Abstract
Action potentials (APs) in plants are involved in fast leaf or trap closure as well as elongation, respiration, photosynthesis, and fertilization regulation. Here, spontaneous APs (SAPs) in relation to endogenous stem movement named circumnutation (CN) have been investigated in Helianthus annuus in different light conditions in freely circumnutating and immobilized plants. Extracellular electrical measurements and time-lapse photography were carried out simultaneously. The parameters of CN (trajectory length, period, and direction) and the number and transmission direction of SAPs were analysed. In continuous light (25–40 μmol m−2 s−1), all plants circumnutating vigorously in a regular elliptical manner and no SAPs were observed. In light/dark conditions, the plants circumnutated in a daily pattern, most SAPs were observed in the dark and freely circumnutated sunflowers had two times more SAPs (10 SAPs/24 h/plant) than the immobilized plants (5 SAPs/24 h/plant). In continuous very low light (5 μmol m−2 s−1), the plants circumnutated weakly and irregularly and SAPs appeared without the circadian pattern. 3–5 SAPs/24/plant occurred in the freely circumnutating and immobilized plants. In light/dark and continuous very low light conditions, an ultradian rhythm of SAPs was observed and the mean spacing between SAPs was approx. 121–271 min in the freely circumnutating and immobilized plants. Under all light conditions, more SAPs were transmitted basipetally than acropetally. One-hour lasting series of 3–4 min spaced SAPs locally propagated were observed as well in very low light. Basipetal and acropetal SAPs passing along the stem motor region accompany irregularity, changes in the CN trajectory direction, and stem torsion. These results demonstrate that APs and CN changes play a role in plant adaptation to light conditions and that there is an ultradian rhythm of SAPs beside ultradian CN rhythm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In plants, APs—long-distance electrical signals—are induced by a variety of stimuli, e.g. light, shadow, wounding, touch, temperature, or chemicals (glutamate, potassium chloride) (Dziubinska 2003; Fromm and Lautner 2007; Król et al. 2010; Mousavi et al. 2013; Salvador-Recatala et al. 2014; Salvador-Recatala 2016). They are involved in fast leaf or trap closure, elongation, regulation of respiration and fertilization, and gene expression (Dziubinska 2003; Fromm and Lautner 2007; Król et al. 2010). The involvement of APs in fast leaf and trap closure in sensitive plants Mimosa pudica and Dionaea muscipula is the best-visible and evident effector response in plants (Sibaoka 1991; Krol et al. 2012). Besides the stimuli-induced APs, there are SAPs described 20 years ago by Zawadzki et al. (1995). They appear under lack of external stimuli and their source is unknown. Spontaneous APs (SAPs) have lately been observed in Solanum lycopersicum plants (Macedo et al. 2015) and in Helianthus annuus (Zawadzki et al. 1995), which is also known to exhibit intense and varied endogenous movements named circumnutation (CN). CNs are commonly observed in growing plants (Darwin and Darwin 1880); they vary during different stages of plant growth, and exhibit an ultradian, daily, and circadian rhythm as well as complex geometrical patterns (Buda et al. 2003; Charzewska and Zawadzki 2006). CN is an endogenous movement modulated by multiple stimuli, e.g. light, wounding, touch, temperature, chemicals, and gravity (Buda et al. 2003; Hayashi et al. 2004; Charzewska and Zawadzki 2006; Stolarz et al. 2010). The same stimuli also trigger APs, as mentioned above; therefore, the search for relationships between endogenous CN and excitation in plants is advisable. Our previous studies showed the effect of glutamate and lithium on CN. Glutamate-induced series of APs differ in vigorously and weakly circumnutating sunflower seedlings treated with lithium (Stolarz et al. 2015). Sunflowers with a lithium-induced decrease in the circumnutation intensity had an increased number of APs in Glu-induced series. Recently, we have shown that the ultradian oscillations of the membrane potential (depolarisation and hyperpolarisation) accompany CN as well (Kurenda et al. 2015). Additionally, the same ion channel and proton pump inhibitors modulating excitation in plants modulate CN as well (Millet and Badot 1996; Krol and Trebacz 2000; Krol et al. 2006; Kurenda et al. 2015).
