diagnosed with breast cancer. The comprehensive search strategy included a combination of keywords, synonyms, Medical Subject Headings (MeSH), and Emtree terms relating to concepts of clinical decision-making tools, survivorship, treatment, web-based, personalized, and breast cancer (Supplemental Table 2). A trained librarian (GB) at the National Institutes of Health pilot tested 50 articles and refined our search strategy based on the initial search results. We searched PubMed, PsycInfo, Embase, Scopus, Web of Science, and Cochrane Database of Systematic Reviews for relevant articles. After screening all the articles from the database searches, we reviewed the reference lists of the articles to identify any additional tools that may have been missed, and these additional relevant articles were screened based on inclusion/exclusion criteria. The date of our most recent search was May 12, 2023.
Inclusion and exclusion criteria
For all articles, the inclusion criteria included: (1) female or male adults (≥ 18 years) diagnosed with breast cancer, (2) breast cancer treatment or survivorship, (3) online, web-based risk prediction models and interactive, personalized, or individualized tools developed from 2013 to 2023, (4) primary empirical research studies, and (5) articles written in English. We limited our search to include tools from 2013 to 2023, as these tools are more likely to consider the most up-to-date information on breast cancer treatment and survivorship care. Additional information is provided in Supplemental Table 3.
Data screening, extraction, and assessment of articles and tools
All titles and abstracts from articles retrieved from the databases were initially screened for eligibility by four authors (KW, DK, JZ, LS) based on the inclusion and exclusion criteria. A second round of screening using the same criteria was conducted via a full text review of the remaining articles. Screening was done using Covidence, an online application that helps streamline the review process [40]. Disagreements between authors were resolved through discussions.
We visited each tool’s publicly available website and tested each tool with pseudo patient characteristics to identify patient inputs used for personalization and breast cancer outcomes included in the tool. For tools that did not have publicly available websites, we reviewed screenshots and examined the tool development section in the methods of each corresponding article to retrieve information. We contacted the corresponding author for missing information. We used the articles, websites, and relevant screenshots to extract information about each tool, including the name and purpose, target population for tool development, interventions, data source and methods, input factors (e.g., individual, clinical, genomic, behavioral, contextual) used for personalization, breast cancer outcome/s, target user/s, and date of last update.
We also reviewed articles that provided information on tool validation, usability, feasibility, and acceptability testing. Personalized, web-based decision-making tools typically use statistical and/or simulation models to estimate outcomes associated with various input factors. After model development, these models are validated in independent, external samples to evaluate model performance and generalizability [41]. Usability testing is designed to capture the user experience and understanding of the tool, while feasibility testing helps infer the likelihood that the decision-making tool will be used to enhance the patient-physician interaction [32,33,34]. Acceptability testing is conducted to evaluate user satisfaction with the tool [32,33,34]. We extracted information on the distribution of race and ethnicity, education, income, marital status, and insurance in the sample of individuals included in validation, usability, feasibility, and acceptability testing of the tools. Data were extracted using Covidence and Excel [40].
Quality assessment
We used the International Patient Decision Aid Standard (IPDAS) instrument to assess the quality of each tool included in our study [42]. The IPDAS collaboration considers a decision aid to be any tool that helps people make decisions about health care [43]. The IPDAS instrument was selected for the quality assessment since it was established to provide a standardized framework and a set of criteria to evaluate the content, development, and implementation of decision tools used to support health care decisions [43]. These criteria may be useful to a wide range of individuals who may use decision tools such as patients, healthcare providers, tool developers, researchers, and policymakers [42, 43].
Accordingly, the IPDAS instrument checklist evaluates tools based on the presentation of information, ability to clarify patient values, tool development process, story usage, the impact of the tool on decision processes, and decision quality [42, 43]. The full IPDAS instrument checklist is accessible in Supplemental Table 4. In our study, the tools were scored from a range of 0 to 63, with increasing scores representing the increasing number of items from the IPDAS instrument checklist represented in each tool. Finally, we summarized the overall strengths and weaknesses of each tool considering the IPDAS instrument checklist [42].
Results
Search results
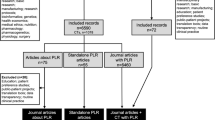
A total of 5,237 records were identified through PubMed, PsycInfo, Embase, Scopus, Web of Science, and Cochrane Database of Systematic Reviews. After removing duplicates, irrelevant, and ineligible articles, a total of 46 relevant articles were included in this study (Fig. 1). These articles described 54 tools, including 11 tools that provided personalized breast cancer treatment outcomes based on individual factors (e.g., age, tumor characteristics). The remaining 43 tools provided breast cancer outcomes associated with individual factors but did not include treatment-specific personalized breast cancer outcomes.
Article identification process using research framework. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71
Personalized tools for treatment outcomes (N = 11)
These tools varied by target population, inputs, outcomes, and treatment considerations (Table 1) [13,14,15, 17, 44,45,46,47,48,49]. The tools were developed for adult women (≥ 18 years) with early-stage breast cancer [13,14,15, 17, 44, 45, 47,48,49] or ductal carcinoma in situ (DCIS) [46] considering different types of treatment. The target users for two tools were only patients [15, 17], while four tools were developed for physicians only [47,48,49], and nine tools were developed for both physicians and patients [13, 14, 44,45,46]. Two tools were developed specifically for older women [45, 49]. Four tools predicted treatment outcomes for local–regional or distant recurrence risk [13, 14, 44, 45], and five tools predicted breast cancer mortality [44,45,46, 49], while the other tools predicted other treatment outcomes. Several tools (N = 4) included multiple outcomes [14, 44,45,46]. For example, ‘BTxChoice’ provided estimates for the 10-year risk of distant recurrence and life-years gained with and without chemotherapy treatment [14].
The tools varied by inputs used to estimate breast cancer treatment outcomes. All tools included individual and clinical characteristics, such as age and tumor size. Two tools considered genomic features measured by the 21-gene recurrence score [13, 14], and two tools considered health behaviors [15, 45]. No tools considered the impact of contextual factors, such as insurance status or access to a treatment facility. One tool helped elicit patient preferences and values by providing a brief survey outlining patients’ thoughts and feelings about treatment options [15]. We found one tool considering the variation of breast cancer outcomes based on race and ethnicity [46].
