Abstract
Background
Frailty is a common condition present in older Emergency Department (ED) patients that is associated with poor health outcomes. The Clinical Frailty Scale (CFS) is a tool that measures frailty on a scale from 1 (very fit) to 9 (terminally ill). The goal of this scoping review was to describe current use of the CFS in emergency medicine and to identify gaps in research.
Methods
We performed a systemic literature search to identify original research that used the CFS in emergency medicine. Several databases were searched from January 2005 to July 2021. Two independent reviewers completed screening, full text review and data abstraction, with a focus on study characteristics, CFS assessment (evaluators, timing and purpose), study outcomes and statistical methods.
Results
A total of 4818 unique citations were identified; 34 studies were included in the final analysis. Among them, 76% were published after 2018, mainly in Europe or North America (79%). Only two assessed CFS in the pre-hospital setting. The nine-point scale was used in 74% of the studies, and patient consent was required in 69% of them. The main reason to use CFS was as a main exposure (44%), a potential predictor (15%) or an outcome (15%). The most frequently studied outcomes were mortality and hospital admission.
Conclusion
The use of CFS in emergency medicine research is drastically increasing. However, the reporting is not optimal and should be more standardized. Studies evaluating the impact of frailty assessment in the ED are needed.
Registration
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty is a physiological state where small perturbations in health result in disproportionate adverse effects due to an underlying decline in reserve of multiple physiological systems [1,2,3]. It is common in older Emergency Department (ED) patients with reported prevalence rates between 21 and 62 [4,5,6,7]. Frailty is associated with a wide range of adverse outcomes, including mortality [8], hospitalization [9], delirium [7] and diminished quality of life [10]. People often present to the ED due a change in health status, this offers a unique opportunity to alter their health trajectory. To meet the needs of the growing population of older adults with frailty presenting to the ED, there is advocacy for the integration of ED frailty evaluation [11, 12]. However, the benefit and harms associated with frailty screening in the ED are largely unknown [13, 14]. Furthermore, frailty identification in the ED is not common [15]. Cited barriers included feasibility of tools in the time pressured ED environment, lack of formal clinical frailty guidelines for the ED and geriatric expertise [11, 13, 15, 16].
Previous scoping reviews on frailty in the acute care setting have included multiple medical disciplines including geriatrics, emergency medicine, general medicine, cardiology and orthopedics [14, 17]. Van Dam et al. recently completed a narrative review of frailty assessment in the ED [18]. They focused on the predictive accuracy of frailty screening tools, the use of clinical gestalt to determine frailty, and the rationale for and implementation of frailty assessment in the ED. However, some of included studies have used tools that were initially designed to predict risk of adverse outcome (ie ISAR, TRST) and not frailty specifically [5, 19].
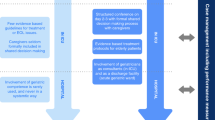
There are 89 different measures that have been used to evaluate frailty in the acute care literature [20]. The Clinical Frailty Scale (CFS) is one of the most commonly used tools. The CFS was initially a seven-point scale used as a judgment-based tool to assess frailty [21]. In 2007, it was expanded to a nine-point scale, from 1 (very fit) to 9 (terminally ill) (Fig. 1). Compared to other frailty tools, the CFS seems to be the ideal choice for measuring frailty in emergency medicine, because it is easier and faster to use, without giving up any prognostic accuracy [22]. There are no studies that exclusively synthesize information on the use of CFS in emergency medicine. This scoping review is intended to fill this gap, by focusing strictly on the CFS literature in the emergency medicine setting. We aimed to describe the current evidence and identify gaps in knowledge including: version of CFS, timing of CFS evaluation, who is completing the evaluation, goals of frailty evaluation, the prevalence of frailty, and the outcomes associated with frailty identification using the CFS.
Materials and methods
A protocol for this scoping review was developed and published on the Open Science Framework, where the study was registered before performing the search strategy (https://doi.org/10.17605/OSF.IO/W2F8N) [23]. We have followed the PRISMA-ScR Statement for reporting scoping reviews [24].
Eligibility criteria
Based on the population, concept, and context (PCC) framework for scoping reviews [25], inclusion criteria were: (1) adult (≥ 18 years) population; (2) use of the CFS; (3) emergency medicine setting (intra-hospital or pre-hospital); and (4) original research. We did not language restrict.
Studies not reporting frailty or reporting frailty using another tool (such as Fried [26], ISAR [27]) exclusively were excluded. We also excluded conference abstracts, editorials, commentaries, position papers, narrative and systematic reviews, and case studies, that did not report on original research.
Search strategy
The MEDLINE search strategy was developed by a health science librarian and peer-reviewed by another librarian [28]. Databases searched were MEDLINE(R) ALL via Ovid, Embase Classic + Embase via Ovid, EBM Reviews—Cochrane Central Register of Control Trials via Ovid, CINAHL via EBSCOhost, Ageline via EBSCOhost, and Scopus. The main search concepts were comprised of terms related to emergency department or pre-hospital settings and frailty. The date of publication was limited from 2005 to 2021. This limit was applied as the Clinical Frailty Scale (CFS) was introduced in 2005. The search strategy was developed in MEDLINE (Appendix 1) and translated to other databases. All databases were searched on July 6th, 2021. Additionally, a manual search of all eligible articles’ reference lists was completed to identify any additional literature.