The ability to respond to changing light environmental conditions and light stimuli is a key feature in a photosynthesising organism. Light intensity and photoperiod changes mainly affect not only photosynthesis but also organ movements and excitability (Zawadzki et al. 1995; Stankovic et al. 1998; Buda et al. 2003; Charzewska and Zawadzki 2006). The aim of this study was to investigate the relation between CN (Appendix S1) and SAPs in different light conditions using extracellular electrical measurements and time-lapse photography in H. annuus plants. To our knowledge, the influence of light on plant spontaneous excitation and its relation to CN movement have not been described so far. We have shown for the first time an ultradian rhythm of the long-distance SAPs beside the CN rhythm and SAPs accompanying the irregularity of the CN trajectory in very low light conditions. Additionally, it was shown that the number of SAPs is higher in freely circumnutating sunflower, compared with immobilized plants.
Materials and methods
Experimental plants
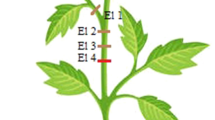
The studies were carried out on 20–30-day-old Helianthus annuus L. plants (PNOS, Ożarów Maz. Poland) grown in a vegetation room in pots filled with garden soil. They were watered with tap water and no other treatment was applied. A 16:8-h light:dark (04:00–20:00) photoperiod was maintained. The intensity of white light in the PAR range (Power Star HQT-T400W/DOSRAM GmbH, Munich, Germany) at the level of plant leaves was approximately 70 μmol m−2 s−1. The vegetation room was air-conditioned; the temperature was 24 ± 1 °C and humidity 50–70%. Approximately 20–35-cm-high plants with one or two pairs of developed leaves were taken for the experiments. To examine the relationship between CN and SAPs, freely circumnutating and immobilized sunflowers were used for measurements (Fig. 1a). The stem of the immobilized sunflowers was tied to a wooden pole for prevention of CN.
CN and SAPs in H. annuus. a Example of a H. annuus plant, top and side view (an example of a circumnutating plant is shown in Appendix S1), and electrode arrangement in extracellular SAP measurements. Four Ag/Cl electrodes (1, 2, 3, 4) were inserted across the stem. The reference (ref) electrode was inserted at the base of the stem. An immobilized sunflower—in the lower right corner. b Top view of CN regular (continuous light) and irregular (continuous very low light) stem apex trajectory, examples of approx. 24-h records (Appendix S2). c Examples of basipetally and acropetally transmitted SAPs along the stem
Circumnutation measurements—time-lapse method
The H. annuus plant was filmed in a Faraday cage with simultaneous electrophysiological measurements. A top view monochromatic camera (Mintron MTV-1368CD, Mintron Enterprise Co. Ltd, Taipei, Taiwan) was used to record the trajectory movement of the stem apex. For filming in darkness, very dim green light with intensity <0.1 μmol m−2 s−1 PAR (25 1.I Pila, Poland) was used. Time-lapse images were recorded at a rate of one frame per minute or five minutes by Gotcha!Multicam software (Prescient System Inc., West Chester, PA, USA). The system was calibrated by a millimetre scale placed at the level of the pot. The time-lapse images were digitized using Circumnutation Tracker (Stolarz et al. 2014). Experimental points (coordinates x, y of the stem apex on the horizontal plane) were determined at 1- or 5-min intervals. The CN length (the distance that the organ apex covers during a single CN cycle) and the CN period (the time that the organ apex needs to trace a single circumnutation cycle) were calculated by Circumnutation Tracker software (Stolarz et al. 2014).
Electrophysiological measurements—extracellular method
The measurements were carried out in the laboratory in a Faraday cage. Changes in the electrical potential were measured with four extracellular Ag/AgCl electrodes (silver wire, 0.2 mm diameter, World Precision Instruments, Sarasota, FL, USA) inserted across the sunflower stem and then interfaced with a multi-channel data acquisition system composed of a differential amplifier (ME-4600 Meilhaus, Germany) and RealView software (Abacom, Germany). During the preparation of the Ag/AgCl electrodes, the silver wire was electrolytically coated with silver chloride. A reference electrode of the same type as the measuring electrodes was placed in the base of the stem. The long-distance SAPs pass by electrodes 1, 2, 3, 4 and the reference, basal electrode; therefore, the reference electrode is a relative reference electrode because they pass through it. The local, apical SAPs pass only by electrodes 1 and 2 and they never reach the reference, basal electrode; hence, the reference electrode in this case is an absolute reference electrode. The frequency of sample recording was 1 Hz. The arrangement of the electrodes on the sunflower is shown in Fig. 1a. The SAP interval, i.e. the time spacing between two subsequent SAPs was calculated.