Validation, usability, feasibility, and acceptability testing
Six tools were externally validated [13,14,15, 46,47,48], three tools were internally validated [13, 14, 44], and three tools did not undergo any validation [17, 45, 49]. Five tools provided results from usability, feasibility, and/or acceptability testing [14, 15, 17, 45, 46]. ‘BreastCHOICE’ had a high mean usability score of 6.3, which was measured using the Computer System Usability Questionnaire, providing a score ranging from 1.0 (lowest) to 7.0 (highest) [15, 50]. ‘Which treatment for DCIS is right for you?’ had a mean usability score of 3.7 out of 5.0 measured using the System Usability Scale and the Preparation for Decision-Making Scale [46, 51, 52]. ‘BTxChoice’ and ‘Radiotherapy for Older Women’ did not report results from usability testing, but the authors stated that the tools were in the process of undergoing testing [14, 45]. ‘BRECONDA’ underwent acceptability and feasibility testing; it was assessed for usefulness and relevancy on a Likert-scale from 1 (lowest) to 5 (highest), with the tool receiving mean scores of 4.8 and 4.4, respectively [17]. Follow-up studies confirmed acceptability of the tool [53, 54].
Supplemental Table 5 provides the distribution of race and ethnicity, income, education, marital status, and insurance status of the individuals included in the validation and usability testing of the tools. Most patients included in validation and usability testing were White (68.2–83.9%) and married (71.1–86.0%).
Personalized tools for other outcomes (N = 43)
A total of 43 tools included models to estimate breast cancer outcomes associated with individual, tumor, and contextual characteristics, but did not include treatment-specific personalized breast cancer outcomes (Table 2) [16, 55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]. These tools were created for adult (≥ 18 years) female and/or male breast cancer patients who had undergone treatment for DCIS or invasive breast cancer. Ten tools were developed for patients with bone or lung metastases after a breast cancer diagnosis [56, 57, 69, 73, 75]. Four tools were created for young breast cancer patients (18–40 years) [16, 68, 78], and another three were created for elderly patients (≥ 65 years) [64, 65, 74]. Three tools were developed specifically for male breast cancer patients with bone metastases [73]. The target user for four tools was patients [55, 78, 80, 81], while 31 were developed only for physicians [16, 56,57,58,59, 62,63,64,65, 67,68,69,70,71, 74,75,76,77, 79, 82,83,84, 86, 87], and eight were developed for both physicians and patients [60, 61, 66, 72, 73, 85]. The most common outcomes estimated in these tools included overall survival (N = 20) [16, 57, 62,63,64, 66,67,68,69,70,71,72,73,74,75,76,77, 79, 85, 86], breast-cancer specific survival (N = 7) [16, 57, 65, 70, 73, 75, 79], and risk of bone metastasis (N = 3) [56, 69, 73]. The ‘After Cancer Education and Support Operations’ tool was the only tool developed to support breast cancer survivors by providing health alerts and follow-up care recommendations after treatment [55].
All tools considered individual and clinical factors such as age and tumor stage [16, 55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]. Four tools considered health behaviors, such as smoking status [59, 60, 78, 82]. Twenty-one tools incorporated contextual factors, including marital status (N = 20) [16, 57, 62,63,64,65, 69, 70, 72,73,74,75,76, 79], insurance status (N = 4) [16, 69], education (N = 1) [78], employment status (N = 1) [78], and financial status (N = 1) [78]. Only two tools included components to incorporate patient preferences or values into decision-making [78, 80]. We found 17 tools considering Black, White, and other race categories to estimate breast cancer outcomes [16, 56, 57, 59, 62,63,64,65, 69, 70, 75, 76]. One tool considered Hispanic and non-Hispanic ethnicities [59].
Validation, usability, feasibility, and acceptability testing
We found that 15 tools were externally validated [55, 56, 58, 59, 61, 65, 68, 69, 71, 76, 79, 84], and 35 tools were internally validated [16, 56, 57, 59, 60, 62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77, 79, 83, 84, 86, 87]. Five tools did not undergo any validation testing [78, 80,81,82, 85]. Only four tools underwent usability, feasibility, and/or acceptability testing [55, 78, 80, 81]. ‘After Cancer Education and Support Operations’ assessed usability and acceptability using ‘Consistency’, ‘Stressfulness’, and ‘Simplicity’ with scores ranging from 1 (most positive) to 7 (most negative) [55, 88]. Consistency refers to the ability to use the tool in the same way over time, while stressfulness refers to the amount of worry or tension caused by the use of the tool, and simplicity refers to the ease of use of the tool [55, 89]. Users reported a mean consistency score of 1.2, a mean stressfulness score of 1.2, and a mean simplicity score of 1.4 for this tool [55]. The tool developed by Graetz et al. was tested for feasibility based on reports from physicians and nurses who used it; providers stated that the tool was easy to implement and did not significantly impact workflow [81]. The ‘Resources and Education for Adherence to Cancer Hormonal Therapy’ tool was assessed for feasibility and acceptability [80], where the study aimed to have 80% of eligible individuals enroll with 80% completing at least one online session. Both goals were exceeded for this tool, with 85.4% of eligible individuals enrolling and 83.7% of individuals completing at least one session [80]. Acceptability was measured using the ‘Client Satisfaction Questionnaire’ and the ‘Intervention Feedback Questionnaire’ [90]. The ‘Resources and Education for Adherence to Cancer Hormonal Therapy’ tool had a mean acceptability score of 3.0 (range 1–4) and 3.4 (range 1–5) on both questionnaires, respectively [80].
Supplemental Table 5 provides the distribution of race and ethnicity, income, education, marital status, and insurance status of the individuals included in validation, usability, feasibility, and acceptability testing of these tools. Most patients were White (0–93.0%), married (41.4–94.0%), and had insurance (93.7–94.9%).