Selection of source of evidence
Search results were imported into Covidence and de-duplicated [29]. Screening and data abstraction were also completed in Covidence. First, team members screened a sample of 50 citations. Conflicts were reviewed and discussed. As the agreement on the pilot test was low (< 90%), another pilot was performed, with success. Then, two reviewers independently screened all remaining citations. Disagreements were resolved by consensus. Second-level screening was performed using a similar strategy (pilot, double independent screening). The study screening form can be found in Appendix 2.
Data charting process and data items
Data were abstracted, using a pre-specified data abstraction form. To ensure consistency between reviewers, all reviewers initially abstracted the same five citations. Any discrepancies were resolved by consensus. The form was then adapted (Appendix 3), and data abstraction was completed independently by two reviewers. We collected data on publication characteristics (authors, country, year of publication, journal), study characteristics (design, sample size, setting, patients’ age and sex), frailty [version of CFS used, cut-off used to define frail people, type of categorization of CFS, purpose of the assessment (outcome, screening, descriptive, exposure, covariate, potential predictor), assessor, prevalence of frailty] and outcomes under study. When composite outcomes were studied, we collected each outcome of the composite outcome individually.
Critical appraisal of individual sources of evidence
As the main goal of this study was to report on the contextual features of frailty in emergency medicine literature, no critical appraisal was performed on the individual studies.
Synthesis of results
Results of the search and the screening process are presented using a flow diagram. Outcomes were grouped according to essential themes for the purpose of analysis.
Results
Figure 2 presents the study flow diagram. From the 7164 records, we identified 4757 unique citations after deduplication. Sixty-one studies were also identified from references of included articles. Following first-level screening, 4575 were deemed irrelevant. Second-level screening excluded a further 209 citations. Thirty-four manuscripts (33 full manuscript and one research letter) underwent complete data abstraction and are presented in this manuscript (Appendix 4). No potentially relevant studies were excluded.
Table 1 presents characteristics of the included studies. All studies were published in English and the primary author affiliation was mainly from North America [7, 30,31,32,33,34,35,36,37,38,39,40,41,42,43] (44%) and Europe [44,45,46,47,48,49,50,51,52,53,54,55] (35%). No papers had been published before 2015, and most of the papers (76%) were published beginning 2019. Studies were published in emergency medicine journals (41%) [7, 30, 32, 33, 36, 40, 46, 48,49,50, 53, 54, 56, 57], geriatric journals (38%) [31, 34, 35, 38, 39, 41, 42, 45, 47, 56, 58, 59] or other types of journals (21%) [37, 43, 51, 52, 55, 60, 61].
Two-thirds of the studies were prospective cohorts [7, 30, 33, 35,36,37,38, 40, 42, 43, 47,48,49,50, 52, 54,55,56,57,58,59, 62], while the remaining were retrospective cohorts (24%) [34, 39, 41, 45, 46, 53, 60, 61], intervention studies (9%) [31, 44, 51] or cross-sectional studies (3%) [32] (Table 1). One study [45] was performed in pre-hospital setting only, and another one [43] included both pre-hospital and ED patients. Overall, the median sample size was 612, with an important variability from one study to the other (IQR 330–1309). The median or mean age varied between 75 and 85, while the proportion of female patients varied between 36 and 77%. Patient consent was required in 20 studies and not required in four studies [36, 45, 53, 54]. The 10 remaining studies [32, 34, 41, 43, 46, 50, 51, 59,60,61] did not mention patient consent.
The majority (74%) of the studies used the nine-point CFS [32,33,34, 36, 39,40,41, 44,45,46, 48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. For three studies [30, 43, 47], it was not possible to assess which CFS version was used. Only two studies excluded patients with CFS score of nine. [33, 49] Thirteen studies reported frailty prevalence, with a median (using authors’ cut-off) of 36.8% (IQR 31.8–57.6). Frailty was assessed mostly during patient work-up (65%) [31,32,33, 35,36,37,38, 40, 42, 44, 45, 47,48,49, 51, 53, 55, 56, 58, 59, 61, 62], while some authors assessed it at triage (18%) [41, 46, 50, 52, 54, 60], at patient disposition (9%) [7, 34, 57] or at other times (9%) [30, 39, 43]. Table 2 shows the different types of assessors. Research staff (35%) [7, 30, 31, 35, 37, 38, 43, 47, 49, 56, 59, 62], nurse (32%) [36, 40, 41, 44, 46, 48, 50, 52, 54, 58, 60] and ED physician (20%) [32, 33, 36, 40, 42, 46, 57] were the most frequent.
CFS was most commonly used as a main exposure (44%) [7, 33, 37,38,39, 41, 42, 46, 49, 52, 53, 55, 57, 60]. Other frequent purposes included potential predictor (15%) [30, 35, 45, 56, 62] and outcome (15%) [32, 36, 40, 43, 48]. Only two studies used it as an eligibility criterion. When CFS was used as a main exposure or a predictor (20 studies), the most frequent studied outcomes (either alone or in composite) were mortality (10 studies, 50%) [33, 39, 46, 49, 55,56,57,58, 60, 62] and hospital admission (7 studies, 35%) (Table 3) [33, 35, 41, 49, 53, 55, 60]. For mortality, several time points were used, including 1 month [33, 39, 49, 55, 57, 60], 3 months [56, 62] or 1 year. Three papers used it as a time-to-event variable [39, 46, 49]. Four papers considered patient-oriented outcomes (alone or included in a composite outcome), such as quality of life [37, 58], functional decline [38, 42] or need for community service following discharge [58]. In the case of use as the main exposure, a sample size calculation was reported only in three studies [7, 49, 52]. Different methods to deal with the CFS variable as exposure or predictor were used for the statistical analysis: binarization (35%) [7, 33, 38, 55, 56, 58, 62], categorisation in 3 or more groups (30%) [35, 37, 39, 42, 46, 49] or continuous (20%) [41, 45, 53, 57]. One study [60] used different methods and two studies [30, 52] did not mention their analytic approach. Among the 15 studies looking for an association between a main exposure and an outcome, only 3 (20%) mentioned a sample size calculation [7, 49, 52]. Finally, these 15 studies found a statistically significant association. Three studies did not incorporate any covariate in the model [41, 42, 52]. For the other ones, age (10 studies [7, 33, 38, 46, 49, 53, 55, 57, 58, 60]), sex or gender (9 studies [33, 38, 46, 49, 53, 55, 57, 58, 60]) and comorbidities (7 studies [37, 38, 46, 53, 57, 58, 60]) were the most frequent covariates used for adjustment (Table 4).
