Abstract
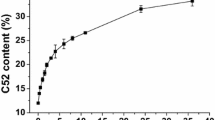
A packed-bed reactor (length 6.5 cm; id 4.65 mm) has been used to enrich docosahexaenoic acid (DHA) via the lipase-catalyzed esterification of the fatty acid from tuna oil with ethanol. Lipozyme RM IM (from Rhizomucor miehei) was used for the esterification reaction because of its ability to discriminate between different fatty acids, and several reaction parameters, including the temperature, molar ratio of substrates, and water content were explored as a function of residence time. In this way, the optimum conditions for the enrichment process were determined to be a temperature of 20 °C, a molar ratio of 1:5 (i.e., fatty acid to ethanol), and a water content of 1.0 % (based on the total substrate weight). Under these conditions, a residence time of 90 min gave a DHA concentration of 70 wt% and a DHA recovery yield of 87 wt% in the residual fatty acid fraction.







Similar content being viewed by others
References
Williams CM, Burdge G (2006) Long-chain n-3 PUFA: plant v. marine sources. Proc Nutr Soc 65:42–50
Yamazaki K, Fujikawa M, Hamazaki T, Yano S, Shono T (1992) Comparison of the conversion rates of α-linolenic acid [18:3 (n-3)] and stearidonic acid [18:4 (n-3)] to longer polyunsaturated fatty acids in rats. BBA Lipids Lipid Metab 1123:18–26
Wanasundara U, Shahidi F (1998) Lipase-assisted concentration of n-3 polyunsaturated fatty acids in acylglycerols from marine oils. J Am Oil Chem Soc 75:945–951
Wanasundara UN, Shahidi F (1999) Concentration of omega 3-polyunsaturated fatty acids of seal blubber oil by urea complexation: optimization of reaction conditions. Food Chem 65:41–49
Vázquez L, Akoh C (2011) Concentration of stearidonic acid in free fatty acid and fatty acid ethyl ester forms from modified soybean oil by winterization. J Am Oil Chem Soc 88:1775–1785
Huang YS, Pereira SL, Leonard AE (2005) Handbook of industrial biocatalysis. In: Hou CT (ed) Enzymes for the transgenic production of long-chain polyunsaturated fatty acid-enriched oils. Taylor & Francis/CRC, Boca Raton, pp 1–13
Haraldsson G, Kristinsson B, Sigurdardottir R, Gudmundsson G, Breivik H (1997) The preparation of concentrates of eicosapentaenoic acid and docosahexaenoic acid by lipase-catalyzed transesterification of fish oil with ethanol. J Am Oil Chem Soc 74:1419–1424
Tanaka Y, Hirano J, Funada T (1992) Concentration of docosahexaenoic acid in glyceride by hydrolysis of fish oil with Candida cylindracea lipase. J Am Oil Chem Soc 69:1210–1214
Haraldsson G, Kristinsson B (1998) Separation of eicosapentaenoic acid and docosahexaenoic acid in fish oil by kinetic resolution using lipase. J Am Oil Chem Soc 75:1551–1556
Hoshino T, Yamane T, Shimizu S (1990) Selective hydrolysis of fish oil by lipase to concentrate n-3 polyunsaturated fatty acids. Agric Biol Chem 54:1459–1467
McNeill G, Ackman R, Moore S (1996) Lipase-catalyzed enrichment of long-chain polyunsaturated fatty acids. J Am Oil Chem Soc 73:1403–1407
Shimada Y, Sugihara A, Nakano H, Kuramoto T, Nagao T, Gemba M, Tominaga Y (1997) Purification of docosahexaenoic acid by selective esterification of fatty acids from tuna oil with Rhizopus delemar lipase. J Am Oil Chem Soc 74:97–101
Shimada Y, Maruyama K, Sugihara A, Moriyama S, Tominaga Y (1997) Purification of docosahexaenoic acid from tuna oil by a two-step enzymatic method: hydrolysis and selective esterification. J Am Oil Chem Soc 74:1441–1446
Hills M, Kiewitt I, Mukherjee K (1990) Enzymatic fractionation of fatty acids: enrichment of γ-linolenic acid and docosahexaenoic acid by selective esterification catalyzed by lipases. J Am Oil Chem Soc 67:561–564
Halldorsson A, Kristinsson B, Glynn C, Haraldsson G (2003) Separation of EPA and DHA in fish oil by lipase-catalyzed esterification with glycerol. J Am Oil Chem Soc 80:915–921
Laudani CG, Habulin M, Knez Ž, Porta GD, Reverchon E (2007) Immobilized lipase-mediated long-chain fatty acid esterification in dense carbon dioxide: bench-scale packed-bed reactor study. J Supercrit Fluids 41:74–81
Chen YH, Huang YH, Lin RH, Shang NC (2010) A continuous-flow biodiesel production process using a rotating packed bed. Bioresour Technol 101:668–673
Du W, Li W, Sun T, Chen X, Liu D (2008) Perspectives for biotechnological production of biodiesel and impacts. Appl Microbiol Biotechnol 79:331–337
Xu X, Balchen S, Høy CE, Adler-Nissen J (1998) Production of specific-structured lipids by enzymatic interesterification in a pilot continuous enzyme bed reactor. J Am Oil Chem Soc 75:1573–1579