Quality assessment
The sum of the scores for each tool in each dimension on the IPDAS instrument checklist is reported in Supplemental Table 6. The tools could receive scores ranging from 0 (lowest quality) to 63 (highest quality). Most tools provided information about options (N = 48) and outcome probabilities (N = 48), were written in plain language (N = 49), and were easy to navigate online (N = 51). However, only six tools provided disclosure information about funding or conflicts of interest, and only two tools used stories. In our sample, the average quality assessment score for the tools was 16 (range: 6–46; potential maximum: 63). The tool with the highest IPDAS instrument score was ‘BreastCHOICE’, with 46 points. ‘BreastCHOICE’ provided information on different options and the development process while also sufficiently incorporating patient values and preferences into the decision-making tool by asking patients what matters most to them, what their concerns were, and how they feel about different treatments [15].
Summary: strengths and weaknesses
We provided a list of strengths and weaknesses of the web-based decision-making tools included in our study in Table 3. In terms of strengths, we found that most tools were written in plain language (N = 49), were validated (N = 45), and provided information about breast cancer outcomes (N = 48). However, usability, feasibility, and acceptability of the tools were evaluated using different measures. As a result, it was not possible to compare the performance of the tools. There was also limited information on the validity and usability testing of the tools in underserved (e.g., uninsured, low education) and underrepresented (e.g., Alaska Native, Pacific Islanders) populations.
Discussion
Breast cancer care decisions are complex and often require the consideration of individual, clinical, genetic, health behavioral, and contextual characteristics, as well as personal preferences and values, to achieve optimal treatment outcomes. In this scoping review, we identified 54 web-based, personalized, interactive decision-making tools that could be used to support breast cancer care in clinical settings.
Comparison with other literature
Previous studies have reviewed up to 21 tools, including risk prediction models, to support breast cancer treatment decisions [7, 91, 92]. In contrast, we identified a broader set of tools that could potentially be useful to support breast cancer treatment and survivorship care decisions in clinical settings. Like previous reviews, we also found that most tools still need to undergo usability, feasibility, and acceptability testing [7, 91, 92]. However, in this study, in addition to an appraisal of tool validity, usability, feasibility, and acceptability, we also evaluated the inclusion of underrepresented and underserved populations in tool development and testing. We found that individuals included in post-testing of the tools were mostly White, insured, married, and had higher levels of education. Moreover, previous reviews have provided limited information on health behaviors and contextual factors that may also influence breast cancer outcomes [7, 91, 92]. To our knowledge, this is the first to provide a detailed and comprehensive evaluation of the web-based decision tools considering health behaviors, contextual factors, and the characteristics of the populations included in validity and usability testing of these tools.
Summary of main findings
Tool validation is a necessary step in decision-making tool development, as it provides critical information on the tools’ ability to accurately estimate outcomes of interest in independent cohorts [93]. A tool’s performance (e.g., sensitivity, specificity) may vary based on the distribution of individual, clinical, and contextual characteristics of a given cohort [94]. Therefore, it is important to test the external validity of the decision-making tools (and related algorithms) in independent cohorts prior to the introduction of these tools into practice settings. Validation could also help identify additional important features that may have been missed in the initial development of the tool, which could help further increase the accuracy of the prediction. The validation samples for the tools in our review included mostly White, married, and insured populations. For example, ‘BTxChoice’ was validated in two populations, both with a White majority (73.0–83.9%) [14]. These findings were consistent with previous studies reporting that only 14% of decision tools were tested with a significant representation of underserved and underrepresented groups [95]. The lack of representation in validation samples could limit the ability to assess the performance of these tools in diverse settings [95]. Importantly, if the tools are unable to generate accurate estimates for certain subgroups of the population, using them to guide clinical decisions could perpetuate disparities in cancer care and outcomes. Therefore, it is necessary to develop and validate tools in diverse cohorts including underserved and underrepresented individuals.
Usability testing is a necessary step in tool development to help identify and fix problems with the website/mobile application, [96] but few tools in our review had undergone usability testing. During usability testing, tool developers should assess the tools’ ease of use and the presentation of information considering health literacy and numeracy [97, 98]. Studies have shown that tools that are difficult to use are often neglected despite their utility [99]. Usability testing that includes individuals with different levels of health literacy and numeracy could potentially enhance the long-term utility of these tools in clinical settings [96,97,98].
Several tools considered health behaviors, such as smoking status and alcohol intake. Health behaviors are important predictors of breast cancer mortality and survivorship [100]. While physical activity was not considered a health behavior in most of the decision-making tools included in our study [78], previous studies have shown that increased physical activity could lower breast cancer recurrence and mortality [101, 102]. Current smoking, dietary intake, sedentary behavior, and poor sleep are also known to be associated with breast cancer mortality [103,104,105]. Inclusion of these factors in breast cancer decision-making tools could potentially help patients identify resources (e.g., smoking cessation interventions for quitting) to improve behavior and help physicians develop survivorship care plans considering these factors.
Few tools considered patients’ preferences and values by asking patients their thoughts and concerns about different treatments and what matters most to them. Patients may have a wide range of preferences and values when considering the benefits and harms of treatment. Patients who receive their preferred treatment have been shown to be half as likely to stop treatment, and patients who are actively involved in decision making throughout their cancer care by voicing their preferences and values report a higher quality of life [18, 106]. Additionally, tools that incorporate patient values, such as cultural values, spirituality, and community, often improve the communication between patients and physicians, leading to improved shared decision making [107].
The debate over whether to include race and ethnicity in risk prediction models is ongoing, and not many tools included race or ethnicity as input variables. Race-based medicine has been used to deliver healthcare for years based on epigenetics, but it has a deeply problematic history used to reinforce and justify slavery and perpetuate racial discrimination [108]. Furthermore, racial categories change over time, which may mean that older tools that have not been updated may not be as relevant or accurate [108]. Currently, there is a push to consider race as an input factor only when it is directly connected to racism and contextual factors [109]. Studies have shown that contextual factors such as lack of health insurance, income, food insecurity, and access to treatment facilities contribute to the racial and ethnic disparities in breast cancer mortality [110, 111]. Therefore, the consideration of these factors in decision-making tools could potentially provide a means to reduce racial and ethnic disparities in breast cancer outcomes in the U.S. [112].