Experimental variants
The measurements were carried out in three different light conditions: light/dark: 16 h of 25–40 μmol m−2 s−1/8 h darkness, continuous light: 25–40 μmol m−2 s−1, and continuous very low light: 5 μmol m−2 s−1 in PAR range white light (Power Star HQT-T400W/DOSRAM GmbH, Munich, Germany) and a temperature of 24 ± 1 °C. Freely circumnutating sunflowers and those with circumnutation prevented by stem immobilization were investigated.
Data analyses
Ten plants were investigated in each of the variants of the experiments and the results are presented as the mean ± SE. The data were analysed using Statistica ver. 12 software (StatSoft, Inc. 2014). The data set was first tested for normality using Shapiro–Wilk test and homogeneity of variance was assessed with Levene’s test. All data had non-normal distribution and unequal variance; therefore, non-parametric Kruskal–Wallis ANOVA was used. Multiple comparisons of the mean ranks for all groups (pairwise analysis) were used. The level of statistical significance for all tests was set at p < 0.05.
Results
The sunflower plants circumnutated (Appendix S1) in an approx. 2–3 h ultradian rhythm and the CN trajectory had a regular or irregular pattern (Fig. 1b; Appendix S2). Simultaneously, spontaneous electrical changes in a form of a 5–60 mV 1-min-long-lasting SAPs were recorded. A typical graphic example of SAPs transmitted basipetally and acropetally along the sunflower stem is shown in Fig. 1c. The CN length and period as well as the number and transmission direction of SAPs were different in the different light conditions. Vigorous CN or their arrest was observed (Fig. 2) and no SAPs or single acropetally or basipetally transmitted SAPs (Fig. 1c) and even series of APs were recorded. The CN and SAP parameters in light/dark cycles, continuous light (25–40 μmol m−2 s−1) and continuous very low light (5 μmol m−2 s−1) in freely circumnutating and immobilized sunflowers are shown in Table 1 (Supplementary Table 1 and Supplementary Table 2).
Examples of CN in different light conditions in freely circumnutating H. annuus. The plot is an x- or y-coordinate projection on the time course from a top-view recording. a Light/dark: 16 h of 25–40 μmol m−2 s−1/8 h darkness, light-on 04:00 light-off 20:00. b Continuous light: 25–40 μmol m−2 s−1. c Continuous very low light: 5 μmol m−2 s−1
Ultradian and daily rhythm of CN and SAPs in light/dark conditions
In light/dark conditions, the ultradian rhythm and daily CN pattern appeared; an example is shown in Fig. 2a. Small daily and large nocturnal CNs were observed (Fig. 3a). In those plants, approx. 9.8 ± 1 (N = 30) SAPs/day/plant were generated (Table 1) mainly during the dark period, which is shown in Fig. 3a. The number of SAPs emerging during 3-h intervals was calculated, and an evident daily rhythm was revealed. The greatest number of SAPs preceded the maximal-length CNs by approx. 3 h. It was found that the interval between SAPs in the light/dark conditions was on average 121 ± 9 min (n = 266) and was nearing the CN period, which was 143 ± 1 min (n = 293) (Table 1). In the immobilized sunflower, the number of SAPs/day/plant was halved significantly (4.6 ± 0.6 (N = 28) SAPs/day/plant) and the SAPs interval was doubled [237 ± 30 min (n = 118)]. In free CN, similar to the immobilized plants, approx. 60–70% of the SAPs were generated in the upper part of the plant (leaves and stem apex) and approx. 25% in the lower part of the plant. Beside acropetally or basipetally propagated SAPs, occasional acropetal–basipetal SAPs were recorded (10–20%, Table 1). The acropetal–basipetal SAPs originated from some place along the stem and propagated from that place acropetally and basipetally simultaneously, presumably through some phloem vessels or in one direction only, and later in the opposite direction along different vessels that were not excited before. Refractoriness would not allow basipetal transmission followed promptly by acropetal transmission (or vice versa). Propagation velocity of basipetally and acropetally propagated SAPs was approx. 8–19 cm min−1 (Supplementary Table 2). It is known that light-off and light-on evoke CN disturbance (Stolarz et al. 2008) and AP induction (Stankovic et al. 1998; Stahlberg et al. 2006; Król et al. 2010); therefore, to exclude this stimulus and ensure constant environmental conditions, we applied continuous light to study the relation between SAPs and CN.
