Abstract
Background
The intent of plain-language resources (PLRs) reporting medical research information is to advance health literacy among the general public and enable them to participate in shared decision-making (SDM). Regulatory mandates coupled with academic and industry initiatives have given rise to an increasing volume of PLRs summarizing medical research information. However, there is significant variability in the quality, format, readability, and dissemination channels for PLRs. In this scoping review, we identify current practices, guidance, and barriers in developing and disseminating PLRs reporting medical research information to the general public including patients and caregivers. We also report on the PLR preferences of these intended audiences.
Methods
A literature search of three bibliographic databases (PubMed, EMBASE, Web of Science) and three clinical trial registries (NIH, EMA, ISRCTN registry) was performed. Snowball searches within reference lists of primary articles were added. Articles with PLRs or reporting topics related to PLRs use and development available between January 2017 and June 2023 were identified. Evidence mapping and synthesis were used to make qualitative observations. Identified PLRs were quantitatively assessed, including temporal annual trends, availability by field of medicine, language, and publisher types.
Results
A total of 9116 PLRs were identified, 9041 from the databases and 75 from clinical trial registries. The final analysis included 6590 PLRs from databases and 72 from registries. Reported barriers to PLR development included ambiguity in guidance, lack of incentives, and concerns of researchers writing for the general public. Available guidance recommendations called for greater dissemination, increased readability, and varied content formats. Patients preferred visual PLRs formats (e.g., videos, comics), which were easy to access on the internet and used short jargon-free text. In some instances, older audiences and more educated readers preferred text-only PLRs. Preferences among the general public were mostly similar to those of patients. Psychology, followed by oncology, showed the highest number of PLRs, predominantly from academia-sponsored research. Text-only PLRs were most commonly available, while graphical, digital, or online formats were less available. Preferred dissemination channels included paywall-free journal websites, indexing on PubMed, third-party websites, via email to research participants, and social media.
Conclusions
This scoping review maps current practices, recommendations, and patients’ and the general public’s preferences for PLR development and dissemination. The results suggest that making PLRs available to a wider audience by improving nomenclature, accessibility, and providing translations may contribute to empowerment and SDM. Minimizing variability among available guidance for PLR development may play an important role in amplifying the value and impact of these resources.
Plain Language Summary
Plain-language resources (PLRs) can help people understand medical research information. This will allow them to make informed decisions about their health. However, PLRs vary in quality, format, and ways in which they are shared. In this study, researchers looked at how PLRs are made and publicly shared. They also studied what makes PLRs useful for the public and patients. Creating PLRs is not easy because of unclear guidelines on writing for the public. Using different formats and languages can make PLRs readable. Patients preferred PLRs as videos and comics. Older and educated readers liked text-only PLRs. The fields of psychology and oncology had the highest number of PLRs. Text-only PLRs were more common than digital or online formats. PLRs should be easily and freely accessible. Open-access journal websites, PubMed, third-party websites, email, and social media can be used to share PLRs. This study showed that PLRs can be helpful, but there are challenges in creating and sharing them. Good PLRs can inform patients and help them make better health-related decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain-language resources (PLRs) are texts, videos, graphics and other content that summarize medical research information for patients, carers, and the general public. |
The quality, format, and disseminations channels of PLRs vary significantly, impacting their effectiveness in empowering individuals and facilitating shared decision-making. |
Patient preferences for PLRs vary, with some favoring visual formats such as videos and comics, while others prefer text-only versions. Accommodating different audience preferences is crucial for effective PLR development and dissemination. |
1 Introduction
Shared decision-making (SDM) between patients and healthcare professionals is becoming an increasingly important concept in modern medicine [1]. The move toward a more inclusive, patient-centric approach can enable patients to be active participants and decision-makers in their own healthcare [2, 3]. Research has shown that engaged patients lead to improved health outcomes, higher satisfaction with the care experience, and reduced healthcare costs [3,4,5,6]. Health literacy levels influence the ability of people to seek, understand, and utilize healthcare information; thus, health literacy plays a defining role in how patients, caregivers, and the general public derive benefit from their interactions with healthcare professionals, manage self-care, and navigate the healthcare system [7]. For patients, caregivers, and the general public to actively participate in SDM, medical research information needs to be readily accessible in a jargon-free, easy-to-understand form [8, 9].
The findings of medical research are predominantly disseminated to healthcare professionals and members of the research community through articles in peer-reviewed journals and presentations at conferences; these are typically data dense and text heavy [10, 11]. These formats of disseminating information are hardly suited to the needs of a nonscientific audience [9, 10, 12, 13]. Taking this into consideration, the US and EU regulatory bodies first expressed interest in making available clinical trial lay summaries on web-based registries in 2007, while stronger recommendations or mandates came in the following years [14,15,16]. Complementing these efforts, many journal publishers have advocated for the development of plain language summaries of scientific articles or conference presentations [17,18,19]. Beyond these, there are initiatives by various stakeholders such as public health bodies, research hospitals, and patient organizations to make available plain language materials in various formats including text, graphic, and video summaries and through different channels [20,21,22]. For the purpose of this paper, the term “plain-language resources” (PLRs) encompasses all of the above types of materials.
PLRs summarize medical research information in a form that is easily understood by a nonscientific audience such as the general public, including patients and caregivers. PLRs can fill the significant unmet need for timely, reliable, and accessible medical research information; they have the potential to facilitate SDM in disease management and enable self-management by patients [8]. In addition to improving patient engagement, PLRs may also help patients and the general public to critically evaluate and identify biased information or disinformation.
Since 2017, there has been a steady increase in the development and dissemination of PLRs by clinical trial sponsors [9, 23, 24]. However, as available guidance varies significantly or is ambiguous and open to interpretation, there is significant variability in the standards of content development [23, 25, 26]. Dissemination channels or hosting locations are also varied, with some nonprofit clinical trial sponsors releasing results in nontechnical language through institutional websites and press releases [27]. Published in-depth analysis of the PLR landscape is limited to qualitative assessment of text-only PLRs of journal articles [9]. However, for PLRs to fulfil their potential, a deeper understanding is needed on what adds the most value to the general public including patients [28,29,30].
The objectives of this scoping review were: (1) to quantitatively examine the origin, types, and characteristics of PLRs on medical research information currently available; (2) to identify current practices, and barriers to developing and disseminating PLRs on medical research information to the general public including patients and caregivers; (3) to study the preferences of these intended audiences and map available guidance on PLRs to health literacy themes.
2 Methods
We searched three bibliographic databases, PubMed, EMBASE, and Web of Science. This was complemented by searches on three clinical trial registries—the National Institutes of Health (NIH) Clinical Trials Registry, the European Medicines Agency (EMA) Clinical Trials Register, and the BioMed Central’s ISRCTN registry (the primary registry of the World Health Organization Registry Network). English-language articles from January 2017 to June 2023 were considered. Reference lists of primary studies published in English during this period were also included. We used the framework of Arksey and O’Malley to identify and select eligible articles and collated and summarized the results [31]. This analysis and manuscript were prepared in accordance with the PRISMA Extension for Scoping Reviews (PRISMA-ScR), where applicable [32].