Less than half of the decision-making tools personalized breast cancer outcomes based on individual contextual factors such as insurance, education, employment, marital status, and financial status/burden. We considered marital status as a contextual factor due to the marriage protection theory [113], which posits that marriage may lead to improved breast cancer survival through the strengthening of interpersonal relationships, providing social and financial support, and reducing risky behaviors [114, 115]. Studies have also shown that living in highly segregated neighborhoods in the U.S. are associated with lower rates of breast cancer survival [116, 117]. The inclusion of these factors in decision-making tools may provide an opportunity for physicians to discuss, advocate, and ensure that patients’ full range of circumstances are accounted for when making informed decisions about breast cancer care.
Strengths and limitations
Our review has several limitations that should be considered when evaluating our findings. We did not consider web tools created prior to 2013 or in any language other than English because we wanted to limit our review to include the most recent, relevant tools. However, this means that our search likely did not encompass the full range of personalized decision-making web tools that are currently available for breast cancer care. Additionally, we only assessed tools that were developed in the U.S., Europe, Australia, Japan, and Korea. Because of this, tools may not be generalizable or applicable to all populations. We were unable to access 23 tools due to payment barriers or because only screenshots with incomplete information were available in the publications. As a result, we were unable to assess the quality of all the components of those tools that were not easily accessible. Also, we were unable to report the characteristics of the samples included in the validation, usability, feasibility, and acceptability testing of 18 tools, as this information was not readily available in the original studies.
Despite these limitations, we conducted a robust search for personalized web-based clinical tools and identified a significant number of tools that assessed breast cancer treatment and survivorship outcomes. To our knowledge, this is the first scoping review providing a detailed assessment and comparison of the web-based decision tools available to support breast cancer care in clinical settings.
Conclusions
There was wide variation in the characteristics, validity, usability, and quality of web-based, interactive decision-making tools available to support breast cancer care. We found that the quality assessment tool (i.e., the IPDAS instrument checklist) did not include components to evaluate contextual factors which may influence patient decisions, the ability to seek health care, and patient outcomes [42]. The inclusion of contextual factors in the IPDAS instrument checklist could motivate tool developers to include these factors in new decision-making tools.
We expect the quality and the use of these tools to increase with the new U.S. FDA regulation [26]. However, it is important to concurrently provide training to patients and physicians to ensure that these tools are used for their intended purposes [27,28,29, 118]. Further, integrating decision tools into electronic medical records systems could improve clinical workflow, the speed and quality of decision making, and communication between physicians and their patients [119].
Data availability
Data sharing is not applicable to this article as no datasets were analyzed or generated during the current study. All the studies summarized in this scoping review are listed in the data supplement.
References
Krzyszczyk P, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018;6(3–4):79–100.
Cirillo D, Valencia A. Big data analytics for personalized medicine. Curr Opin Biotechnol. 2019;58:161–7.
Mesko B, et al. Digital health is a cultural transformation of traditional healthcare. Mhealth. 2017;3:38.
Cancela, J., et al., Digital health in the era of personalized healthcare: opportunities and challenges for bringing research and patient care to a new level. Digital Health. 2021. https://doi.org/10.1016/B978-0-12-820077-3.00002-X
Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–4.
Peppercorn JM, et al. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29(6):755–60.
Zhao A, et al. A scoping review of interactive and personalized web-based clinical tools to support treatment decision making in breast cancer. Breast. 2022;61:43–57.
care that fits. 2021. Available from: https://carethatfits.org/. Accessed 2023.
Austin CA, et al. Tools to promote shared decision making in serious illness: A systematic review. JAMA Intern Med. 2015;175(7):1213–21.
Montori VM, Kunneman M, Brito JP. Shared decision making and improving health care: The answer is not in. JAMA. 2017;318(7):617–8.
Stacey D, Legare F, Lewis KB. Patient decision aids to engage adults in treatment or screening decisions. JAMA. 2017;318(7):657–8.
Jayasekera, J., et al., Benefits and harms of mammography screening in 75 + women to inform shared decision-making: a simulation modeling study. J Gen Intern Med., 2023. https://doi.org/10.1007/s11606-023-08518-4
Sparano JA, et al. Development and validation of a tool integrating the 21-gene recurrence score and clinical-pathological features to individualize prognosis and prediction of chemotherapy benefit in early breast cancer. J Clin Oncol. 2021;39(6):557–64.
Jayasekera J, et al. Development and validation of a simulation model-based clinical decision tool: Identifying patients where 21-gene recurrence score testing may change decisions. J Clin Oncol. 2021;39(26):2893–902.
Politi MC, et al. A randomized controlled trial evaluating the BREASTChoice tool for personalized decision support about breast reconstruction after mastectomy. Ann Surg. 2020;271(2):230–7.
Sun Y, et al. Nomograms for prediction of overall and cancer-specific survival in young breast cancer. Breast Cancer Res Treat. 2020;184(2):597–613.
Sherman KA, et al. BRECONDA: development and acceptability of an interactive decisional support tool for women considering breast reconstruction. Psychooncology. 2014;23(7):835–8.
Hack TF, et al. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2005;15:9–19.
Sutton RT, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17.
Elwyn G, et al. Implementing shared decision making in the NHS. BMJ. 2010;341: c5146.
Polacek GN, Ramos MC, Ferrer RL. Breast cancer disparities and decision-making among U.S. women. Patient Educ Couns. 2007;65(2):158–65.
Alcaraz KI, et al. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31–46.
Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177(3):537–48.
Mishra SI, et al. Social determinants of breast cancer screening in urban primary care practices: a community-engaged formative study. Womens Health Issues. 2012;22(5):e429–38.
Espinoza Suarez NR, et al. Using shared decision-making tools and patient-clinician conversations about costs. Mayo Clin Proc Innov Qual Outcomes. 2020;4(4):416–23.