Comparison of the CN length and number of SAPs in H. annuus. The number of SAPs for 3-day extracellular measurements was calculated at 3-h intervals (12:00–15:00, 15:00–18:00, 18:00–21:00…) in ten plants (column). The CN length is the length of the trajectory of CN, the plot is a moving average of a 3-h window from ten plants (line). a Light/dark: 16 h of 25–40 μmol m−2 s−1/8 h darkness, light-on 04:00 light-off 20:00. b Continuous very low light: 5 μmol m−2 s−1
Vigorous CNs in continuous light but no SAPs
In continuous light (25–40 μmol m−2 s−1), the sunflower circumnutates vigorously in a regular elliptical manner (Fig. 2b; Table 1) but does not generate SAPs. This complete lack of SAPs under continuous light in the presence of vigorous CN indicates that SAP does not play a role in the basic mechanism generating CN and SAPs are not induced by CN at this light intensity. In the immobilized sunflower, SAPs were absent as well. To evoke the SAP phenomenon, continuous very low light was applied in the next experiments.
SAP ultradian rhythm and CN irregularity in continuous very low light conditions
In continuous very low light (5 μmol m−2 s−1), the CNs were weak and irregular as shown in Appendix S3 and Appendix S4 and in Table 1, Figs. 2c, 3b and 4. During the second day, there were many CN disturbances and during the third day CNs were usually arrested. The number of SAPs on average was approx. 4–5 SAPs/24 h/plant during all days of the experiments, even during the third day of the CN arrest. The relation of the CN length vs. the SAP number is shown in Fig. 3b. Examples of an irregular CN trajectory and simultaneous SAP emergence are shown in Fig. 4a–d. Many SAPs emerge during maximum stem bending. The examples of SAP occurrence followed by CN direction changes are shown in Fig. 4e–h and, respectively, in Appendix S5 and Appendix S6. It is noticeable, therefore, that in very low light SAPs accompany the irregular CNs. Another type of stem movement changes is the 2–4-mm stem “torsion” shown in video Appendix S7 and Appendix S8 (marked by a red point) accompanied by SAPs propagating acropetally along the stem. “Torsion” is a sudden twitch against the background of the slow circumnutation movement of the plant.
The CN and appearance of SAPs in H. annuus plants in very low light (5 μmol m−2 s−1). The plots are the x- and y-coordinate projection on the time course (a, c, e, f) or the top view of the CN trajectory (b, d, f, h). Black point marks the moment of SAP appearance. The arrow marks the beginning of CN, dashed line—trajectory of CN after SAP. a–d Examples of weak and irregular CN associated with SAPs passing along the stem motor region. Highly excitable plants are presented with more SAPs than the average SAP number. Examples of circumnutating plants are shown in Appendix S3 and Appendix S4, respectively. e–h Examples of CN direction changes after acropetal SAP passing along the motor region. Examples of circumnutating plants are shown in Appendix S5 and Appendix S6, respectively
It was found that in very low light conditions in a free-CN sunflower the number of SAPs/day/plant was 4.8 ± 0.6 (N = 29) and the interval between all SAPs was on average 259 ± 22 min (n = 120) and these were similar as in the immobilized sunflower (Table 1). In the freely CN plants, two kinds of series of SAPs were observed: ultradian series of long-distance transmitted SAPs and series of local SAPs. The series consisting of 2–7 SAPs with, on average, 159 ± 8 min (n = 75) intervals between them were recorded and named ultradian series of SAPs (Table 2). Examples of the ultradian SAPs are presented in Fig. 5. The intervals between the SAPs were the same as the CN period, i.e. 171 ± 6 min (n = 152) (Table 1). The ultradian SAPs propagated throughout whole stem; they were registered by electrodes 1, 2, 3, 4 and the reference electrode (Fig. 5). In low-light condition, 73% of APs were generated in the upper part of the sunflower (leaves and stem apex) and transmitted basipetally and 20% of APs were generated in the lower part of the plant and transmitted acropetally. Additionally, during the third day of the experiment, series of locally propagated APs appeared in some plants as shown in Fig. 6 and Table 2. They persisted on average for 66 ± 7 min and consisted of 18 ± 2 SAPs (n = 15 series); there was a correlation between the duration of the series and the number of APs (Fig. 7). During the approx. 1-h-long local series, the sunflower generated SAPs at ca. 3–4-min intervals.