Discussion
We conducted a scoping review that explored the use of the CFS in adult patients in emergency medicine. We found there is increasing use of the CFS in the emergency setting. Most of the studies using it have been published in recent years. The revised version of the CFS with nine points was the most frequently used; however, the purpose and timing of the CFS, who performed the assessment and the analytic approach differed between studies. The cut-off used to define frailty not reported in almost half of studies and the most frequent use of CFS was as an exposure, to look at an association with an outcome.
Our study adds to the work of Church et al., and van Dam et al. [18, 63]. Van Dam et al. completed a narrative review of frailty assessment in the ED. Their study evaluated multiple tools and only included three studies that used the CFS. Church, on the other hand, focused exclusively on use of the CFS, but only six were in the ED. While there are some similarities, including trend over time, assessors and outcomes under study, our findings contribute significantly to our understanding of the current use of the CFS in the ED, as we focused on the ED setting and we examined additional characteristics, such as consent and statistical analysis.
This research showed that consent was required for study inclusion most of the time. While we acknowledge the importance to seek patient consent to participate in a study, studies looking at the impact of frailty assessment or association with outcomes that exclude patients that cannot give informed consent are at risk of, in the very least, limiting the generalizability of the results but in the worst case biasing their results. The impact of patient selection based on consent on study results has been shown in other vulnerable populations, including patients with delirium and stroke [64, 65]. As there appears to be a relation between frailty and ability to give informed consent, the risk of bias in this patient population is high [66]. Therefore, it would be optimal to get a waiver of consent for minimum risk studies.
Another important finding of this study is suboptimal reporting regarding CFS. It was occasionally difficult to determine who completed the CFS assessment, when the assessment took place, which version of the CFS was used or how the CFS was considered in the analysis. A lack of standardized reporting is a crucial issue in research as it could impact interpretation and reproducibility of results [20].
Regarding the analysis, our study highlights several issues that should be mentioned. Studies that reported frailty prevalence or used frailty as a binary variable in their analysis, did not use a consistent CFS cut-off, some authors used four and more whereas other authors used five and more, likely because of the recent change of wording (“vulnerable” to “very mild frailty”). Although binarization is never the best solution, there needs to be consensus regarding a standardized cut-off if the CFS is to be dichotomized. While many studies consider frailty as a binary variable, some authors used it as a continuous one. Such analysis should be performed with caution as it is unlikely that regression fundamental assumptions would be met, such as linearity of the log-odds. Using categories, or even more advanced methods such as restricted cubic spline, could improve the rigor in this part of a study [67]. Almost all authors chose to adjust the main association. Age and comorbidities were frequently chosen. It can be argued that, because the CFS is a multi-faceted tool, incorporating already such aspects, there is a risk of collinearity.
Some limitations of this scoping review should be acknowledged. Our search strategy was developed for our specific question, however there is the possibility that studies could have been missed, especially studies with CFS used as inclusion criteria, baseline characteristics or covariates as they are frequently not mentioned in the abstract. Therefore, the results regarding the purpose of the CFS assessment in the ED could be biased, with a risk of underestimating the use of CFS for those purposes. We decided a priori to include only studies with patients, as our goal was to see how the CFS was used in the ED. There are, however, some papers on the reliability of the CFS that were based on clinical vignettes. Those studies were excluded. Finally, to ensure the homogeneity of our results, we excluded papers that included both ED patients and ward patients, as the finding could have biased our results, if the CFS was not assessed in the ED environment.
This scoping review has strengths. To our knowledge, this is the first exhaustive review on the CFS in the ED. The results from this review will help to define future research questions. Secondly, we used rigorous methodology for the sources (several databases, published papers and conferences abstract), the search strategy (more comprehensive than previous studies), the screening (pilot testing, double independently review) and the data extraction. This process reinforces the internal validity of our results. Finally, this scoping review was registered, its protocol is available, and all amendments to this protocol are listed to increase the transparency of our work.
Based on this review, we identify several gaps that could be considered in future research projects. From a global perspective, there needs to be a move toward common data elements (including cut-off point where appropriate) and core outcome measures [68]. Consensus on data elements and outcome measures for the CFS in the ED could be achieved using the Delphi methodology [69]. We identified multiples studies that looked at the association between CFS level and outcomes. Robust synthesis, including bias assessment and meta-analysis should be performed. From a clinical perspective, there are currently few studies looking at the added value of the systematic use of the CFS in the ED. Evaluation of the impact of ED frailty screening with this tool is therefore needed. Studies comparing frailty screening to no screening are required before advocating for a large implementation of frailty screening. Other important questions include who should complete the frailty evaluation and what is the optimal timing of frailty assessment during the ED course. While it has been shown in the ICU that assessment based on chart review, with family or directly to the patient were quite similar [70], the research on this issue within emergency medicine is scarce. It is likely that assessing frailty at triage versus at disposition could have a different impact. Finally, we found only one study performed exclusively in the pre-hospital setting. When paramedic attend at patients’ home, they could have a better perspective of their environment and could therefore have a more accurate assessment of their frailty.