2.1 Literature Searches
The search terms used for PubMed are presented in Supplementary Information Table S1 and were modified for use with other bibliographic databases in combination with database-specific filters. An example of the search terms used for the NIH clinical trial registry is presented in Supplementary Information Table S2.
We included (a) articles with associated PLRs (within the main text of the article or accompanying the text); (b) standalone published PLRs; (c) clinical trial result summaries accompanied by a PLR from the three clinical trial registries; (d) standalone PLRs of journal articles published in a different journal; (e) peer-reviewed articles reporting on the types, formats, and dissemination strategies for PLRs; and (f) qualitative, quantitative, and mixed-method articles proposing recommendations or sharing experience on plain language content development or dissemination. With the aim to screen out articles that may not purely be focusing on medical research, we excluded reports of preclinical or basic research, manufacturing, generic treatments (not original research), research in health system models, and those discussing general topics around health literacy, health education, public–patient projects, public health or health policy, research protocols, allied fields such as bioinformatics, genetics, health economics, pharmacology, pharmacogenetics, physiology, surgery, and nutrition (Supplementary Information Table S3). Additional details are provided in the protocol posted on an open-source platform (details at the end of this manuscript).
The titles and abstracts of articles found were screened independently by one researcher (AP, supported by AY and AK who are mentioned under the acknowledgements) to confirm that they met the inclusion criteria and to eliminate duplicates. Full texts were then independently assessed for eligibility by the same researcher (AP), while extraction and cross-checking were done independently by two researchers (AP, TW). Disagreement was resolved through discussion.
2.2 Evidence Mapping and Synthesis
Identified articles were classified according to their topic (i.e., quality standards, barriers to development and dissemination, impact of PLRs) or their type (i.e., associated with a journal article, associated with a clinical trial, or standalone PLRs in a different journal than where the original article was published). We defined full set as all relevant records from bibliographic databases that met the inclusion criteria above and clinical trial set as PLRs reporting clinical trial results.
2.3 Data Extraction
Two data extraction forms were designed by AP and reviewed by IA and TW. Both data extraction forms compiled published attributes of PLRs such as bibliographic information, digital object identifier (DOI) number, web location, abstract/summary, and article type. The first data extraction form additionally compiled medical specialty, sponsors (based on author affiliation), publisher, and category (journal article with PLR, journal article about PLRs, standalone PLRs of journal articles published in a different journal). PLRs reporting clinical trials were identified based on tagging as a clinical trial on the bibliographic databases and the use of terms “study” or “trial” in the article titles.
The second data extraction form was used to additionally compile study type (qualitative, quantitative), publisher, nomenclature of PLR used, formats of PLRs evaluated, recommendations, or guidance or current practice sharing on the quality attributes of PLRs (language, structure, readability, format), preferences of intended audiences (patients or general public), and impact on knowledge and understanding, dissemination channels, barriers to the development and dissemination of PLRs, and potential impact and limitations of research reported in the articles. Articles that reported qualitative or quantitative assessments or experience (either methodological or involving public or patient respondents) on development and dissemination of PLRs were classified as current practices. Articles that provided consensus or collective or multistakeholder recommendations from academic or industry affiliated organizations on development and dissemination of PLRs, or indicated guidance or recommendation in their titles were classified as guidance. The above extracted information was categorized using the interpretation of health literacy from a patient perspective of Jordan et al. [7], supported by the definitions of health literacy of Abel et al. [33] and Sykes et al. [34] and the association between health literacy and empowerment as interpreted by Crondahl et al. [35]. The categories were mapped to the following broad health literacy themes and specific subthemes:
-
Accessibility: Ease of searchability, discoverability; user-friendly, pro-disability content; language characteristics not limited to readability
-
Understanding: Ease of understanding based on how the content is structured
-
Knowledge: Enablement of knowledge gain based on format of information presentation
-
Utilization/empowerment: Self-identification of opportunities for utilization of knowledge
2.4 Statistical Analysis
Microsoft Excel-based formulae were used to store and analyze the retrieved articles. Charts of selected data were generated using Microsoft Excel. We performed (1) a trend analysis of PLRs as part of the descriptive quantitative analysis and (2) a qualitative overview of available PLR reporting quality standards/attributes and analyzed them in the context of health literacy principles [33,34,35]. Post hoc analyses based on summative content analysis methodology as described by Hsieh and Shannon 2005 [36] were performed on PLRs reporting clinical trial results to evaluate the various terminology used in the published literature describing PLRs, and the frequency of PLRs describing positive study results. There is inconsistent naming of PLRs, which is a known challenge with the potential to reduce accessibility [37] and bias toward submission and publication of studies with positive results over negative results [38, 39]. For this purpose, a predefined list of 35 terms (Supplementary Information Table S4) identified by Abola and Prasad 2016 [40] and Vinkers et al. 2015 [41] were searched and quantified across the scientific abstracts.
3 Results
3.1 Origin and Types of PLRs
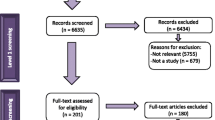
A total of 9116 PLRs were identified, 9041 from the bibliographic databases and 75 from clinical trial registries. Of these, 6662 were included in the final analysis (6590 from databases and 72 from registries; Fig. 1). There were a total of 55 standalone PLRs and 6532 PLRs accompanying journal articles or clinical trial results. There were 55 articles that discussed the development and dissemination of PLRs. In total, 15.7% of the PLRs reported clinical trial results.
Flowchart of the search strategy and screening of articles according to PRISMA [32]. CT clinical trial, PLR plain language resource. Note: Identified articles were classified according to their topic (i.e., quality standards, barriers to development and dissemination, impact of PLRs) or their type (i.e., associated with a journal article, associated with a clinical trial, or a standalone PLR article in a different journal than where the original article was published)
Of the 55 journal articles about PLRs discussing current practices and barriers [8, 9, 12, 21, 23, 24, 37, 42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89], ten articles sharing guidance on PLRs development or related topics were identified [23, 24, 47, 66, 67, 75, 76, 79, 84, 86]. Ten articles were narratives (e.g., commentaries, letters, or short communications) [43, 47, 58,59,60, 66, 74, 76, 79, 81], and six were literature reviews, including systematic reviews [9, 24, 37, 57, 61, 71]. Seven articles described case studies and case series [21, 48, 50, 55, 84, 86, 88], six described randomized studies [12, 51,52,53, 63, 82], and 19 reported comparative or correlational research [44,45,46, 49, 54, 62, 63, 65, 68,69,70, 72, 77, 78, 80, 83, 85, 87, 89]. For more information on the 55 articles, please refer to Table 1.