Ultradian series of long-distance-propagated SAPs in H. annuus plants in very low light (5 μmol m−2 s−1). Examples of traces from an extracellular electrical recording. Electrode (1, 2, 3, 4) arrangement shown in Fig. 1a. Arrow up—acropetally transmitted SAP, arrow down—basipetally transmitted SAP. a, b Ultradian, long-distance-propagated SAPs, c, d Ultradian, long-distance-propagated SAPs and series of local SAPs. Examples of a local series of SAPs are marked by asterisks
Series of local SAP H. annuus plants emerging during the third day of very low light (5 μmol m−2 s−1). Electrode arrangement shown in Fig. 1a. Arrow up—acropetally transmitted SAP, arrow down—basipetally transmitted SAP
When transferred back to the vegetation room, plants kept for 3 days in the very low light began flowering after 2 months.
Discussion
SAP-associated light/dark sensing
Active movements in plants are a way of adaptation to the changing light conditions in the environment. The examples include, e.g. the widely spread phenomena of tropism and nastic movements as in the pulvinus-driven leaf movement after illumination or darkening in Samanea, Phaseolus, Oxalis, and Desmodium (Moshelion et al. 2002; Moran 2007). H. annuus is a common sun-tracing plant, which moves its stem apex to follow the relative position of the sun (Vandenbrink et al. 2014) and shows an ultradian, daily and circadian rhythm of CN and CN disturbance after illumination and darkening (Buda et al. 2003; Charzewska and Zawadzki 2006; Stolarz et al. 2008). There are reports that AP is a light/dark-guided signal in plants (Król et al. 2010). Lately, in S. lycopersicum plants the highest number of SAPs was observed in the early morning (Macedo et al. 2015). Shade and darkness-induced APs were reported also in H. annuus (Stankovic et al. 1998; Stahlberg et al. 2006). Here we have checked the effect of light on plant spontaneous APs and their relation to CN. The light conditions applied here reflect the conditions present in the natural environment and imitate the effect of prolonged shading or cloudy days on plants. The 3-day-long very low light conditions applied here constituted well-tolerated stress because after the treatment plants underwent typical vegetative and generative development after the return to the vegetation room. It has been shown here using long-lasting simultaneous time lapse and extracellular recordings that stem movements (CN) and spontaneous excitation (SAPs) in sunflower plants are light dependent (Table 1). The CN study has shown a regular elliptical or irregular CN trajectory pattern (Fig. 1b; Appendix S2). Regular ellipses dominated under continuous light while irregularity was observed under light/dark and very low light conditions. The CN irregularity is illustrated in Appendix S3 and Appendix S4 and Fig. 4a–d, respectively. This regular and irregular pattern is related to the absence of SAPs and presence of many SAPs, respectively. The greatest number of SAPs preceded the maximal-length CNs by 3 h (light/dark, Fig. 3a) and many SAPs appeared at the absence of CNs in very low light (Figs. 3b, 4a, c) and in immobilized plants (Table 1). The daily rhythm of the SAPs number in light/dark condition and the lack of SAPs under continuous light (Table 1) indicate that the light conditions influence the number of SAPs. This is also consistent with the opinion that stronger light decreases excitability (Król et al. 2010). The difference in the number of SAPs in different light conditions (Tables 1, 2) should be a suggestion about the information-encoding role of APs in plants.