In summary, this scoping review found increasing use of the Clinical Frailty Scale in studies with adults presenting to the ED. The majority of studies used it as a predictor for adverse outcomes, most commonly admission to hospital and mortality. The quality of the reporting in future studies must be improved. Future research should look at how patients can benefit from its use in the ED and when, how and by whom the CFS should be used.
Availability of data and material
The data that support the findings of this study are available on the Open Science Framework (https://doi.org/10.17605/OSF.IO/WQRFV).
References
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381(9868):752–762
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I et al (2005) A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 173(5):489–495
WHO Clinical Consortium on Health Ageing (2017) Geneva, Switzerland: World Health Organization
Choutko-Joaquim S, Tacchini-Jacquier N, Pralong D’Alessio G, Verloo H (2019) Associations between frailty and delirium among older patients admitted to an emergency department. Dement Geriatr Cognitive Disord Extra 9(2):236–249
Salvi F, Morichi V, Grilli A, Lancioni L, Spazzafumo L, Polonara S et al (2012) Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 16(4):313–318
Aprahamian I, Arico de Almeida GV, de Vasconcellos Romanin CF, Gomes Caldas T, Antunes Yoshitake NT, Bataglini L et al (2019) Frailty could predict death in older adults after admissionat emergency department? A 6-month prospective study from a middle-income country. J Nutr Health Aging 23(7):641–647
Giroux M, Sirois M-J, Boucher V, Daoust R, Gouin E, Pelletier M et al (2018) Frailty assessment to help predict patients at risk of delirium when consulting the emergency department. J Emerg Med 55(2):157–164
Kojima G, Iliffe S, Walters K (2018) Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing 47(2):193–200
Kojima G (2016) Frailty as a predictor of hospitalisation among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health 70(7):722–729
Crocker TF, Brown L, Clegg A, Farley K, Franklin M, Simpkins S et al (2019) Quality of life is substantially worse for community-dwelling older people living with frailty: systematic review and meta-analysis. Qual Life Res 28(8):2041–2056
Dent E, Hoogendijk EO, Cardona-Morrell M, Hillman K (2016) Frailty in emergency departments. Lancet 387(10017):434
Eagles D, Ellis B, Melady D (2020) Frailty: a key concept to improve older person care. CJEM 22(5):624–625
Elliott A, Phelps K, Regen E, Conroy SP (2017) Identifying frailty in the emergency department-feasibility study. Age Ageing 46(5):840–845
Hogan DB, Maxwell CJ, Afilalo J, Arora RC, Bagshaw SM, Basran J et al (2017) A scoping review of frailty and acute care in middle-aged and older individuals with recommendations for future research. Can Geriatr J 20(1):22–37
Elliott A, Hull L, Conroy SP (2017) Frailty identification in the emergency department-a systematic review focusing on feasibility. Age ageing 46:509–513
Fallon A, Kennelly S, O’Neill D (2016) Frailty in emergency departments. Lancet 387(10029):1720
Theou O, Squires E, Mallery K, Lee JS, Fay S, Goldstein J et al (2018) What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr 18(1):139
van Dam CS, Hoogendijk EO, Mooijaart SP, Smulders YM, Vet RCW, Lucke JA et al (2021) A narrative review of frailty assessment in older patients at the emergency department. Eur J Emerg Med 28:266–276
Meldon SW, Mion LC, Palmer RM, Drew BL, Connor JT, Lewicki LJ et al (2003) A brief risk-stratification tool to predict repeat emergency department visits and hospitalizations in older patients discharged from the emergency department. Acad Emerg Med 10(3):224–232
Theou O, Squires E, Mallery K, Lee JS, Fay S, Goldstein J et al (2018) What do we know about frailty in the acute care setting ? A scoping review. BMC Geriatr. https://doi.org/10.1186/s12877-018-0823-2
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I et al (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ 173(5):489–495
Lewis ET, Dent E, Alkhouri H, Kellett J, Williamson M, Asha S et al (2019) Which frailty scale for patients admitted via Emergency Department? Cohort Study Arch Gerontol Geriatr 80:104–114
Open Science Framework [Available from accessed on July 6 2022 https://osf.io/.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D et al (2018) PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 169(7):467–473
Joanna Briggs Institute (2015) The Joanna Briggs Institute reviewers’ manual 2015. Methodol JBI Scoping Rev Joanna Briggs Inst JB.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146–M156
Dendukuri N, McCusker J, Belzile E (2004) The identification of seniors at risk screening tool: further evidence of concurrent and predictive validity. J Am Geriatr Soc 52(2):290–296
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C (2016) PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75:40–46
Covidence systematic review software [Internet]. Veritas Health Innovation. Available from accessed on July 6 2022 www.covidence.org.