3.2 Frequency of Availability and Published Attributes of PLRs
The annual number of PLRs grew progressively from 369 in 2017 to 2159 articles in 2022, reaching a peak of 2156 articles in 2021 (Fig. 2A). The number of PLRs peaked between 2020 and 2022, which appeared to coincide with the COVID-19 pandemic; however, any predominance of infectious diseases PLRs was not apparent. Considering the ten most common medical specialties (psychology, oncology, hepatology, infectious diseases, cardiology, reproductive health, neurology, gastroenterology, endocrinology, immunology), PLRs reporting results of psychology research (n = 1329) predominate over other fields (Fig. 2B). Oncology and hepatology, have the next highest availability of PLRs (n = 1012 and n = 932, respectively), with oncology having the highest number (n = 243) among PLRs reporting clinical trial results.
By sponsor types, PLRs reporting results of academia-sponsored research were more numerous (n = 5614) than those supported by pharmaceutical organizations (n = 469) or nonprofits (n = 463) in both the overall set and clinical trials. When assessed by journal publishers, Wiley had published the highest number of PLRs (n = 1374), followed by Elsevier, Future Medicine, and Springer Nature (Fig. 2C).
Post hoc analysis of the PLRs reporting clinical trial results (n = 1078) showed that 79% of the PLRs did not use the predefined positive terms to describe their conclusions. The frequency of use of the positive terms in the clinical trials set is summarized in Fig. 2D. “Novel” (n = 66) was the most commonly used term, followed by “promising” (n = 35) and “unique” (n = 32).
Lay summary and plain language summary (PLS) were the most frequently used terms to describe PLRs, with others including (in decreasing frequency) lay abstract and plain English summary (Fig. 3). Overall, this analysis found 26 different terms for PLRs, 20 of which were only found in single instances.
3.3 Current Practices to Improve Accessibility, Understanding, Knowledge, and Utilization of PLRs
Current practices in PLR development were reported in a subset of articles describing methodological approaches to PLR development [37, 46, 57, 69, 80, 83, 87]. Accessibility to PLRs was increased by making them freely available even when they are included within articles (even if the main article is paywalled), or through sharing on social media or hosting them alongside the abstract in PubMed [37, 83]. PLRs were also made more accessible by adapting the content to the expected education level of the audience by using appropriate language level and by minimizing jargon and use of passive voice [37, 57, 69, 80, 87].
Understanding of content among the target audience was improved by using PLR text of appropriate length and structure. While journal recommendations for length for text PLR vary widely (e.g., from 80 words to up to 850), there is flexibility to use longer text in PLR describing more complex or conflicting findings and using more structured formats with predefined subheadings or Q&A [57, 80]. These articles reported improvement in PLRs through use of readability tools and calculators but concluded that these should not be seen as a replacement for patient’s or general public’s feedback [23, 24, 67, 75, 84, 86]. Knowledge transfer from PLR content was also increased compared to scientific literature through text-only formats, audio formats, and graphics/infographics, and graphic formats were reported to be particularly useful for informing an autistic audience [83, 87]. Practices promoting empowerment included the inclusion of laypeople or patients in PLR development and writing in a focused and clear style that answers the audience’s medical questions [37, 46, 80, 87].
The most commonly discussed format in articles about PLRs included text-only PLRs (of various levels of detail up to and including scientific abstracts) [8, 9, 12, 21, 42, 44,45,46, 48, 49, 51, 52, 54, 57, 58, 61,62,63,64,65, 68, 70,71,72, 74, 75, 80, 83,84,85,86,87,88,89]. Some articles discussed visual forms of PLRs, including text and graphic PLRs (including infographics) [9, 49,50,51, 53, 55, 62, 68, 70, 75, 77, 89], comics [12, 83], or videos [49, 62]. Online forms of PLRs considered included webpages, blogposts, and social media posts [45, 52, 82, 88]. Some articles included scientific abstracts as a comparator to more simplified formats [44, 51, 63, 83, 85, 87]. Seven discussed all formats of PLRs that may accompany research findings [37, 47, 56, 60, 76, 79, 81]. Nineteen studies reported audience preferences for different PLR formats [12, 21, 44,45,46, 48, 49, 51, 52, 63,64,65, 68, 70, 77, 83, 85, 87, 89]. Audience preferences on structure, language, content presentation, and hosting location are presented later under Sect. 3.5.
3.4 Available Guidance on PLR Development and Dissemination
In the ten articles offering guidance for PLR development, available guidance focused on the following needs and components: consistent criteria for selecting studies/datasets for PLR development [23, 67], consistent policy guiding PLR development [23, 67], training and creating awareness among stakeholders about PLRs [47, 67], understanding the target audience’s needs [23, 67], establishing a reliable review process (both for developers and journal publishers) [23, 67, 75, 86], always providing context of the findings and linking back to the source article [75], carefully timing the release of PLRs [47], and monitoring impact [23]. These guidance articles also included detailed advice regarding the accessibility and dissemination of PLRs [23, 76], and recommendations on readability, language, formats, and structure [23, 24, 47, 67, 75, 76, 84, 86]. Common themes for language and structure of content included recommendations to avoid jargon and abbreviations [24], use of concise titles and shorter sentences [24, 84, 86], and to take into account the needs and reading level of the intended audience [23, 24, 67, 76, 84]. Specific recommendations on improving discoverability included pointers to make these materials available via paywall-free, publicly available channels such as websites, social media, and emails [23, 24, 47, 67, 76]. These recommendations are summarized in Supplementary Information Table S5 and are presented in more detail below.
Various options were discussed for dissemination channels [23, 24, 47, 66, 67, 75, 76, 79, 84, 86]. Proposed dissemination channels included paywall-free journal websites, publicly accessible websites, indexing on PubMed, third-party websites (for example, Kudos and Figshare), clinical trial center/institutional websites, or by email to research participants and social media. The guidance articles’ advice on accessibility, discoverability, language, structure, and format were generally consistent with the preferences expressed by the general public and patient audiences (Supplementary Information Table S5), although several options for access in these guidance articles, namely investigator websites and direct emails, were not suggested by patient and general public audiences.
Among the guidance documents identified, some contradictions were evident; for example, several of these recommended a maximum length of 250 words for a text summary [75, 76, 84], but another recommendation for text length ranged from one to three pages [67]. Another source of inconsistency is that although the guidance documents suggest many options to improve the accessibility and readability of PLRs, they are largely nonprescriptive and leave many decisions on format and accessibility open to PLR developers.