Goodman, K.E., A.M. Rodman, and D.J. Morgan, Preparing physicians for the clinical algorithm era. N Engl J Med., 2023. https://doi.org/10.1056/NEJMp2304839.
Elwyn, G., et al., Why do clinicians not refer patients to online decision support tools? Interviews with front line clinics in the NHS. BMJ Open., 2012. 2(6). https://doi.org/10.1136/bmjopen-2012-001530.
Shortliffe EH. Testing reality: the introduction of decision-support technologies for physicians. Methods Inf Med. 1989;28(1):1–5.
O’Neill SC, et al. Multilevel influences on patient-oncologist communication about genomic test results: oncologist perspectives. J Health Commun. 2018;23(7):679–86.
Ankolekar A, et al. The benefits and challenges of using patient decision aids to support shared decision making in health care. JCO Clin Cancer Inform. 2018;2:1–10.
Koon, S., Important considerations for design and implementation of decision aids for shared medical decision making. Perm J., 2020. 24. https://doi.org/10.7812/TPP/19.064.
Benedict C, et al. Development of a web-based decision aid and planning tool for family building after cancer (Roadmap to Parenthood): usability testing. JMIR Cancer. 2022;8(2): e33304.
Jayasekera J, et al. Question prompt list to support patient-provider communication in the use of the 21-gene recurrence test: feasibility, acceptability, and outcomes. JCO Oncol Pract. 2020;16(10):e1085–97.
Brown SA, et al. Patient similarity and other artificial intelligence machine learning algorithms in clinical decision aid for shared decision-making in the Prevention of Cardiovascular Toxicity (PACT): a feasibility trial design. Cardiooncology. 2023;9(1):7.
Arksey, H. and L. O'Malley, Scoping studies: towards a methodological framework. International J Soc Res Methodol., 2005. 8(1). https://doi.org/10.1080/1364557032000119616.
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69.
Peters, M.D.J., et al., JBI manual for evidence synthesis: chapter 11: scoping reviews (2020 version). 2020: JBI. https://doi.org/10.46658/JBIRM-20-01.
Tricco AC, et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Wojcik KM, et al. An evaluation of web-based, interactive, personalized clinical tools designed to support breast cancer treatment and survivorship decisions in clinical settings: a scoping review. 2023. https://osf.io/6kdsv
Covidence systematic review software. 2023. Available from: www.covidence.org. Accessed 2023.
Ramspek CL, et al. External validation of prognostic models: what, why, how, when and where? Clin Kidney J. 2021;14(1):49–58.
Elwyn G, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417.
International Patient Decision Aids Standards (IPDAS) Collaboration. 2019. Available from: http://ipdas.ohri.ca/index.html. Accessed 2023.
Sittenfeld SMC, et al. A multi-institutional prediction model to estimate the risk of recurrence and mortality after mastectomy for T1–2N1 breast cancer. Cancer. 2022;128(16):3057–66.
Wang SY, et al. “Radiotherapy for Older Women (ROW)”: a risk calculator for women with early-stage breast cancer. J Geriatr Oncol. 2020;11(5):850–9.
Fridman I, et al. A web-based personalized decision support tool for patients diagnosed with ductal carcinoma in situ: development, content evaluation, and usability testing. Breast Cancer Res Treat. 2022;192(3):517–27.
Corsi F, et al. Development of a novel nomogram-based online tool to predict axillary status after neoadjuvant chemotherapy in cN+ breast cancer: a multicentre study on 1,950 patients. Breast. 2021;60:131–7.
Meretoja TJ, et al. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol. 2017;35(15):1660–7.
Wyld L, et al. Bridging the age gap in breast cancer: cluster randomized trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices. Br J Surg. 2021;108(5):499–510.
Lewis, J., IBM computer usability satisfaction questionnaires: psychometric evaluation and instructions for use. Int J Hum Comput Interact., 1995. 7(1). https://doi.org/10.1080/10447319509526110.
Brooke, J., Usability evaluation in industry; SUS: a ‘quick and dirty’ usability scale. 1 ed. 1996, Bristol, PA: Taylor & Francis Inc. 6.
Bennett C, et al. Validation of a preparation for decision making scale. Patient Educ Couns. 2010;78(1):130–3.
Sherman KA, et al. Facilitating decision-making in women undergoing genetic testing for hereditary breast cancer: BRECONDA randomized controlled trial results. Breast. 2017;36:79–85.
Sherman KA, et al. Reducing decisional conflict and enhancing satisfaction with information among women considering breast reconstruction following mastectomy: results from the BRECONDA randomized controlled trial. Plast Reconstr Surg. 2016;138(4):592e–602e.
Kapoor A, Nambisan P. Usability and acceptance evaluation of ACESO: a web-based breast cancer survivorship tool. J Cancer Surviv. 2018;12(3):316–25.
Liu WC, et al. Using machine learning methods to predict bone metastases in breast infiltrating ductal carcinoma patients. Front Public Health. 2022;10: 922510.
Wang, K., et al., Web-based dynamic nomograms for predicting overall survival and cancer-specific survival in breast cancer patients with lung metastases. J Pers Med., 2022. 13(1). https://doi.org/10.3390/jpm13010043.
Orucevic A, et al. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat. 2017;163(1):51–61.
Jonczyk MM, et al. Surgical predictive model for breast cancer patients assessing acute postoperative complications: the breast cancer surgery risk calculator. Ann Surg Oncol. 2021;28(9):5121–31.
Kim JYS, et al. Individualized risk of surgical-site infection: an application of the breast reconstruction risk assessment score. Plast Reconstr Surg. 2014;134(3):351e–62e.
Chowdhury M, et al. A model for individualized risk prediction of contralateral breast cancer. Breast Cancer Res Treat. 2017;161(1):153–60.
Meng X, et al. A novel conditional survival nomogram for monitoring real-time prognosis of non-metastatic triple-negative breast cancer. Front Endocrinol (Lausanne). 2023;14:1119105.