Beland E, Nadeau A, Carmichael P-H, Boucher V, Voyer P, Pelletier M et al (2021) Predictors of delirium in older patients at the emergency department: a prospective multicentre derivation study. CJEM 23(3):330–336
Boucher V, Lamontagne M-E, Lee J, Carmichael P-H, Dery J, Emond M (2019) Acceptability of older patients’ self-assessment in the Emergency Department (ACCEPTED)—a randomised cross-over pilot trial. Age Ageing 48(6):875–880
Dresden SM, Platts-Mills TF, Kandasamy D, Walden L, Betz ME (2019) Patient versus physician perceptions of frailty: a comparison of Clinical Frailty Scale scores of older adults in the emergency department. Acad Emerg Med Off J Soc Acad Emerg Med 26(9):1089–1092
Fernando SM, Guo KH, Lukasik M, Rochwerg B, Cook DJ, Kyeremanteng K et al (2020) Frailty and associated prognosis among older emergency department patients with suspected infection: a prospective, observational cohort study. CJEM 22(5):687–691
Hominick K, McLeod V, Rockwood K (2016) Characteristics of older adults admitted to hospital versus those discharged home, in emergency department patients referred to internal medicine. Can Geriatr J CGJ 19(1):9–14
Lee J, Sirois M-J, Moore L, Perry J, Daoust R, Griffith L et al (2015) Return to the ED and hospitalisation following minor injuries among older persons treated in the emergency department: predictors among independent seniors within 6 months. Age Ageing 44(4):624–629
Lo AX, Heinemann AW, Gray E, Lindquist LA, Kocherginsky M, Post LA et al (2021) Inter-rater reliability of clinical frailty scores for older patients in the emergency department. Acad Emerg Med Off J Soc Acad Emerg Med 28(1):110–113
Provencher V, Sirois M-J, Emond M, Perry JJ, Daoust R, Lee JS et al (2016) Frail older adults with minor fractures show lower health-related quality of life (SF-12) scores up to six months following emergency department discharge. Health Qual Life Outcomes 14(101153626):40
Provencher V, Sirois M-J, Ouellet M-C, Camden S, Neveu X, Allain-Boule N et al (2015) Decline in activities of daily living after a visit to a Canadian emergency department for minor injuries in independent older adults: are frail older adults with cognitive impairment at greater risk? J Am Geriatr Soc 63(5):860–868
Pulok MH, Theou O, van der Valk AM, Rockwood K (2020) The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing 49(6):1071–1079
Ringer T, Thompson C, McLeod S, Melady D (2020) Inter-rater agreement between self-rated and staff-rated Clinical Frailty Scale scores in older emergency department patients: a prospective observational study. Acad Emerg Med Off J Soc Acad Emerg Med 27(5):419–422
Serina P, Lo AX, Kocherginsky M, Gray E, Lindquist LA, Post LA et al (2021) The Clinical Frailty Scale and health services use for older adults in the emergency department. J Am Geriatr Soc 69(3):837–839
Sirois M-J, Griffith L, Perry J, Daoust R, Veillette N, Lee J et al (2017) Measuring frailty can help emergency departments identify independent seniors at risk of functional decline after minor injuries. J Gerontol A Biol Sci Med Sci 72(1):68–74
Tong T, Chignell M, Tierney MC, Sirois M-J, Goldstein J, mond M et al (2017) Technology profiles as proxies for measuring functional and frailty status. Procedia Comput Sci 111:77–86
Alakare J, Kemp K, Strandberg T, Castrén M, Jakovljević D, Tolonen J et al (2021) Systematic geriatric assessment for older patients with frailty in the emergency department: a randomised controlled trial. BMC Geriatr 21(1):1–11
Bernard P, Corcoran G, Kenna L, O’Brien C, Ward P, Howard W et al (2021) Is pathfinder a safe alternative to the emergency department for older patients? Obs Anal Age Ageing 50:1854–1858
Elliott A, Taub N, Banerjee J, Aijaz F, Jones W, Teece L et al (2021) Does the Clinical Frailty Scale at triage predict outcomes from emergency care for older people? Ann Emerg Med 77(6):620–627
Griffin A, O’Neill A, O’Connor M, Ryan D, Tierney A, Galvin R (2020) The prevalence of malnutrition and impact on patient outcomes among older adults presenting at an Irish emergency department: a secondary analysis of the OPTI-MEND trial. BMC Geriatr 20(1):455
Jarman H, Crouch R, Baxter M, Wang C, Peck G, Sivapathasuntharam D et al (2021) Feasibility and accuracy of ED frailty identification in older trauma patients: a prospective multi-centre study. Scand J Trauma Resusc Emerg Med. https://doi.org/10.1186/s13049-021-00868-4
Kaeppeli T, Rueegg M, Dreher-Hummel T, Brabrand M, Kabell-Nissen S, Carpenter CR et al (2020) Validation of the Clinical Frailty Scale for prediction of thirty-day mortality in the emergency department. Ann Emerg Med 76(3):291–300
Kemp K, Alakare J, Harjola V-P, Strandberg T, Tolonen J, Lehtonen L et al (2020) National early warning score 2 (NEWS2) and 3-level triage scale as risk predictors in frail older adults in the emergency department. BMC Emerg Med 20(1):83