3.5 General Public and Patient Preferences to Improve Understanding, Knowledge and Accessibility of PLRs
Nineteen of 55 studies compared audience (patients and general public) preferences for PLR formats [8, 12, 21, 45, 48,49,50,51,52,53, 56, 63, 64, 68, 70, 72, 77, 82, 89], five evaluated different text-only formats, eight compared text PLRs with scientific abstracts [8, 21, 48, 53, 64], two compared text PLRs with blogshots [45, 52], and one compared various summary formats with Wikipedia posts [9]. Other comparisons included text PLRs with news reports [66]; five compared text PLRs versus visual formats such as infographics, visual abstracts, infographics, or comics [12, 49, 68, 70, 89]; and one study compared PLRs in different webpage formats [82]. Two of the studies were randomized controlled trials [12, 64].
Patient preferences for PLRs were reported in nine of 55 articles (Table 2) [8, 12, 50, 51, 53, 68, 77, 82, 89] and the general public’s preferences were reported in ten articles (Table 3) [21, 45, 48, 49, 52, 56, 63, 64, 70, 72]. Accessibility preferences of patients included a preference for visual forms of PLRs such as comics and infographics, easy online accessibility, and, in some cases, a preference for text-based summaries of key observations over detailed, quantified data points (Table 2). Regarding understanding, for text-only PLRs, patients preferred shorter jargon-free text written for a lower reading level, with several studies reporting a Q&A style as a preferred format [21, 48, 72, 89]. Patients generally agreed that PLRs were an appropriate tool to enable knowledge sharing and promote greater engagement and discussion with healthcare professionals [12, 68, 77]; health literacy was considered a predictor of the level of understanding [51]. Patients wished for PLRs to be translated into languages other than English to enable wider accessibility [12, 53, 82]. They also expected that limitations of research and more details on specific side effects should be acknowledged in PLRs [77, 89], and that these materials be hosted on websites managed by regulators, healthcare organizations, or patient advocacy groups to improve perceptions of credibility [77]. Patients’ experience suggested that knowledge acquisition was easier or higher and more enjoyable with visual or digital formats and preferred those over text-only summaries [12, 50, 82, 89], although short text-only summaries were deemed satisfactory in most instances [8, 52, 53]. One study, however, reported a preference for text-only PLRs over infographics among older age groups or more educated readers [68]. Patients believed PLRs could empower/be utilized by a more diverse audience through the use of various formats and help patients be more confident in coping with their daily self-management and in participating in discussions with their physicians [8, 12, 50].
Preferences among the general public were similar overall to those of patients (Table 3). Shorter-form and jargon-free text PLRs, videos, and blogshots were considered more accessible than scientific abstracts and PLRs from sources such as the Cochrane Collaboration [45, 64, 70]. Several studies reported Q&A-structured text-only PLRs as an appropriate format [21, 70], with a preference for shorter words and sentences and the definitions of technical terms provided [21, 56, 63, 72]. Provision of links to further information or author contact details were also reported as a preference in some studies [45, 48, 56, 70]. Across all studies, general public stressed the value of PLRs in helping nonexperts understand and share science more rapidly and as a reliable information source for those proactive about self-managements [21, 45, 48, 49, 52, 56, 63, 64, 70, 72].
The inclusion of intended audience members in the development of PLRs was suggested in feedback from both general public and patients [49, 56], as was the provision of non-English translations [12, 53, 63, 82]. The main limitation cited by reports of both patient’s and general public’s preferences was the generalizability of the study findings, as study populations were usually small and often more motivated or educated than the general population [8, 45, 48, 51, 52, 64, 68, 70, 77]. Several patient studies reported gender imbalance or limited ethnic diversity [12, 68, 89].
3.6 Barriers in Developing and Disseminating PLRs
The reported barriers to PLR development included concerns of low readability, resource constraints, insufficient dissemination, exposure to liability, and a lack of incentives for authors to produce PLRs. Readability and audience-related concerns included researchers’ ability to write at an appropriate level and length for the general public [23, 24, 44, 64, 81, 83, 88]. Similarly, the complexity of incorporating the audience’s information preferences or reading ability was also noted [23, 24, 58, 68, 73, 77]. Four articles cited a lack of guidance on the development of appropriate PLR formats for audiences [9, 67, 68, 76], and identification of target audiences was hindered by inconsistent naming of PLRs [37, 76]. Resource constraint barriers included the skills, time, and costs required for the creation of visual formats, especially if additional expertise (e.g., professional graphic designers) would be required [12, 58, 65].
Dissemination barriers discussed in the included articles were the limited platforms for dissemination [67], and the limited options for indexing, tagging and search-engine optimization of PLRs that reduces their discoverability [71, 74, 76]. Other barriers were the inconsistent naming of PLRs as a barrier to discoverability [37, 60, 76] and that digital-only distribution of PLRs may not reach audiences with poor internet access or digital literacy [73, 77]. Concerns related to liability included the disclosure of uncertain, sensitive, misleading, or promotional information [52, 58, 83, 89]. Privacy concerns surrounding patient data were reported [42], as was loss of control if authors outside of the research team were involved in PLRs development [60, 65]. A related concern was content differences between original PLRs and foreign-language translations [47]. Furthermore, a lack of incentives (positive or negative) or guidance to create PLRs was noted [58], including an absence of guidance on the purpose of PLRs, or on which types of articles should have PLRs [88]. The low uptake of PLRs, particularly among high-tier journals, was also perceived as a barrier [54, 76].
4 Discussion
This is, to our knowledge, the first scoping review that extensively captures the evolution in the landscape of PLRs (quantitative evaluation of all formats and dissemination channels) from its formative years until recent developments. We also report an in-depth qualitative analysis of current practices, existing guidance and barriers in disseminating PLRs reporting medical research information. Improving health literacy has been shown to positively influence a patient’s self-care in disease management, outcomes, medication adherence, and help lower healthcare costs [35, 90,91,92,93]. Further, given the complexity of the present healthcare systems, PLRs can help patients make informed choices related to their self-care, derive greater benefit from their interactions with their healthcare providers, and also help them navigate healthcare systems [8, 9, 94]. Hence, we contextualize the findings uniquely by drawing connections between the trends related to PLRs and patient health literacy. To do so, we categorized our findings as per the principles of health literacy from the perspective of patients, as outlined by Jordan et al. [7], which are distinct from the classical health literacy framework originally proposed by Nutbeam [95].
Our analysis shows a progressive increase in the availability of PLRs over the period evaluated, reaching a peak in 2021. The subsequent years showed a dip, but this may be attributed to the time lag associated with the publication of research output. We also noted that not all forms of medical research are equally represented. There were few records from clinical trial registries (Fig. 1), suggesting that many clinical trial PLRs are not available despite the recommendations from key drug regulators [14,15,16]. This leaves a significant gap in public information on what medical research is currently ongoing.