Dai D, Jin H, Wang X. Nomogram for predicting survival in triple-negative breast cancer patients with histology of infiltrating duct carcinoma: a population-based study. Am J Cancer Res. 2018;8(8):1576–85.
Liu X, et al. Risk stratification model for predicting the overall survival of elderly triple-negative breast cancer patients: a population-based study. Front Med (Lausanne). 2021;8: 705515.
Lu X, et al. Nomogram for predicting breast cancer-specific mortality of elderly women with breast cancer. Med Sci Monit. 2020;26: e925210.
Meng X, et al. Conditional survival nomogram predicting real-time prognosis of locally advanced breast cancer: analysis of population-based cohort with external validation. Front Public Health. 2022;10: 953992.
Johnson HM, et al. Refining breast cancer prognosis by incorporating age at diagnosis into clinical prognostic staging: introduction of a novel online calculator. Breast Cancer Res Treat. 2021;187(3):805–14.
Huang X, et al. Survival nomogram for young breast cancer patients based on the SEER database and an external validation cohort. Ann Surg Oncol. 2022;29(9):5772–81.
Huang Z, et al. Risk factors, prognostic factors, and nomograms for bone metastasis in patients with newly diagnosed infiltrating duct carcinoma of the breast: a population-based study. BMC Cancer. 2020;20(1):1145.
Li Y, Ma L. Nomograms predict survival of patients with lymph node-positive, luminal a breast cancer. BMC Cancer. 2021;21(1):965.
Zaorsky NG, et al. Survival after palliative radiation therapy for cancer: the METSSS model. Radiother Oncol. 2021;158:104–11.
Pan J, et al. Survival nomogram for patients with locally advanced breast cancer undergoing immediate breast reconstruction: a SEER population-based study. Clin Breast Cancer. 2023;23(4):e219–29.
Gao B, et al. Risk stratification system and visualized dynamic nomogram constructed for predicting diagnosis and prognosis in rare male breast cancer patients with bone metastases. Front Endocrinol (Lausanne). 2022;13:1013338.
Meng X, et al. Development and validation a survival prediction model and a risk stratification for elderly locally advanced breast cancer. Clin Breast Cancer. 2022;22(7):681–9.
Wang W, et al. An effective tool for predicting survival in breast cancer patients with de novo lung metastasis: nomograms constructed based on SEER. Front Surg. 2022;9: 939132.
Wu J, et al. Prognostic nomogram for female patients suffering from non-metastatic HER2 positive breast cancer: a SEER-based study. Medicine (Baltimore). 2022;101(40): e30922.
Xu Y, et al. Nomogram for predicting overall survival in patients with triple-negative apocrine breast cancer: surveillance, epidemiology, and end results-based analysis. Breast. 2022;66:8–14.
Sella T, et al. Young, empowered and strong: a web-based education and supportive care intervention for young women with breast cancer across the care continuum. JCO Clin Cancer Inform. 2021;5:933–43.
Yin F, et al. Development and validation of nomograms for predicting overall survival and cancer specific survival in locally advanced breast cancer patients: a SEER population-based study. Front Public Health. 2022;10: 969030.
Arch JJ, et al. Randomized controlled pilot trial of a low-touch remotely-delivered values intervention to promote adherence to adjuvant endocrine therapy among breast cancer survivors. Ann Behav Med. 2022;56(8):856–71.
Graetz I, et al. Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: a randomized controlled feasibility trial. J Cancer Surviv. 2018;12(4):431–40.
Beato Tortajada, I., C. Ferrer Albiach, and V. Morillo Macias, Nomogram for the personalisation of radiotherapy treatments in breast cancer patients. Breast., 2021. 60: p. 255–262. https://doi.org/10.1016/j.breast.2021.11.004.
Davey, M.G., et al., A novel surrogate nomogram capable of predicting oncotypeDX recurrence score(c). J Pers Med., 2022. 12(7). https://doi.org/10.3390/jpm12071117.
Sugimoto M, Takada M, Toi M. Development of Web tools to predict axillary lymph node metastasis and pathological response to neoadjuvant chemotherapy in breast cancer patients. Int J Biol Markers. 2014;29(4):e372–9.
Nahm SH, et al. Using three scenarios to explain life expectancy in advanced cancer: attitudes of patients, family members, and other healthcare professionals. Support Care Cancer. 2022;30(9):7763–72.
Jang W, et al. Artificial intelligence for predicting five-year survival in stage IV metastatic breast cancer patients: a focus on sarcopenia and other host factors. Front Physiol. 2022;13: 977189.
Meng X, et al. Nomogram predicting the risk of locoregional recurrence after mastectomy for invasive micropapillary carcinoma of the breast. Clin Breast Cancer. 2021;21(4):e368–76.
Ericsson, K.A. and H.A. Simon, How to study thinking in everyday life: contrasting think-aloud protocols with descriptions and explanations of thinking. Mind Cult Act., 1998. 5(3). https://doi.org/10.1207/s15327884mca0503_3.
Nambisan P. Evaluating patient experience in online health communities: implications for health care organizations. Health Care Manage Rev. 2011;36(2):124–33.
Arch JJ, Mitchell JL. An Acceptance and Commitment Therapy (ACT) group intervention for cancer survivors experiencing anxiety at re-entry. Psychooncology. 2016;25(5):610–5.
Spronk I, et al. The availability and effectiveness of tools supporting shared decision making in metastatic breast cancer care: a review. BMC Palliat Care. 2018;17(1):74.
Modi ND, et al. A literature review of treatment-specific clinical prediction models in patients with breast cancer. Crit Rev Oncol Hematol. 2020;148: 102908.
Sepucha KR, et al. “It’s valid and reliable” is not enough: critical appraisal of reporting of measures in trials evaluating patient decision aids. Med Decis Making. 2014;34(5):560–6.
Gander JC, Gordon EJ, Patzer RE. Decision aids to increase living donor kidney transplantation. Curr Transplant Rep. 2017;4(1):1–12.
Nathan AG, et al. Use of decision aids with minority patients: a systematic review. J Gen Intern Med. 2016;31(6):663–76.
