Abstract
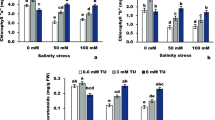
To explore the possible physiological mechanism of salt tolerance in peanut, we investigated the effect of salinity on antioxidant enzyme activity, fatty acid composition, and chlorophyll fluorescence parameters. Seedlings at the initial growth stage had been treated with 0, 100, 150, 200, 250, and 300 mM NaCl for 7 days. Results showed that fresh mass and dry mass decreased with the rise of the NaCl concentration. They decreased significantly when the NaCl concentration was more than 200 mM. The PSII’s highest photochemical efficiency (F v/F m) was not affected before treating 250 mM NaCl. However, the PSII (ΦPSII)’s actual photochemical efficiency of decreased after treating 200 mM NaCl. Both the initial fluorescence (F o) and non-photochemical quenching (NPQ) increased after 200 mM NaCl treatment. PSI oxidoreductive activity (ΔI/I o) was not affected before 200 mM NaCl. The malondialdehyde (MDA) content increased with the rise of the NaCl concentration. The activities of ascorbate peroxidase (APX) and superoxide dismutase (SOD) activities increased first and then decreased, while the content of H2O2 and O −·2 decreased first and then increased. Treated with 150 mM NaCl, the linolenic acid (18:3) and linoleic acid (18:2) of monogalactosyldiacylglycerols (MGDG), digalactosyldiacylglycerols (DGDG), sulphoquinovosyldiacylglycerols (SQDG) as well as phosphatidylglycerols (PG), the ratio of DGDG/MGDG increased, and the opposite results were obtained with 300 mM NaCl. The double bond index (DBI) of MGDG, DGDG, SQDG, and PG also increased after treating 150 mM NaCl. These conclusions verified that increased unsaturated fatty acid content in membrane lipid of peanut leaves could improve salt tolerance by alleviating photoinhibition of PSII and PSI.






Similar content being viewed by others
Abbreviations
- 16:0:
-
Palmitic acid
- 16:1(3t):
-
△3-trans-hexadecenoic
- 18:0:
-
Stearic acid
- 18:1:
-
Oleic acid
- 18:2:
-
Linoleic acid
- 18:3:
-
Linolenic acid
- APX:
-
Ascorbate peroxidase
- DBI:
-
Double bond index
- DGDG:
-
Digalactosyldiacylglycerols
- F o :
-
Initial fluorescence of the dark-adapted state
- F m :
-
Maximum yield of fluorescence
- F s :
-
Steady-state fluorescence
- F v :
-
Variable fluorescence
- F v/F m :
-
Maximal photochemical efficiency of PSII
- F m′:
-
Light-adapted state
- H2O2 :
-
Hydrogen peroxide
- MGDG:
-
Monogalactosyldiacylglycerols
- MDA:
-
Malondialdehyde
- NPQ:
-
Non-photochemical quenching
- O −·2 :
-
Superoxide anion radical
- PVP:
-
Polyvinylpyrrolidon
- PG:
-
Phosphatidylglycerols
- PSI:
-
Photosystem I
- SQDG:
-
Sulphoquinovosyldiacylglycerols
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
- ΦPSII:
-
Actual photochemical efficiency of PSII
- ΔI/I o :
-
PSI oxidoreductive activity
References
Airaki M, Leterrier M, Mateos RM, Valderrama R, Chaki M, Barroso JB, Del Rio LA, Palma JM, Corpas FJ (2012) Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ 35:281–295
Allakhverdiev SI, Kinoshita M, Inaba M, Suzuki I, Murata N (2001) Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol 125:1842–1853
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6:36–41
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Biol 1:601–639
Baker NR (1991) A possible role for photosystem II in environmental perturbations of photosynthesis. Physiol Plant 81:563–570
Block MA, Dorne AJ, Joyard J, Douce R (1983) Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem 258:13281–13286
Chen Z, Xu C, Chen M, Xu L, Wang K, Lin S, Kuang T (1994) Effect of chilling acclimation on thylakoid membrane protein of wheat. Acta Bot Sin 36:423–429
Cooke DT, Burden RS (1990) Lipid modulation of plasma membrane-bound ATPases. Physiol Plant 78:153–159
Demmig-Adams B, Adams WW (1996) The role of xanthophyll cyclecarotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Deuticke B, Haest C (1987) Lipid modulation of transport proteins in vertebrate cell membranes. Ann Rev Physiol 49:221–235
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. NewPhytol 179:945–963
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59(2):309–314
Gigon A, Matos AR, Laffray D, ZuilyFodil Y, Pham Thi AT (2004) Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype Columbia). Ann Bot 94:345–351
Hamed KB, Youssef NB, Ranieri A, Zarrouk M, Abdelly C (2005) Changes in content and fatty acid profiles of total lipids and sulfolipids in the halophyte Crithmummaritimum under salt stress. J Plant Physiol 162:599–602
Hasegawa PM, Bressan RA, Zhu J, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499
Huflejt M, Tremolieres A, Pineau B, Lang J, Hatheway J, Packer L (1990) Changes in membrane lipid composition during saline growth of the freshwater cyanobacterium Synechococcus 6311. Plant Physiol 94:1512–1521
Im YJ, Han O, Chung GC, Cho BH (2002) Antisense expression of an Arabidopsis omega-3 fatty acid desaturase gene reduces salt/drought tolerance in transgenic tobacco plants. Mol Cells 13:264–271
Jimenez A, Hernandez JA, del Río LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114(1):275–284