The substantially higher number of PLRs for a few specialties such as psychology, oncology, and hepatology when compared with other specialties, such as immunology, might indicate that less reliable, easy-to-understand medical research information is available for these specialties. This imbalance could potentially lead to a spurt in misinformation in these specialties [96]. While publishing trends from our analysis show promise in terms of increasing availability, there is a clear need for wider support, especially by highly indexed journals [76], to increase availability as well as improve the quality of PLR being developed. In the modest number of PLR articles reporting clinical trial results identified, the use of positive terms to report outcomes was not evident; however, this observation should be interpreted with caution given the small sample size.
Our research provides a detailed overview of the preferences of patients and general public audiences when accessing medical research information. As anticipated, these audiences struggle with jargon, unstructured and text-heavy content, or lack of information in a language they are comfortable with. Their searches are often hindered by paywalled content or the variability in nomenclature and dissemination channels of PLRs [37]. Audiences appear to trust independent sources such as websites of drug regulators and patient advocacy groups regarding medical information they find online [77]. For patients and the general public to derive the true value of PLRs and for these resources to be impactful, all of these barriers in accessibility and quality will have to be addressed.
Among the literature on barriers to PLR implementation, a perceived lack of guidance on PLR development is reported [9, 67, 68, 76]. We identified ten articles offering such guidance [23, 24, 47, 66, 67, 75, 76, 79, 84, 86]. This may reflect a lack of awareness of existing guidance or a need for a more structured oversight of the PLR development process. Quality assessment of available guidance was out of scope of this scoping review, hence, there needs to be further investigation on whether current guidance is comprehensive, useful, and is being implemented by PLR developers. Furthermore, the multiple sources of guidance available may add to the problem of inconsistency and lack of uniform standards for the development of PLRs. This highlights the need for harmonization of available or emerging guidance on PLR development, and a move toward more specific guidance for PLRs adapted to the needs of specific audiences. Currently no designated overseer for the quality, timing, or dissemination of PLRs exists, which leaves complete accountability to self-regulation and self-governance; this is a significant gap.
Despite the suggestions included in the guidelines, the limited dissemination options for PLRs were also noted as a barrier [67, 76]. The variable nomenclature of PLRs and the scattered approach to their dissemination have been identified as a barrier to accessibility elsewhere [37]. Overall, this suggests there may be a need for an improved understanding of where patients look for PLRs and adjust dissemination strategies accordingly. Efforts among expert groups to reduce the variability of PLRs nomenclature may help, and specific guidance for search engine optimization of PLRs may also reduce this barrier. The resources required to develop PLRs are a further barrier reported in the literature, however artificial intelligence tools for text generation and review may help reduce this problem in the future [97].
The observations from this scoping review are relevant to English language PLRs only. This reflects the dominance of English language in PLRs and the underlying medical research literature. Translation of PLRs into the audience’s preferred language has been reported elsewhere as an important step in reducing barriers to access and improving trust in healthcare information [98]. Furthermore, audiences who speak different languages may have different patterns of media usage [98]. Therefore, there is a large unmet need, not just for translating PLRs into other languages, but for identifying dissemination channels suitable for non-English-speaking audiences.
Among the literature reporting patient and general public preferences for PLRs, many included small study populations, limiting the generalizability of these observations. The generalizability was further limited as the participants were more educated and more motivated than may be expected in everyday practice [8, 12, 48, 50,51,52, 64, 68, 89]. Thus, there is a possibility of a fundamental mismatch in health literacy levels and associated knowledge, attitude, and preferences. This leaves an important gap in our knowledge of what truly constitutes the preference of patients and general public audiences when it comes to PLRs on medical research information. Thus, future studies should aim to address this unmet need to understand preferences for PLRs among broader and more diverse populations.
To discuss the implications of our findings on PLRs in enabling SDM, it is pertinent to position them in the context of the core objective of SDM, which is to enable a more inclusive and participatory healthcare ecosystem for patients, where healthcare professions have a critical role in supporting them [7, 94, 99]. In fact, patients are best empowered when provided with individualized information that addresses their preferences and supports their disease management journey [100]. In contrast, low health literacy or inaccessibility of information can reduce patient autonomy, reduce their interest to self-acquire information, and negatively affect their confidence to participate in active discussions with their physicians [101, 102]. In the context of SDM, PLRs, when developed using jargon-free and easy-to-understand language, can emerge as one of the critical enablers in the hands of both patients and healthcare professionals.
The progressively increasing numbers of PLRs across the various medical specialties is reassuring as they fill key knowledge gaps; specialties where information in plain language is still scarce indicate areas with high unmet need and potentially at risk of a spurt in misinformation. The comparatively low number of clinical trial PLRs points at the necessity to address information needs of potential patients who are looking for options beyond commercially available treatments. Albeit a post hoc analysis, nevertheless, the objective reporting of medical research information in the included PLRs is reassuring; this reflects on the potential of PLRs as unbiased sources of reliable information. Our research indicates that there are reliable guidance and best practice documents available for developers of PLRs; however, further harmonization will definitely aid in improving quality and accessibility [94]. While there are also published reports sharing current practices and learnings related to accessibility, readability, data presentation formats, and usability of PLRs, we should perhaps focus more on reports that specifically describe the preferences of patients or the general public and work on alleviating the associated barriers. These practical insights can complement and enhance existing guidance for PLR development and dissemination, thereby enabling health literacy and improving the ability of patients and the general public to participate in SDM.
4.1 Strengths and Limitations
We acknowledge several strengths of this analysis. Firstly, this scoping review encompasses a period of five and a half years starting from the period when PLR development started gaining more prominence [15, 16, 103, 104]. To our knowledge, this is the longest timeframe available in published literature. Secondly, it includes all formats of PLRs and supplements knowledge gaps in the current PLR landscape, which is predominantly focused on text-based PLRs. Thirdly, it uniquely captures an in-depth analysis of audience preferences on content and dissemination channels for the first time. Finally, the observations are contextualized uniquely from a health literacy perspective.
The first limitation of our analysis is that the search was limited to the databases and registries included in the scoping review and does not claim to be comprehensive. Secondly, the study population demographics (age, gender) analysis was not performed because a preliminary assessment of the tagging available via PubMed or bibliography databases showed inaccuracies and the manual approach of classification was too cumbersome due to the high volume of literature. Finally, analyses of trends among the PLRs for clinical trials were challenging to interpret, given the modest size of the subset (n = 1078).
5 Conclusions
This scoping review provides an extensive landscape analysis capturing PLRs. The results have identified evidence gaps that might be a basis for future research and exploration. These results highlight barriers to sharing medical research information with key stakeholders, provide insight into the current formats and channels used to disseminate PLRs, and inform on existing guidance and best practices. We aspire that the results will inform regulators, sponsors, medical societies, journal publishers, and patient organizations about the recommended content standards that apply and the channels that can be used for dissemination of PLRs reporting medical research information. There has been a visible increase in the development and dissemination of PLRs in the years included in our analysis. However, for PLRs to contribute to patient-centric healthcare systems that encourage SDM and to be perceived of value to patients, caregivers, and the general public, they might need to be more accessible and maintain the highest levels of scientific rigor and reliability.