Lynch PJ, Horton S. Web style guide: basic design principles for creating web sites. 3rd ed. Yale University Press: New Haven, CT; 2008.
Monkman H, Kushniruk A. Applying usability methods to identify health literacy issues: an example using a personal health record. Stud Health Technol Inform. 2013;183:179–85.
Coughlin SS, et al. Health literacy and patient web portals. Int J Med Inform. 2018;113:43–8.
Insfran, E. and A. Fernandez, A systematic review of usability evaluation in web development, in Web Informations Systems Engineering—WISE 2008 Workshops. 2008. p. 81–91. https://doi.org/10.1007/978-3-540-85200-1_10.
Oberguggenberger A, et al. Health behavior and quality of life outcome in breast cancer survivors: prevalence rates and predictors. Clin Breast Cancer. 2018;18(1):38–44.
Spei ME, et al. Physical activity in breast cancer survivors: a systematic review and meta-analysis on overall and breast cancer survival. Breast. 2019;44:144–52.
Duan W, et al. Smoking and survival of breast cancer patients: a meta-analysis of cohort studies. Breast. 2017;33:117–24.
Thomson CA. Diet and breast cancer: understanding risks and benefits. Nutr Clin Pract. 2012;27(5):636–50.
Trudel-Fitzgerald C, et al. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br J Cancer. 2017;116(9):1239–46.
Godinho-Mota, J.C.M., et al., Sedentary behavior and alcohol consumption increase breast cancer risk regardless of menopausal status: a case-control study. Nutrients., 2019. 11(8). https://doi.org/10.3390/nu11081871.
Swift JK, Callahan JL. The impact of client treatment preferences on outcome: a meta-analysis. J Clin Psychol. 2009;65(4):368–81.
Alden DL, et al. Cultural targeting and tailoring of shared decision making technology: a theoretical framework for improving the effectiveness of patient decision aids in culturally diverse groups. Soc Sci Med. 2014;105:1–8.
Wright, J.L., et al., Eliminating race-based medicine. Pediatrics., 2022. 150(1). https://doi.org/10.1542/peds.2022-057998.
Yearby R. Race based medicine, colorblind disease: how racism in medicine harms us all. Am J Bioeth. 2021;21(2):19–27.
Wright JL, et al. Achieving equity through science and integrity: dismantling race-based medicine. Pediatr Res. 2022;91(7):1641–4.
Gehlert, S., D. Hudson, and T. Sacks, A critical theoretical approach to cancer disparities: breast cancer and the social determinants of health. Front. Public Health., 2021. 9. https://doi.org/10.3389/fpubh.2021.674736.
Campbell JB. Breast cancer-race, ethnicity, and survival: a literature review. Breast Cancer Res Treat. 2002;74(2):187–92.
August KJ, Sorkin DH. Marital status and gender differences in managing a chronic illness: the function of health-related social control. Soc Sci Med. 2010;71(10):1831–8.
Guner N, Kulikova Y, Llull J. Marriage and health: selection, protection, and assortative mating. Eur Econ Rev. 2018;104:138–66.
Ding W, et al. Dynamic changes in marital status and survival in women with breast cancer: a population-based study. Sci Rep. 2021;11(1):5421.
Jayasekera J, et al. Opportunities, challenges, and future directions for simulation modeling the effects of structural racism on cancer mortality in the United States: a scoping review. J Natl Cancer Inst Monogr. 2023;2023(62):231–245. https://doi.org/10.1093/jncimonographs/lgad020.
Goel N, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275(4):776–83.
Khalifa M. Clinical decision support: strategies for success. Procedia Comput Sci. 2014;37:422–7.
Mills S. Electronic health records and use of clinical decision support. Crit Care Nurs Clin North Am. 2019;31(2):125–31.
Wyld L, et al. Age gap decision tool—compare surgery and primary endocrine therapy. 2021. Available from: https://agegap.shef.ac.uk/surgery_vs_pet/comparisons/new. Accessed 2023.
Wyld L, et al. Age gap decision tool—compare surgery with and without chemotherapy. 2021. Available from: https://agegap.shef.ac.uk/surgery_and_chemotherapy/comparisons/new. Accessed 2023.
Lee CN, et al. Integrating a patient decision aid into the electronic health record: a case report on the implementation of BREASTChoice at 2 sites. MDM Policy Pract. 2022;7(2):23814683221131316.
Sherman KA, et al. Breconda. 2014. Available from: https://breconda.bcna.org.au/. Accessed 2023.
Corsi F, et al. LinfoNeo. 2021. Available from: https://app.linfoneo.com/#/home. Accessed 2023.
Sittenfeld SMC, et al. Outcomes predictor after mastectomy with N1 breast cancer. 2022. Available from: https://riskcalc.org/BreastPMRT/. Accessed 2023.
Wang SY, et al. Radiation for older women (ROW). 2020. Available from: https://rtbreastcancer.org/. Accessed 2023.
Sparano JA, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21.
Nahm SH, et al. 3 scenarios for survival. 2022. Available from: https://ctc.usyd.edu.au/3scenarios/. Accessed 2023.
Sugimoto M, Takada M, Toi M. ADTree model for axillary lymph node metastasis. 2014. Available from: https://www.brca-pm.net/model/model1/prediction.php. Accessed 2023.
Sugimoto M, Takada M, Toi M. ADTree model for pathological response to neoadjuvant therapy. 2014. Available from: https://www.brca-pm.net/model/model2/prediction.php. Accessed 2023.
Kapoor A, Nambisan P. Personal decision support for survivor engagement: formulation and feasibility evaluation of a conceptual framework for implementing online cancer survivorship care plans. BMC Med Inform Decis Mak. 2020;20(1):59.
Liu, W.C., et al. Application of machine learning methods to predict bone metastases in breast infiltrating ductal carcinoma patients. 2022; Available from: https://liuwencaincu-breast-cancer-breast-fyv20f.streamlit.app/. Accessed 2023.