Schmitt-Rozieres M, Deyris V, Comeau LC (2000) Enrichment of polyunsaturated fatty acids from sardine cannery effluents by enzymatic selective esterification. J Am Oil Chem Soc 77:329–332
Shimada Y, Watanabe Y, Samukawa T, Sugihara A, Noda H, Fukuda H, Tominaga Y (1999) Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase. J Am Oil Chem Soc 76:789–793
Shen Z, Wijesundera C (2006) Evaluation of ethanolysis with immobilized Candida antarctica lipase for regiospecific analysis of triacylglycerols containing highly unsaturated fatty acids. J Am Oil Chem Soc 83:923–927
Dordick JS (1989) Enzymatic catalysis in monophasic organic solvents. Enzyme Microb Technol 11:194–211
Boon MA, Janssen AE, van‘t Riet K (2000) Effect of temperature and enzyme origin on the enzymatic synthesis of oligosaccharides. Enzyme Microb Technol 26:271–281
Li N, Zong M-H, Liu X-M, Ma D (2007) Regioselective synthesis of 3′-O-caproyl-floxuridine catalyzed by Pseudomonas cepacia lipase. J Mol Catal B Enzym 47:6–12
Xu X, Fomuso LB, Akoh CC (2000) Synthesis of structured triacylglycerols by lipase-catalyzed acidolysis in a packed bed bioreactor. J Agric Food Chem 48:3–10
Watanabe T, Shimizu M, Sugiura M, Sato M, Kohori J, Yamada N, Nakanishi K (2003) Optimization of reaction conditions for the production of DAG using immobilized 1,3-regiospecific lipase Lipozyme RM IM. J Am Oil Chem Soc 80:1201–1207
Yasufuku Y, Ueji S (1995) Effect of temperature on lipase-catalyzed esterification in organic solvent. Biotechnol Lett 17:1311–1316
Pyo YG, Hong SI, Kim Y, Kim BH, Kim IH (2012) Synthesis of monoacylglycerol containing pinolenic acid via stepwise esterification using a cold active lipase. Biotechnol Prog 28:1218–1224
Phillips RS (1996) Temperature modulation of the stereochemistry of enzymatic catalysis: prospects for exploitation. Trends Biotechnol 14:13–16
López-Martínez JC, Campra-Madrid P, Guil-Guerrero JL (2004) γ-Linolenic acid enrichment from Borago officinalis and Echium fastuosum seed oils and fatty acids by low temperature crystallization. J Biosci Bioeng 97:294–298
Syed Rahmatullah MSK, Shukla VKS, Mukherjee KD (1994) γ-Linolenic acid concentrates from borage and evening primrose oil fatty acids via lipase-catalyzed esterification. J Am Oil Chem Soc 71:563–567
Zaidi A, Gainer JL, Carta G (1995) Fatty acid esterification using nylon-immobilized lipase. Biotechnol Bioeng 48:601–605
Watanabe Y, Yamauchi-Sato Y, Nagao T, Yamamoto T, Tsutsumi K, Sugihara A, Shimada Y (2003) Production of MAG of CLA in a solvent-free system at low temperature with Candida rugosa lipase. J Am Oil Chem Soc 80:909–914
Zaks A, Klibanov AM (1988) The effect of water on enzyme action in organic media. J Biol Chem 263:8017–8021
He Y, Shahidi F (1997) Enzymatic esterification of ω-3 fatty acid concentrates from seal blubber oil with glycerol. J Am Oil Chem Soc 74:1133–1136
Yang T, Xu X, He C, Li L (2003) Lipase-catalyzed modification of lard to produce human milk fat substitutes. Food Chem 80:473–481
Shimada Y, Sugihara A, Minamigawa Y, Higashiyama K, Akimoto K, Fujikawa S, Komemushi S, Tominaga Y (1998) Enzymatic enrichment of arachidonic acid from Mortierella single-cell oil. J Am Oil Chem Soc 75:1213–1217
Lee C-H, Parkin KL (2001) Effect of water activity and immobilization on fatty acid selectivity for esterification reactions mediated by lipases. Biotechnol Bioeng 75:219–227
Chulalaksananukul W, Condoret JS, Delorme P, Willemot RM (1990) Kinetic study of esterification by immobilized lipase in n-hexane. FEBS Lett 276:181–184
Mukherjee K, Kiewitt I, Hills M (1993) Substrate specificities of lipases in view of kinetic resolution of unsaturated fatty acids. Appl Microbiol Biotechnol 40:489–493
Jachmanián I, Schulte E, Mukherjee KD (1996) Substrate selectivity in esterification of less common fatty acids catalysed by lipases from different sources. Appl Microbiol Biotechnol 44:563–567
Acknowledgments
This study was supported by the Food High Pressure Technology Development Project, Korea Food Research Institute and the Rural Development Administration (Korea, project number PJ009247).
Author information
Authors and Affiliations
Corresponding author
Additional information
S. I. Hong, N. Ma, and D. S. No contributed equally to this research.
About this article
Cite this article
Hong, S.I., Ma, N., No, D.S. et al. Enrichment of DHA from Tuna Oil in a Packed Bed Reactor via Lipase-Catalyzed Esterification. J Am Oil Chem Soc 91, 1877–1884 (2014). https://doi.org/10.1007/s11746-014-2536-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2536-9