References
Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172–9.
Vahdat S, Hamzehgardeshi L, Hessam S, Hamzehgardeshi Z. Patient involvement in health care decision making: a review. Iran Red Crescent Med J. 2014;16(1): e12454.
Krist AH, Tong ST, Aycock RA, Longo DR. Engaging patients in decision-making and behavior change to promote prevention. Stud Health Technol Inform. 2017;240:284–302.
Cluley V, Ziemann A, Feeley C, et al. Mapping the role of patient and public involvement during the different stages of healthcare innovation: a scoping review. Health Expect. 2022;25(3):840–55.
Kershaw VF, Chainrai M, Radley SC. Patient initiated follow up in obstetrics and gynaecology: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2022;272:123–9.
Pruette CS, Amaral S. Empowering patients to adhere to their treatment regimens: a multifaceted approach. Pediatr Transplant. 2021;25(1): e13849.
Jordan JE, Buchbinder R, Osborne RH. Conceptualising health literacy from the patient perspective. Patient Educ Couns. 2010;79(1):36–42.
Pushparajah DS, Manning E, Michels E, Arnaudeau-Bégard C. Value of developing plain language summaries of scientific and clinical articles: a survey of patients and physicians. Ther Innov Regul Sci. 2018;52(4):474–81.
Stoll M, Kerwer M, Lieb K, Chasiotis A. Plain language summaries: a systematic review of theory, guidelines and empirical research. PLoS ONE. 2022;17(6): e0268789.
Committee on Strategies for Responsible Sharing of Clinical Trial Data, Board on Health Sciences Policy, Institute of Medicine. Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk Washington (DC): National Academies Press (US) Copyright 2015 by the National Academy of Sciences. All rights reserved.; 2015 [cited 2023 August 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK269030/
Lumley M, Perera D. Patient-level data: a paradigm shift in clinical trial transparency? Interv Cardio. 2013;6(6):619–21.
Kearns C, Eathorne A, Kearns N, et al. How best to share research with study participants? A randomised crossover trial comparing a comic, lay summary, and scientific abstract. J Vis Commun Med. 2022;45(3):172–81.
Rakedzon T, Segev E, Chapnik N, et al. Automatic jargon identifier for scientists engaging with the public and science communication educators. PLoS ONE. 2017;12(8): e0181742.
Food and Drug Administration. Amendments Act of 2007, Public Law No. 110–85 § 801 [cited 2023 August 7]. Available from: https://www.govinfo.gov/app/details/PLAW-110publ85/summary
ClinicalTrials.gov. FDAAA 801 and the Final Rule 2023 [cited 2023 August 7]. Available from: https://classic.clinicaltrials.gov/ct2/manage-recs/fdaaa
European Medicines Agency. Publication and access to clinical-trial data (Policy/0070) 2013 [cited 2023 September 25]. Available from: https://www.ema.europa.eu/en/documents/other/draft-policy-70-publication-access-clinical-trial-data_en.pdf
Taylor & Francis Online. Articles with a Plain Language Summary (PLS) 2023 [cited 2023 August 7]. Available from: https://www.tandfonline.com/topic/article-features/plain-language-summary?_ga=2.39289636.664843318.1691384765-60020648.1684563006&_gl=1*13svo7d*_ga*NjAwMjA2NDguMTY4NDU2MzAwNg..*_ga_0HYE8YG0M6*MTY5MTM4NDc2NS4zLjEuMTY5MTM4NDgyNS4wLjAuMA
New England Journal of Medicine. Search Results: Research Summaries 2023 [cited 2023 August 7]. Available from: https://www.nejm.org/search?q=%22Research+Summary%22&startPage=1&objectType=nejm-media&isFiltered=true&sortBy=pubdate-descending
Sage Publications. Plain Language Summaries 2023 [cited 2023 August 7]. Available from: https://us.sagepub.com/en-us/nam/plain-language-summaries
The Cochrane Collaboration. Updated template and guidance for writing Plain Language Summaries in Cochrane Reviews now available 2023 [cited 2023 August 7]. Available from: https://community.cochrane.org/news/updated-template-and-guidance-writing-plain-language-summaries-cochrane-reviews-now-available
Maurer M, Siegel JE, Firminger KB, et al. Lessons learned from developing plain language summaries of research studies. Health Lit Res Pract. 2021;5(2):e155–61.
Center for Information and Study on Clinical Research Participation, Inc. Trial Results Summaries 2023 [cited 2023 August 7]. Available from: https://www.ciscrp.org/services/health-communication-services/trial-result-summaries/
Dormer L, Schindler T, Williams LA, et al. A practical “How-To” Guide to plain language summaries (PLS) of peer-reviewed scientific publications: results of a multi-stakeholder initiative utilizing co-creation methodology. Res Involv Engagem. 2022;8(1):23.
Sedgwick C, Belmonte L, Margolis A, et al. Extending the reach of science - talk in plain language. Epilepsy Behav Rep. 2021;16: 100493.
TranCelerate Biopharma Inc. Recommendations for drafting non-promotional lay summaries of clinical trial results 2015 [cited 2023 September 25]. Available from: https://www.transceleratebiopharmainc.com/wp-content/uploads/2015/04/TransCelerate-Non-Promotional-Language-Guidelines-v10.2.pdf
Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard. MRCT Return of Aggregate Results Toolkit 2017 [cited 2023 September 25]. Available from: https://mrctcenter.org/resource/return-of-aggregate-results-to-participants-toolkit-version-3-1/
National Institute for Health and Care Research. Plain English summaries 2021 [cited 2023 September 25]. Available from: https://www.nihr.ac.uk/documents/plain-english-summaries/27363
Gaskarth M, King K, Magee R, et al. Where are biomedical research plain-language summaries (PLS)? Presented at the International Society for Medical Publication Professionals (ISMPP) Annual Meeting, London, UK, January 22–23, 2019. 2021.
Shepherd C, Fisher G, Gardner J. Could PubMed be a viable route to discovering plain language summaries? Presented at the 2020 European meeting of the International Society for Medical Publication Professionals (ISMPP), London, UK, January 21–22, 2020. 2020.
Walker J, Dormer L. Publishing Plain Language Summaries of Publications as standalone journal articles: a publisher’s case study. Presented at the 2021 International Society for Medical Publication Professionals, Virtual meeting, April 12–14, 2021. 2021.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and Explanation. Ann Intern Med. 2018;169(7):467–73.
Abel T, Benkert R. Critical health literacy: reflection and action for health. Health Promot Int. 2022;37(4):daac114.
Sykes S, Wills J, Rowlands G, Popple K. Understanding critical health literacy: a concept analysis. BMC Public Health. 2013;13:150.