Wang K, et al. Breast cancer lung metastasis CSS nomogram. 2022. Available from: https://cssnomogram-xiangyahospital.shinyapps.io/DynNomapp/. Accessed 2023.
Wang, K., et al. Breast cancer lung metastasis OS nomogram. 2022. Available from: https://nomogram-xiangyahospital.shinyapps.io/BCLMOSnomogram/. Accessed 2023.
Orucevic, A., et al. Updated breast cancer nomograms: prediction for a low-risk and a high-risk oncotype DX recurrence score. 2017. Available from: https://utgsm.shinyapps.io/OncotypeDXCalculator/. Accessed 2023.
Orucevic A, et al. Nomogram update based on TAILORx clinical trial results—oncotype DX breast cancer recurrence score can be predicted using clinicopathologic data. Breast. 2019;46:116–25.
Jonczyk, M.M., et al. Breast cancer surgery risk calculator. 2021. Available from: https://www.breastcalc.org/. Accessed 2023.
Kim JYS, et al. Breast reconstruction risk assessment (BRA) score—extended length. 2014. Available from: http://www.brascore.org/. Accessed 2023.
Chowdhury M, et al. CBCRisk: contralateral breast cancer (CBC) risk predictor. 2017. Available from: https://cbc-predictor-utd.shinyapps.io/CBCRisk/. Accessed 2023.
Chowdhury M, et al. Validation of a personalized risk prediction model for contralateral breast cancer. Breast Cancer Res Treat. 2018;170(2):415–23.
Liu X, et al. Dynamic nomogram. 2021. Available from: https://xiaozhuliu.shinyapps.io/dynnomapp/. Accessed 2023.
Lu X, et al. Dynamic nomogram. 2020. Available from: https://bcsd.shinyapps.io/DynNomapp/. Accessed 2023.
Meng X, et al. Dynamic nomogram for breast IMPC after mastectomy. 2021. Available from: https://impcofmxd.shinyapps.io/DynNomapp/. Accessed 2023.
Meng X, et al. Dynamic nomogram for predicting survival of locally advanced breast cancer (web version). 2022. Available from: https://impcofmxd.shinyapps.io/LABC/. Accessed 2023.
Johnson HM, et al. Equation. 2021. Available from: https://docs.google.com/spreadsheets/d/1bVaMe6bI_MpolSJhqJys8P_83aAoH1fwJbzJHXdKt7U/edit?rm=minimal#gid=0. Accessed 2023.
Zaorsky NG, et al. Survival after palliative radiation therapy. 2021. Available from: https://cl865.github.io/surv/. Accessed 2023.
Christ SM, et al. Validation and extension of the METSSS score in a metastatic cancer patient cohort after palliative radiotherapy within the last phase of life. Clin Transl Radiat Oncol. 2022;34:107–11.
Nieder C, Mannsaker B, Yobuta R. Independent external validation of the METSSS model predicting survival after palliative radiotherapy. Anticancer Res. 2022;42(3):1477–80.
Pan J, et al. Dynamic nomogram. 2023. Available from: https://dcpanfromsh.shinyapps.io/NomforLABCafterIBR/. Accessed 2023.
Gao B, et al. Dynamic nomogram—CSS. 2022. Available from: https://gaobing191.shinyapps.io/Nomogram_of_CSS_in_MBCBM/. Accessed 2023.
Gao B, et al. Dynamic nomogram—MBCBM. 2022. Available from: https://gaobing191.shinyapps.io/Nomogram_for_Diagnosis_of_MBCBM/. Accessed 2023.
Gao B, et al. Dynamic nomogram—OS. 2022. Available from: https://gaobing191.shinyapps.io/Nomogram_of_OS_in_MBCBM/. Accessed 2023.
Meng X, et al. Nomogram predicting survival of elderly locally advanced breast cancer (web version). 2022. Available from: https://impcofmxd.shinyapps.io/ElderlyLABC/. Accessed 2023.
Beato Tortajada I, Ferrer Albiach C, Morillo Macias V. CLINGEN rating scale. 2021. Available from: https://form.jotformeu.com/cgarcia84/clingen. Accessed 2023.
Jang W, et al. Stage 4—breast cancer. 2022. Available from: http://ai-wm.khu.ac.kr/BreastCancer/. Accessed 2023.
Davey MG, et al. 2022. Available from: https://mattdavey93.shinyapps.io/RSsurrogate/. Accessed 2023.
Funding
Jinani Jayasekera was supported by the Division of Intramural Research at the National Institute on Minority Health and Health Disparities of the National Institutes of Health and the National Institutes of Health Distinguished Scholars program. Kaitlyn M. Wojcik, Dalya Kamil, and Oliver W.A. Wilson were supported by the Division of Intramural Research at the National Institute on Minority Health and Health Disparities of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
KW and JJ contributed to the conception and design of this study. KW, DK, JZ, and LS completed screening and data extraction of articles. All authors contributed to the acquisition, analysis, and interpretation of data, drafting the work, or revising it critically for important intellectual content, and final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval
This review used study-level data, so no ethical approval is required.
Competing interests
Claudine Isaacs has received research funding to her institution from Novartis, Pfizer, Genentech, and GSK, and she has served in a consulting or advisory role for Genentech, PUMA, Seattle Genetics, AstraZeneca, Novartis, Pfizer, ION, and Gilead. She receives royalties from Kluwer and McGraw Hill. Allison Kurian has received research funding to her institution from Myriad Genetics. All other authors declare no financial or non-financial competing interests.
Disclaimer
The contents and views in this manuscript are those of the authors and should not be construed to represent the views of the National Institutes of Health.
Opinions and comments expressed in this paper belong to the authors and do not necessarily reflect those of the US Government, Department of Health and Human Services, National Institutes of Health, or the National Institute on Minority Health and Health Disparities. The study funders had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wojcik, K.M., Kamil, D., Zhang, J. et al. A scoping review of web-based, interactive, personalized decision-making tools available to support breast cancer treatment and survivorship care. J Cancer Surviv (2024). https://doi.org/10.1007/s11764-024-01567-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-024-01567-6