Spontaneous action potentials
The circumstances of the occurrence of SAPs and their function are still unclear. It was shown here that SAPs appeared singly or in groups forming ultradian series of long-distance SAPs (Fig. 5) and spontaneous approx. 1-h series of local SAPs (Fig. 6) (Table 2). Here we have shown for the first time the slow approx. 2–4-h ultradian rhythm of SAPs, beside the slow ultradian CN rhythm. Additionally, another series of local SAPs (Fig. 6) at 3–4-min intervals was recorded and they resembled those recorded after glutamate injection (Stolarz et al. 2010, 2015). This confirmed the assumption that there are spontaneous series of APs in plants and that an AP frequency code may exist in plants (Favre et al. 1999; Paszewski et al. 1982). In our study, we observed acropetally or basipetally transmitted SAPs (Fig. 1c). It is probable that SAPs are propagated along the vascular bundle similar to stimuli-induced APs (Dziubińska et al. 2001; Dziubinska 2003; Fromm and Lautner 2007; Salvador-Recatala et al. 2014; Hedrich et al. 2016). Propagation velocity was approx. 10–20 cm min−1, typical for sunflower (Zawadzki et al. 1995). Additionally, occasional acropetal–basipetal SAPs were also recorded. They were similar to SAPs described previously by Zawadzki et al. (1995) and APs evoked by glutamate (Stolarz et al. 2010).
SAPs vs. CN
Zawadzki et al. (1995) found and our present study confirmed the SAP phenomenon, whose physiological function in the sunflower is not determined. Herein, CN changes accompanied by SAPs were revealed. A daily rhythm of SAPs and CNs (Fig. 3a) and the ultradian interval of the SAP and CN period (Table 1) were shown. Additionally, the example in Fig. 4 shows that SAPs accompany CN irregularity and a decrease in the CNs and even changes in the CN trajectory direction from cw to ccw or vice versa. The sunflower stem “torsion” associated with acropetal SAP passing throughout the motor region was presented in the films (Appendix S7 and Appendix S8). The AP-associated elongation/contraction in H. annuus (Stankovic et al. 1998) and the growth rate inhibition in Luffa cylindrica (Shiina and Tazawa 1986) reported previously support the suggestion that the ultradian SAPs passing throughout the motor region of the stem could disturb a growth-dependent CN. Simultaneously, experiments with immobilized plants showed that freely circumnutating plants had more SAPs than the immobilized plants. This showed that CN increased the number of SAPs in the sunflower plants. The experiments with inhibited CN (free-CN and immobilized sunflowers in continuous very low light) demonstrate that CNs are not necessary to appearance of SAPs. On the other hand, sunflower immobilization in light/dark conditions halved the number of SAPs (the number was similar as in the free-CN and immobilized sunflower in continuous very low light); thus, CN could increase the number of SAPs. This suggested that in light/dark conditions CN could evoke APs.
The present investigation shows that external conditions can modulate the number of SAPs in H. annuus plants and there is a complex relation between the CN and SAP phenomena and light conditions. The answers to the question if the CN changes can evoke APs and if APs could evoke CN changes (as an effector response) need a future investigation.
Conclusions
In the present work, it has been shown that there is the ultradian SAP rhythm beside the CN rhythm and the number of SAPs and CN trajectory changes depends on light conditions. In continuous light, no SAPs under vigorous regular CN are observed. In continuous very low light conditions, the irregularity of the CN trajectory is accompanied by SAPs. Presented results showed that SAP appearance and CN changes play a role in adaptation to very low light conditions in H. annuus plants. We believe that in future studies SAPs will be found in other plant species and other stress conditions and that the CN changes could be a helpful phenomenon for future investigations of the role of long-distance electrical signals in plant behaviour.
Author contribution statement
MS designed and carried out the experiments, collected and analysed the results, and wrote the manuscript. HD helped in the analysis of the results and editing the manuscript.