Crondahl K, Eklund KL. The nexus between health literacy and empowerment: a scoping review. SAGE Open. 2016;6(2):2158244016646410.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
FitzGibbon H, King K, Piano C, et al. Where are biomedical research plain-language summaries? Health Sci Rep. 2020;3(3): e175.
West JD, Bergstrom CT. Misinformation in and about science. Proc Natl Acad Sci USA. 2021;118(15):e1912444117
Mlinarić A, Horvat M, Šupak SV. Dealing with the positive publication bias: why you should really publish your negative results. Biochem Med (Zagreb). 2017;27(3): 030201.
Abola MV, Prasad V. The use of superlatives in cancer research. JAMA Oncol. 2016;2(1):139–41.
Vinkers CH, Tijdink JK, Otte WM. Use of positive and negative words in scientific PubMed abstracts between 1974 and 2014: retrospective analysis. BMJ. 2015;351: h6467.
Aldinger CE, Ligibel J, Shin IH, et al. Returning aggregate results of clinical trials: empirical data of patient preferences. J Clin Transl Sci. 2018;2(6):356–62.
Anand G, Joshi M. Harmonising terminology with MedDRA for plain language summaries. Indian. J Med Ethics. 2021;VII(3):255.
Anderson HL, Moore JE, Millar BC. Comparison of the readability of lay summaries and scientific abstracts published in CF Research News and the Journal of Cystic Fibrosis: Recommendations for writing lay summaries. J Cyst Fibros. 2022;21(1):e11–4.
Anzinger H, Elliott SA, Hartling L. Comparative usability analysis and parental preferences of three web-based knowledge translation tools: multimethod study. J Med Internet Res. 2020;22(3): e14562.
Banić A, Fidahić M, Šuto J, et al. Conclusiveness, linguistic characteristics and readability of Cochrane plain language summaries of intervention reviews: a cross-sectional study. BMC Med Res Methodol. 2022;22(1):240.
Barnes A, Patrick S. Lay summaries of clinical study results: an overview. Pharmaceut Med. 2019;33(4):261–8.
Barnfield S, Pitts AC, Kalaria R, et al. “Is all the stuff about neurons necessary?” The development of lay summaries to disseminate findings from the Newcastle Cognitive Function after Stroke (COGFAST) study. Res Involv Engagem. 2017;3:18.
Bredbenner K, Simon SM. Video abstracts and plain language summaries are more effective than graphical abstracts and published abstracts. PLoS ONE. 2019;14(11): e0224697.
Bruce IA, Ezgü FS, Kampmann C, et al. Addressing the need for patient-friendly medical communications: adaptation of the 2019 recommendations for the management of MPS VI and MPS IVA. Orphanet J Rare Dis. 2022;17(1):91.
Buljan I, Malički M, Wager E, et al. No difference in knowledge obtained from infographic or plain language summary of a Cochrane systematic review: three randomized controlled trials. J Clin Epidemiol. 2018;97:86–94.
Buljan I, Tokalić R, Roguljić M, et al. Comparison of blogshots with plain language summaries of Cochrane systematic reviews: a qualitative study and randomized trial. Trials. 2020;21(1):426.
Buljan I, Tokalić R, Roguljić M, et al. Framing the numerical findings of Cochrane plain language summaries: two randomized controlled trials. BMC Med Res Methodol. 2020;20(1):101.
Carvalho FA, Elkins MR, Franco MR, Pinto RZ. Are plain-language summaries included in published reports of evidence about physiotherapy interventions? Analysis of 4421 randomised trials, systematic reviews and guidelines on the Physiotherapy Evidence Database (PEDro). Physiotherapy. 2019;105(3):354–61.
Dormer L, Walker J. Plain language summary of publication articles: helping disseminate published scientific articles to patients. Future Oncol. 2020;16(25):1873–4.
Edgell C, Rosenberg A. Putting plain language summaries into perspective. Curr Med Res Opin. 2022;38(6):871–4.
Gainey KM, Smith J, McCaffery KJ, et al. What author instructions do health journals provide for writing plain language summaries? A scoping review Patient. 2023;16(1):31–42.
Getz K, Farides-Mitchell J. Assessing the adoption of clinical trial results summary disclosure to patients and the public. Expert Rev Clin Pharmacol. 2019;12(7):573–8.
Gudi SK, Tiwari KK, Panjwani K. Plain-language summaries: an essential component to promote knowledge translation. Int J Clin Pract. 2021;75(6): e14140.
Habr D, Wolf Gianares B, Schuler KW, Chari D. Patients at the heart of the scientific dialogue: an industry perspective. Oncol Ther. 2023;11(1):15–24.
Helmer SM, Matthias K, Mergenthal L, et al. Dissemination of knowledge from Cochrane Public Health reviews: a bibliographic study. Syst Rev. 2023;12(1):113.
Hinckley J, El-Khouri C. Why and how to publish aphasia-friendly research summaries. J Commun Disord. 2023;104: 106338.
Kerwer M, Chasiotis A, Stricker J, et al. Straight from the scientist’s mouth—plain language summaries promote laypeople’s comprehension and knowledge acquisition when reading about individual research findings in psychology. Collabra Psychol. 2021;7(1):18898.
Kerwer M, Stoll M, Jonas M, et al. How to put it plainly? Findings from two randomized controlled studies on writing plain language summaries for psychological meta-analyses. Front Psychol. 2021;12: 771399.
Kirkpatrick E, Gaisford W, Williams E, et al. Understanding plain English summaries. A comparison of two approaches to improve the quality of plain English summaries in research reports. Res Involv Engagem. 2017;3:17.
Kuehn BM. The value of a healthy relationship. Elife. 2017;6:e25412
Lobban D, Gardner J, Matheis R. Plain language summaries of publications of company-sponsored medical research: what key questions do we need to address? Curr Med Res Opin. 2022;38(2):189–200.
Martínez Silvagnoli L, Shepherd C, Pritchett J, Gardner J. Optimizing readability and format of plain language summaries for medical research articles: cross-sectional survey study. J Med Internet Res. 2022;24(1): e22122.
McGrath L, Millar BC, Moore JE. Using plain language to communicate with clinical trials participants: comparison of readability calculators. Contemp Clin Trials. 2022;123: 106995.
Penlington M, Goulet P, Metcalfe B. Improving knowledge and trust in vaccines: a survey-based assessment of the potential of the European Union Clinical Trial Regulation No 536/2014 plain language summary to increase health literacy. Vaccine. 2022;40(6):924–33.
Penlington M, Silverman H, Vasudevan A, Pavithran P. Plain language summaries of clinical trial results: a preliminary study to assess availability of easy-to-understand summaries and approaches to improving public engagement. Pharmaceut Med. 2020;34(6):401–6.
Raynor DK, Myers L, Blackwell K, et al. Clinical trial results summary for laypersons: a user testing study. Ther Innov Regul Sci. 2018;52(5):606–28.