McGrath J, Almeida P, Law R (2019) The Whittington frailty pathway: improving access to comprehensive geriatric assessment: an interdisciplinary quality improvement project. BMJ open quality 8(4):e000798
O’Caoimh R, Costello M, Small C, Spooner L, Flannery A, O’Reilly L et al (2019) Comparison of frailty screening instruments in the emergency department. Int J Environ Res Pub Health. 16(19):3626
O’Shaughnessy I, Romero-Ortuno R, Edge L, Dillon A, Flynn S, Briggs R et al (2021) Home FIRsT: interdisciplinary geriatric assessment and disposition outcomes in the Emergency Department. Eur J Intern Med 85(9003220):50–55
Shrier W, Dewar C, Parrella P, Hunt D, Hodgson LE (2020) Agreement and predictive value of the Rockwood Clinical Frailty Scale at emergency department triage. Emerg Med J EMJ 38:868–873
Simon NR, Jauslin AS, Rueegg M, Twerenbold R, Lampart M, Osswald S et al (2021) Association of frailty with adverse outcomes in patients with suspected COVID-19 infection. J Clin Med 10(11):2472
Cardona M, Lewis ET, Kristensen MR, Skjot-Arkil H, Ekmann AA, Nygaard HH et al (2018) Predictive validity of the CriSTAL tool for short-term mortality in older people presenting at emergency departments: a prospective study. Eur Geriatr Med 9(6):891–901
Ishikawa S, Miyagawa I, Kusanaga M, Abe T, Shiraishi A, Fujishima S et al (2021) Association of frailty on treatment outcomes among patients with suspected infection treated at emergency departments. Eur J Emerg Med Off J Eur Soc Emerg Med 28(4):285–291
Lewis ET, Dent E, Alkhouri H, Kellett J, Williamson M, Asha S et al (2019) Which frailty scale for patients admitted via emergency department? A cohort study. Arch Gerontol Geriatr 80:104–114
Liu H, Shang N, Chhetri JK, Liu L, Guo W, Li P et al (2020) A frailty screening questionnaire (FSQ) to rapidly predict negative health outcomes of older adults in emergency care settings. J Nutr Health Aging 24(6):627–633
Clark S, Shaw C, Padayachee A, Howard S, Hay K, Frakking TT (2019) Frailty and hospital outcomes within a low socioeconomic population. QJM Mon J Assoc Phys 112(12):907–913
Shen VW-C, Yang C, Lai L-L, Chen Y-J, Huang H-H, Tsai S-H et al (2021) Emergency department referral for hospice and palliative care differs among patients with different end-of-life trajectories: a retrospective cohort study. Int J Environ Res Pub Health 18(12):6286
Cardona M, O’Sullivan M, Lewis ET, Turner RM, Garden F, Alkhouri H et al (2019) Prospective validation of a checklist to predict short-term death in older patients after emergency department admission in Australia and Ireland. Acad Emerg Med Off J Soc Acad Emerg Med 26(6):610–620
Church S, Rogers E, Rockwood K, Theou O (2020) A scoping review of the Clinical Frailty Scale. BMC Geriatr. https://doi.org/10.1186/s12877-020-01801-7
Tu JV, Willison DJ, Silver FL, Fang J, Richards JA, Laupacis A et al (2004) Impracticability of informed consent in the registry of the Canadian Stroke Network. N Engl J Med 350(14):1414–1421
Adamis D, Martin FC, Treloar A, Macdonald AJ (2005) Capacity, consent, and selection bias in a study of delirium. J Med Ethics 31(3):137–143
Ruske J, Sharma G, Makie K, He K, Ozaki CK, Menard MT et al (2021) Patient comprehension necessary for informed consent for vascular procedures is poor and related to frailty. J Vasc Surg 73(4):1422–1428
Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Kimura M (2020) A U-shaped relationship between the prevalence of frailty and body mass index in community-dwelling Japanese older adults: the Kyoto-Kameoka study. J Clin Med 9(5):1367
Muscedere J, Afilalo J, Araujo De Carvalho I, Cesari M, Clegg A, Eriksen HE et al (2019) Moving towards common data elements and core outcome measures in frailty research. J Frailty Aging. https://doi.org/10.14283/jfa.2019.43
Hsu C-C, Sandford BA (2007) The delphi technique: making sense of consensus. Pract Assess Res Eval 12:10
Shears M, Takaoka A, Rochwerg B, Bagshaw SM, Johnstone J, Holding A et al (2018) Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care 45:197–203
Acknowledgements
We thank Sarah Visintini, MLIS (Berkman Library, University of Ottawa Heart Institute) for peer review of the MEDLINE search strategy.
Funding
Open access funding provided by University of Geneva. No specific funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: CAF, CHN, DE. Data curation: CAF, NL, DE. Formal analysis: CAF, DE. Investigation: CAF, ZA-N, EC, DE. Methodology: CAF, CHN, NL, DE. Project administration: DE. Supervision: DE. Validation: CAF, DE. Writing—original draft: CAF, DE. Writing—review & editing: CAF, CHN, ZA-N, EC, NL, DE.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Previous presentation
The manuscript was previously presentend in SAEM2022, New-Orleans, Louisiane, USA, May 2022.