Abbreviations
- CN:
-
Circumnutation
- cw:
-
Clockwise
- ccw:
-
Counterclockwise
- SAP:
-
Spontaneous action potential
References
Buda A, Zawadzki T, Krupa M, Stolarz M, Okulski W (2003) Daily and infradian rhythms of circumnutation intensity in Helianthus annuus. Physiol Plant 119:582–589
Charzewska A, Zawadzki T (2006) Circadian modulation of circumnutation length, period, and shape in Helianthus annuus. J Plant Growth Regul 25:324–331
Darwin C, Darwin F (1880) The power of movement in plants. John Murray, London
Dziubinska H (2003) Ways of signal transmission and physiological role of electrical potentials in plants. Acta Soc Bot Polon 72:309–318
Dziubińska H, Trębacz K, Zawadzki T (2001) Transmission route for action potentials and variation potentials in Helianthus annuus L. J Plant Physiol 158:1167–1172
Favre P, Zawadzki T, Dziubinska H, Trebacz K, Greppin H, Degli Agosti R (1999) Repetitive action potentials induced in the liverwort Conocephalum conicum (L.). Arch Sci 52:187–198
Fromm J, Lautner S (2007) Electrical signals and their physiological significance in plants. Plant Cell Environ 30:249–257
Hayashi Y, Nishiyama H, Tanoi K, Ohya T, Nihei N, Tanioka K, Nakanishi TM (2004) An aluminum influence on root circumnutation in dark revealed by a new super-HARP (high-gain avalanche rushing amorphous photoconductor) camera. Plant Cell Physiol 45:351–356
Hedrich R, Salvador-Recatala V, Dreyer I (2016) Electrical wiring and long-distance plant communication. Trends Plant Sci 21:376–387
Krol E, Trebacz K (2000) Ways of ion channel gating in plant cells. Ann Bot 86:449–469
Krol E, Dziubinska H, Stolarz M, Trebacz K (2006) Effects of ion channel inhibitors on cold- and electrically-induced action potentials in Dionaea muscipula. Biol Plant 50:411–416
Krol E, Plachno BJ, Adamec L, Stolarz M, Dziubinska H, Trebacz K (2012) Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann Bot 109:47–64
Król E, Dziubińska H, Trębacz K (2010) What do plants need action potentials for? In: DuBois ML (ed) action potential. Nova Science Publisher, New York, pp 1–28
Kurenda A, Stolarz M, Zdunek A (2015) Electrical potential oscillations—movement relations in circumnutating sunflower stem and effect of ion channel and proton pump inhibitors on circumnutation. Physiol Plant 153:307–317
Macedo FCO, Dziubinska H, Trebacz K, Oliveira RF, Moral RA (2015) Action potentials in abscisic acid-deficient tomato mutant generated spontaneously and evoked by electrical stimulation. Acta Physiol Plant 37:207
Millet B, Badot P (1996) The revolving movement mechanism in Phaseolus; New approaches to old questions. In: Greppin H, Degli Agosti R, Bonzon M (eds) Vistas on Biorhythmicity. University of Geneva, Geneva, pp 77–98
Moran N (2007) Osmoregulation of leaf motor cells. FEBS Lett 581:2337–2347
Moshelion M, Becker D, Czempinski K, Mueller-Roeber B, Attali B, Hedrich R, Moran N (2002) Diurnal and circadian regulation of putative potassium channels in a leaf moving organ. Plant Physiol 128:634–642
Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) Glutamate receptor –like genes mediate leaf-to-leaf wound signalling. Nature 500:422–426. doi:10.1038/nature12478
Paszewski A, Dziubinska H, Trebacz K, Zawadzki T (1982) Electrical activity of the liverwort Conocephalum conicum. Method of investigation and general characteristics of excitation. Physiol Plant 54:83–87
Salvador-Recatala V (2016) The AKT2 potassium channel mediates NaCl induced depolarization in the root of Arabidopsis thaliana. Plant Signal Behav 11(4):e1165381. doi:10.1080/15592324.2016.1165381
Salvador-Recatala V, Tjallingii WF, Farmer EE (2014) Real-time, invivo intracellular recordings of caterpillar-induced depolarization waves in sieve elements using aphid electrodes. New Phytol 203:674–684
Shiina T, Tazawa M (1986) Action potential in Luffa cylindrica and its effects on elongation growth. Plant Cell Physiol 27:1081–1089
Sibaoka T (1991) Rapid plant movements triggered by action potentials. Bot Mag Tokyo 104:73–95
Stahlberg R, Stephens NR, Cleland RE, Van Volkenburgh E (2006) Shade-induced action potentials in Helianthus annuus L. originate primarily from the epicotyl. Plant Signal Behav 1:15–22
Stankovic B, Witters DL, Zawadzki T, Davies E (1998) Action potentials and variation potentials in sunflower: an analysis of their relationships and distinguishing characteristics. Physiol Plant 103:51–58
Stolarz M, Krol E, Dziubinska H, Zawadzki T (2008) Complex relationship between growth and circumnutations in Helianthus annuus stem. Plant Signal Behav 3:376–380