Raza MZ, Bruhn H, Gillies K. Dissemination of trial results to participants in phase III pragmatic clinical trials: an audit of trial investigators intentions. BMJ Open. 2020;10(1): e035730.
Rodgers P. Writing for different readers. Elife. 2017;6.
Rosenberg A, Baróniková S, Feighery L, et al. Open Pharma recommendations for plain language summaries of peer-reviewed medical journal publications. Curr Med Res Opin. 2021;37(11):2015–6.
Rosenberg A, Walker J, Griffiths S, Jenkins R. Plain language summaries: enabling increased diversity, equity, inclusion and accessibility in scholarly publishing. Learn Publ. 2023;36(1):109–18.
Ruzich E, Ritchie J, Ginchereau Sowell F, et al. A powerful partnership: researchers and patients working together to develop a patient-facing summary of clinical trial outcome data. J Am Med Inform Assoc. 2024;31(2):363–74.
Schmitz B. Improving accessibility of scientific research by artificial intelligence–an example for lay abstract generation. Digit Health. 2023;9:20552076231186244.
Shailes S. Something for everyone. Elife. 2017;6:e25411
Shiely F, Daly A. Trial lay summaries were not fit for purpose. J Clin Epidemiol. 2023;156:105–12.
Smith R. Improving and spreading plain language summaries of peer-reviewed medical journal publications. Curr Med Res Opin. 2021;37(11):2017–8.
South A, Joharatnam-Hogan N, Purvis C, et al. Testing approaches to sharing trial results with participants: the Show RESPECT cluster randomised, factorial, mixed methods trial. PLoS Med. 2021;18(10): e1003798.
Stricker J, Chasiotis A, Kerwer M, Günther A. Scientific abstracts and plain language summaries in psychology: a comparison based on readability indices. PLoS ONE. 2020;15(4): e0231160.
Wada M, Sixsmith J, Harwood G, et al. A protocol for co-creating research project lay summaries with stakeholders: guideline development for Canada’s AGE-WELL network. Res Involv Engagem. 2020;6:22.
Wen J, He S, Yi L. Easily readable? Examining the readability of lay summaries published in autism research. Autism Res. 2023;16(5):935–40.
Whiting P, Leeflang M, de Salis I, et al. Guidance was developed on how to write a plain language summary for diagnostic test accuracy reviews. J Clin Epidemiol. 2018;103:112–9.
Yi L, Yang X. Are lay abstracts published in autism readable enough for the general public? A short report. Autism. 2023;27(8):2555–9.
Zając JF, Bała MM. Public media as a tool for dissemination of evidence-based information. Int J Evid Based Healthc. 2019;17(Suppl 1):S32-s33.
Zimmerman KO, Perry B, Hanlen-Rosado E, et al. Developing lay summaries and thank you notes in paediatric pragmatic clinical trials. Health Expect. 2022;25(3):1029–37.
Barello S, Palamenghi L, Graffigna G. The mediating role of the patient health engagement model on the relationship between patient perceived autonomy supportive healthcare climate and health literacy skills. Int J Environ Res Public Health. 2020;17(5):1741.
Centers for Disease Control and Prevention. Patient Engagement 2021 [cited 2023 21 August]. Available from: https://www.cdc.gov/healthliteracy/researchevaluate/patient-engage.html.
Náfrádi L, Nakamoto K, Csabai M, et al. An empirical test of the Health Empowerment Model: does patient empowerment moderate the effect of health literacy on health status? Patient Educ Couns. 2018;101(3):511–7.
National Voices. Knowledge is power: public perspectives on open access publishing. 2017 [cited 2023 8 August]. Available from: https://s42139.pcdn.co/wp-content/uploads/a_new_relationship_with_people_and_communities_0.pdf
Pal A, Klingmann I, Wangmo T, Elger B. Publishing clinical trial results in plain language: a clash of ethical principles? Curr Med Res Opin. 2024;40(3):493–503.
Nutbeam D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259–67.
Schulz PJ, Nakamoto K. The perils of misinformation: when health literacy goes awry. Nat Rev Nephrol. 2022;18(3):135–6.
Anderson LB, Kanneganti D, Houk MB, et al. Generative AI as a tool for environmental health research translation. Geohealth. 2023;7(7):e2023GH000875.
Clayman ML, Manganello JA, Viswanath K, et al. Providing health messages to Hispanics/Latinos: understanding the importance of language, trust in health information sources, and media use. J Health Commun. 2010;15(Suppl 3):252–63.
Halvorsen K, Dihle A, Hansen C, et al. Empowerment in healthcare: a thematic synthesis and critical discussion of concept analyses of empowerment. Patient Educ Couns. 2020;103(7):1263–71.
Aujoulat I, d’Hoore W, Deccache A. Patient empowerment in theory and practice: polysemy or cacophony? Patient Educ Couns. 2007;66(1):13–20.
Smith SK, Dixon A, Trevena L, et al. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med. 2009;69(12):1805–12.
Volandes AE, Paasche-Orlow MK. Health literacy, health inequality and a just healthcare system. Am J Bioeth. 2007;7(11):5–10.
Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Draft FDA Guidance on Provision of Plain Language Summaries 2017 [cited 2023 September 25]. Available from: https://downloads.regulations.gov/FDA-2017-D-5478-0001/attachment_1.pdf.
European Commission. Regulation (EU) No 536/2014 2014. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0536.
Acknowledgements
A detailed description of the methods used has been posted on the Center for Open Science’s OSF Registries website (https://osf.io/geq7z?revisionId=63ee2e4246ee090472141344). The authors would like to thank Danielle Birchall, for critical review and feedback on the protocol and initial literature searches, and Avinash Yerramsetti and Aakash Katdare for re-extraction of the quantitative data. Medical editorial assistance and submission support were provided by Alister Smith, PhD of Morphogen Medical Communications.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by University of Basel. Support for medical editorial and submission assistance was funded by the authors.
Competing Interests
AP is a full-time employee of Novartis Pharma AG. However, this work is independent of his employment and is part of his doctoral research at the Institute for Biomedical Ethics, University of Basel. IA, TW, and BE have no competing interests to disclose.
Availability of Data and Materials
Data can be provided upon reasonable request.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Author Contributions
Avishek Pal: Conceptualization, methodology, formal analysis and investigation, writing—original draft preparation, writing—review and editing writing, resources. Isabelle Arnet: Conceptualization, methodology, writing—review and editing writing, supervision. Tenzin Wangmo: Conceptualization, methodology, formal analysis and investigation, writing—review and editing writing, supervision, resources. Bernice Elger: Conceptualization, methodology, writing—review and editing writing, supervision.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pal, A., Arnet, I., Elger, B.S. et al. Practices and Barriers in Developing and Disseminating Plain-Language Resources Reporting Medical Research Information: A Scoping Review. Patient (2024). https://doi.org/10.1007/s40271-024-00700-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s40271-024-00700-y