Amendments
Some amendments were done to our protocol. We dropped the language restriction and we adapted screening form and data extraction form following initial training.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Search strategy draft Ovid MEDLINE(R) ALL < 1946 to July 02, 2021 >
# | Searches | Results |
|---|---|---|
1 | ((emergenc* or accident) adj3 (department? or room? or ward? or unit? or service? or hospital? or care? or medicine? or treatment? or technician* or practioner* or rescu* or triag*)).ti,ab,kf | 180,881 |
2 | (Out of hospital or Prehospital or pre-hospital or paramedic* or ambulance* or dispatch* or first responder*).ti,ab,kf | 45,798 |
3 | (Emergenc* adj2 (medical or health) adj2 service*).ti,ab,kf | 11,447 |
4 | "observation unit?".ti, ab, kf | 886 |
5 | exp Emergency Medical Services/ | 150,742 |
6 | Emergencies/ | 41,625 |
7 | exp Emergency Service, Hospital/ | 85,732 |
8 | exp Emergency Medicine/ | 14,435 |
9 | Emergency Medical Technicians/ | 5820 |
10 | exp Emergency Treatment/ | 125,715 |
11 | or/1–10 | 409,059 |
12 | CFS.ti, ab, kf | 7384 |
13 | frail*.ti, ab, kf | 26,761 |
14 | Frailty/ | 4442 |
15 | Frail Elderly/ | 12,681 |
16 | or/12–15 | 38,245 |
17 | 11 and 16 | 1375 |
18 | limit 17 to year = "2005-Current" | 1218 |
Appendix 2: Screening form
Question | Answer | Decision |
|---|---|---|
1st-level screening (Title and abstract) | ||
Does the study concern emergency medicine patients (Emergency department, pre-hospital field, paramedics)? | No | Exclusion |
Yes/Unsure | Go-on screening | |
Does this study report original research? | No | Exclusion |
Yes/Unsure | Go-on screening | |
Does the title or the abstract mention CFS or frailty? | No | Exclusion |
Yes | Inclusion | |
2nd-level screening (Full text screening) | ||
Does the study report original research? | No (systematic or scoping review) | Exclusion |
No (editorial, letter, etc.) | Exclusion | |
Yes (intervention, cohort, case control, secondary analysis, etc.) | Go on screening | |
Does the study report the assessment of frailty using the CFS (inclusion criteria, Table 1, exposure, results, etc.)? | No | Exclusion |
Yes / Doubt | Go on screening | |
Are the patients assessed in the pre-hospital field or in the ED? | No | Exclusion |
Doubt/Yes | Go on screening | |
Appendix 3: Extraction form
Type | Full text / Letter |
|---|---|
First author name | Free text |
Country of first affiliation | Free text |
Email of corresponding authors | Free text |
Year of publication | XXXX |
Journal | Free text |
Study design | Not mentioned/Unclear/Intervention/Prospective cohort/retrospective cohort/Case control/Other (Free text) |
Sample size | XXX |
Setting | ED only/Prehospital only/Mixed/Other (Free text) |
Patient’s age (mean or median) | Not mentioned/XXX |
Female proportion (%) | Not mentioned/XXX |
Version of CFS used | 7/9/Not mentioned |
Cut-off to define frail patients | Not mentioned/Free text |
Purpose of the assessment | Eligibility criteria/Main exposure/Co-variate/Outcome/Predictor/Descriptive only/Other (Free text) |
If main exposure or covariate, how was the variable analyzed | Continuous |
Binarization | |
Categorization | |
Transformed | |
Other | |
If main exposure, sample size calculation performed | Yes/No/Not mentioned |
Assessor | Not mentioned/Nurse/ED physician/Geriatric physician/Research staff/Administrative staff/Other (Free text) |
Time of assessment | Triage |
Patient’s work-up | |
Disposition | |
Other (Free text) | |
Prevalence of frailty (%) | Not mentioned/XXX |
Primary outcome | Not mentioned/Free text |
Statistically significant association between frailty and the outcome | Not mentioned/Yes/No |
Secondary outcomes | Free text |
Confounders adjusted association | Yes/No |
If confounders: | Free text |
Appendix 4: Studies included in the analysis
-
Alakare J, Kemp K, Strandberg T, et al. Systematic geriatric assessment for older patients with frailty in the emergency department: a randomised controlled trial. BMC Geriatrics. 2021;21(1):1–11.
-
Beland E, Nadeau A, Carmichael P–H, et al. Predictors of delirium in older patients at the emergency department: a prospective multicentre derivation study. CJEM. 2021;23(3):330–336.
-
Bernard P, Corcoran G, Kenna L, et al. Is Pathfinder a safe alternative to the emergency department for older patients? An observational analysis. Age and ageing. 2021(0,375,655, 2xr).
-
Boucher V, Lamontagne M-E, Lee J, Carmichael P–H, Dery J, Emond M. Acceptability of older patients' self-assessment in the Emergency Department (ACCEPTED)-a randomised cross-over pilot trial. Age and ageing. 2019;48(6):875–880.
-
Cardona M, Lewis ET, Kristensen MR, et al. Predictive validity of the CriSTAL tool for short-term mortality in older people presenting at Emergency Departments: a prospective study. European geriatric medicine. 2018;9(6):891–901.
-
Cardona M, O'Sullivan M, Lewis ET, et al. Prospective Validation of a Checklist to Predict Short-term Death in Older Patients After Emergency Department Admission in Australia and Ireland. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2019;26(6):610–620.
-
Clark S, Shaw C, Padayachee A, Howard S, Hay K, Frakking TT. Frailty and hospital outcomes within a low socioeconomic population. QJM: monthly journal of the Association of Physicians. 2019;112(12):907–913.
-
Dresden SM, Platts-Mills TF, Kandasamy D, Walden L, Betz ME. Patient Versus Physician Perceptions of Frailty: A Comparison of Clinical Frailty Scale Scores of Older Adults in the Emergency Department. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2019;26(9):1089–1092.
-
Elliott A, Taub N, Banerjee J, et al. Does the Clinical Frailty Scale at Triage Predict Outcomes From Emergency Care for Older People? Annals of emergency medicine. 2021;77(6):620–627.
-
Fernando SM, Guo KH, Lukasik M, et al. Frailty and associated prognosis among older emergency department patients with suspected infection: A prospective, observational cohort study. CJEM. 2020;22(5):687–691.
-
Giroux M, Sirois M-J, Boucher V, et al. Frailty Assessment to Help Predict Patients at Risk of Delirium When Consulting the Emergency Department. The Journal of emergency medicine. 2018;55(2):157–164.