Stolarz M, Krol E, Dziubinska H, Kurenda A (2010) Glutamate induces series of action potentials and a decrease in circumnutation rate in Helianthus annuus. Physiol Plant 138:329–338
Stolarz M, Zuk M, Krol E, Dziubinska H (2014) Circumnutation Tracker: novel software for investigation of circumnutation. Plant Methods 10:24
Stolarz M, Król E, Dziubińska H (2015) Lithium distinguishes between growth and circumnutation and augments glutamate-induced excitation of Helianthus annuus seedlings. Acta Physiol Plant 37:1–9
Vandenbrink JP, Brown EA, Harmer SL, Blackman BK (2014) Turning heads: the biology of solar tracking in sunflower. Plant Sci 224:20–26
Zawadzki T, Dziubinska H, Davies E (1995) Characteristics of action potentials generated spontaneously in Helianthus annuus. Physiol Plant 93:291–297
Acknowledgements
We thank Professor Kazimierz Trębacz for useful discussions and comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Kovacik.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2017_2528_MOESM1_ESM.mp4
Appendix S1 Top and side view of circumnutating Helianthus annuus plants. The film shows an example of 24 h records (MP4 2460 kb)
11738_2017_2528_MOESM2_ESM.mp4
Appendix S2 Top view of regular and irregular circumnutation of the stem of Helianthus annuus plants. Examples of approx. 24-hour records (MP4 2452 kb)
Appendix S3 Example of an effect of continuous very low light (imitation of prolonged shading or cloudy days) on circumnutation of the Helianthus annuus stem. Three-day experiments. The circumnutation was weak and irregular as shown in Fig. 4A and Fig. 4B. Circumnutation strongly decreased on the second and stopped on the third day of very low light. After the experiment, the plants were grown in a vegetation room (light/dark, 70 μmol m−2 s−1) for the next two months and typical vegetative and generative development was observed. Very low light: 5 μmol m−2 s−1 (MP4 4123 kb)
Appendix S4 Example of an effect of continuous very low light (imitation of prolonged shading or cloudy days) on circumnutation of the Helianthus annuus stem. Three-day experiments. The circumnutation was weak and irregular as shown in Fig. 4C and Fig. 4D. Circumnutation strongly decreased on the second and stopped on the third day of very low light. After the experiment, the plants were grown in a vegetation room (light/dark, 70 μmol m−2 s−1) for the next two months and typical vegetative and generative development was observed. Very low light: 5 μmol m−2 s−1 (MP4 4131 kb)
Appendix S5 Example of a circumnutation direction change and decrease in the circumnutation length after acropetal spontaneous action potential transmission along the stem motor region in Helianthus annuus plant. The example is shown in Fig. 4E and Fig. 4F. The acropetal 60 mV spontaneous action potential appeared during stem maximal bending between 2:54 and 2:59 a.m. (red point). The film shows an eight-hour record. The Video example represents 5 similar recordings (MP4 582 kb)
Appendix S6 Example of a circumnutation direction change and decrease in the circumnutation length after acropetal spontaneous action potential transmission along the stem motor region in Helianthus annuus plant. The example is shown in Fig. 4G and Fig. 4H. The acropetal 60 mV spontaneous action potential appeared during stem maximal bending between 1:40 and 1:45 a.m. (red point). The film shows an eight-hour record. The Video example represents 5 similar recordings (MP4 1256 kb)
Appendix S7 Example of stem “torsion” in the Helianthus annuus plant. “Torsion” appeared between 23:55 and 23:56 (red point) and was accompanied by 60 mV acropetal spontaneous action potential transmission along the stem motor region. The film shows one-hour record, one frame per one minute. Light: 5 μmol m−2 s−1. The Video represents 12 similar recordings (MP4 797 kb)
Appendix S8 Example of stem “torsion” in the Helianthus annuus plant. “Torsion” appeared between 7:25 and 7:26 (red point) and was accompanied by 60 mV acropetal spontaneous action potential transmission along the stem motor region. The film shows one-hour record, one frame per one minute. Light: 5 μmol m−2 s−1. The Video represents 12 similar recordings (MP4 829 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stolarz, M., Dziubińska, H. Spontaneous action potentials and circumnutation in Helianthus annuus . Acta Physiol Plant 39, 234 (2017). https://doi.org/10.1007/s11738-017-2528-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2528-0