-
Griffin A, O'Neill A, O'Connor M, Ryan D, Tierney A, Galvin R. The prevalence of malnutrition and impact on patient outcomes among older adults presenting at an Irish emergency department: a secondary analysis of the OPTI-MEND trial. BMC geriatrics. 2020;20(1):455.
-
Hominick K, McLeod V, Rockwood K. Characteristics of Older Adults Admitted to Hospital versus Those Discharged Home, in Emergency Department Patients Referred to Internal Medicine. Canadian geriatrics journal: CGJ. 2016;19(1):9–14.
-
Ishikawa S, Miyagawa I, Kusanaga M, et al. Association of frailty on treatment outcomes among patients with suspected infection treated at emergency departments. European journal of emergency medicine: official journal of the European Society for Emergency Medicine. 2021;28(4):285–291.
-
Jarman H, Crouch R, Baxter M, et al. Feasibility and accuracy of ED frailty identification in older trauma patients: a prospective multi-centre study. Scandinavian journal of trauma, resuscitation and emergency medicine. 2021;29(1):54.
-
Kaeppeli T, Rueegg M, Dreher-Hummel T, et al. Validation of the Clinical Frailty Scale for Prediction of Thirty-Day Mortality in the Emergency Department. Annals of emergency medicine. 2020;76(3):291–300.
-
Kemp K, Alakare J, Harjola V-P, et al. National Early Warning Score 2 (NEWS2) and 3-level triage scale as risk predictors in frail older adults in the emergency department. BMC emergency medicine. 2020;20(1):83.
-
Lee J, Sirois M-J, Moore L, et al. Return to the ED and hospitalisation following minor injuries among older persons treated in the emergency department: predictors among independent seniors within 6 months. Age and ageing. 2015;44(4):624–629.
-
Lewis ET, Dent E, Alkhouri H, et al. Which frailty scale for patients admitted via Emergency Department? A cohort study. Archives of gerontology and geriatrics. 2019;80(8,214,379, 7ax):104–114.
-
Liu H, Shang N, Chhetri JK, et al. A Frailty Screening Questionnaire (FSQ) to Rapidly Predict Negative Health Outcomes of Older Adults in Emergency Care Settings. The journal of nutrition, health & aging. 2020;24(6):627–633.
-
Lo AX, Heinemann AW, Gray E, et al. Inter-rater Reliability of Clinical Frailty Scores for Older Patients in the Emergency Department. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2021;28(1):110–113.
-
McGrath J, Almeida P, Law R. The Whittington Frailty Pathway: improving access to comprehensive geriatric assessment: an interdisciplinary quality improvement project. BMJ open quality. 2019;8(4):e000798.
-
O'Caoimh R, Costello M, Small C, et al. Comparison of Frailty Screening Instruments in the Emergency Department. International journal of environmental research and public health. 2019;16(19).
-
O'Shaughnessy I, Romero-Ortuno R, Edge L, et al. Home FIRsT: interdisciplinary geriatric assessment and disposition outcomes in the Emergency Department. European journal of internal medicine. 2021;85(9,003,220):50–55.
-
Provencher V, Sirois M-J, Emond M, et al. Frail older adults with minor fractures show lower health-related quality of life (SF-12) scores up to six months following emergency department discharge. Health and quality of life outcomes. 2016;14(101,153,626):40.
-
Provencher V, Sirois M-J, Ouellet M-C, et al. Decline in activities of daily living after a visit to a Canadian emergency department for minor injuries in independent older adults: are frail older adults with cognitive impairment at greater risk? Journal of the American Geriatrics Society. 2015;63(5):860–868.
-
Pulok MH, Theou O, van der Valk AM, Rockwood K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age and ageing. 2020;49(6):1071–1079.
-
Ringer T, Thompson C, McLeod S, Melady D. Inter-rater Agreement Between Self-rated and Staff-rated Clinical Frailty Scale Scores in Older Emergency Department Patients: A Prospective Observational Study. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2020;27(5):419–422.
-
Serina P, Lo AX, Kocherginsky M, et al. The Clinical Frailty Scale and Health Services Use for Older Adults in the Emergency Department. Journal of the American Geriatrics Society. 2021;69(3):837–839.
-
Shen VW-C, Yang C, Lai L-L, et al. Emergency Department Referral for Hospice and Palliative Care Differs among Patients with Different End-of-Life Trajectories: A Retrospective Cohort Study. International journal of environmental research and public health. 2021;18(12).
-
Shrier W, Dewar C, Parrella P, Hunt D, Hodgson LE. Agreement and predictive value of the Rockwood Clinical Frailty Scale at emergency department triage. Emergency medicine journal: EMJ. 2020(b0u, 100,963,089).
-
Simon NR, Jauslin AS, Rueegg M, et al. Association of Frailty with Adverse Outcomes in Patients with Suspected COVID-19 Infection. Journal of clinical medicine. 2021;10(11).
-
Sirois M-J, Griffith L, Perry J, et al. Measuring Frailty Can Help Emergency Departments Identify Independent Seniors at Risk of Functional Decline After Minor Injuries. The journals of gerontology Series A, Biological sciences and medical sciences. 2017;72(1):68–74.
-
Tong T, Chignell M, Tierney MC, et al. Technology profiles as proxies for measuring functional and frailty status. Procedia Comput Sci. 2017;111(C):77–86.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fehlmann, C.A., Nickel, C.H., Cino, E. et al. Frailty assessment in emergency medicine using the Clinical Frailty Scale: a scoping review. Intern Emerg Med 17, 2407–2418 (2022). https://doi.org/10.1007/s11739-022-03042-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-022-03042-5