Khamutov G, Fry IV, Huflejt ME, Packer L (1990) Membrane lipid composition, fluidity, and surface charge changes in response to growth of the freshwater cyanobacterium Synechococcus 6311 under high salinity. Arch Biochem Biophys 277:263–267
Kono M, Noguchi K, Terashima I (2014) Roles of the cyclic electron flow around PSI (CEF-PSI) and O −·2 dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol 55(5):990–1004
Kooten O, Snel JF (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Lin ZF, Li SS, Lin GZ, Sun GZ, Guo JY (1984) Super oxide dismutase activity and lipid peroxidation in relation to senescence of rice leaves. Bull Bot 26:605–615
Liu XY, Li B, Yang JH, Sui N, Yang XM, Meng QW (2008) Overexpression of tomato chloroplast omega-3 fatty acid desaturase gene alleviates the photoinhibition of photosystems 2 and 1 under chilling stress. Photosynthetica 46:185–192
Matos MC, Campos PS, Ramalho JC, Medeira MC, Maia MI, SemedoJM Marques NM, Matos A (2002) Photosynthetic activity and cellular integrity of the Andean legume Pachyrhizus ahipa (Wedd.) Parodi under heat and water stress. Photosynthetica 40:493–501
Mikami K, Murata N (2003) Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog Lipid Res 42:527–543
Minoda A, Sonoike K, Okada K, Sato N, Tsuzuki M (2003) Decrease in the efficiency of the electron donation to tyrosine Z of photosystem II in an SQDG-deficient mutant of Chlamydomonas. FEBS Lett 553(1–2):109–112
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot 55:1105–1113
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279
Pick U, Gounaris K, Weiss M, Barber J (1985) Tightly bound sulpholipids in chloroplast CF0–CF1. BBA-Bioenergetics 808(3):415–420
Qin LQ, Li L, Bi C, Zhang YL, Wan SB, Meng JJ, Meng QW, Li XG (2011) Damaging mechanisms of chillingand salt stress to Arachis hypogaea L. leaves. Photosynthetica 49:37–42
Ramani B, Zorn H, Papenbrock J (2004) Quantification and fatty acid profiles of sulfolipids in two halophytes and a glycophyte grown under different salt concentrations. Z Naturforsch C 59:835–842
Ritter D, Yopp JH (1993) Plasma membrane lipid composition of the halophilic cyanobacterium Aphanothece halophytica. Arch Microbiol 159:435–439
Sairam R, Srivastava G (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Schansker G, Srivastava A, Strasser RJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30:785–796
Schuler I, Milon A, Nakatani Y, Ourisson G, Albrecht AM, Benveniste P, Hartman MA (1991) Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc Natl Acad Sci 88:6926–6930
Siegenthaler PA, Eichenberger W (1984) Structure, function, and metabolism of plant lipids. In: Proceedings of the 6th international symposium on the structure, function, and metabolism of plant lipids held in Neuchâtel, vol 9. Elsevier Science Publishers, Switzerland, pp 16–20
Song XS, Hu WH, Mao WH, Ogweno JO, Zhou YH, Yu JQ (2005) Response of ascorbate peroxidase isoenzymes and ascorbate regeneration system to abiotic stresses in Cucumis sativus L. Plant PhysiolBiochem 43:1082–1088
Stepien P, Klobus G (2005) Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol Plant 125:31–40
Sui N, Han GL (2014) Salt-induced photoinhibition of PSII is alleviated in halophyte Thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol Plant 36:983–992
Sui N, Li M, Liu XY, Wang N, Fang W, Meng QW (2007a) Response of xanthophyll cycle and chloroplastic antioxidant enzymes to chilling stress in tomato over-expressing glycerol-3-phosphate acyltransferase gene. Photosynthetica 45:447–454
Sui N, Li M, Shu DF, Zhao SJ, Meng QW (2007b) Antisensemediated depletion of tomato chloroplast glycerol-3-phosphate acyltransferase affects male fertility and increases thermal tolerance. Physiol Plant 130:301–314
Sui N, Li M, Zhao SJ, Li F, Liang H, Meng QW (2007c) Overexpression of glycerol-3-phosphate acyltransferase gene improves chilling tolerance in tomato. Planta 226:1097–1108
Sui N, Li M, Li K, Song J, Wang BS (2010) Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica 48:623–629
Takagi D, Takumi S, Hashiguchi M, Sejima T, Miyake C (2016) Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol 171:1626
Wang AG, Luo GH (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Comm 6:55–57
Wicke B, Sikkema R, Dornburg V, Faaij A (2011) Exploring land use changes and the role of palm oil production in Indonesia and Malaysia. Land Use Policy 28:193–206
Xu Y, Siegenthaler PA (1997) Low temperature treatments induce an increase in the relative content of both linolenic and λ3-hexadecenoic acids in thylakoid membrane phosphatidylglycerol of squash cotyledons. Plant Cell Physiol 38:611–618
Xu DQ, Wu S (1996) Three phases of dark-recovery course from photoinhibition resolved by the chlorophyll fluorescence analysis in soybean leaves under field conditions. Photosynthetica 32:417–423
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Zhang M, Barg R, Yin M, GuetaDahan Y, LeikinFrenkel A, Salts Y, Shabtai S, Ben Hayyim G (2005) Modulated fatty acid desaturation via overexpression of two distinct ω-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J 44:361–371
Acknowledgements
We are grateful for financial support from the NSFC (National Natural Science Research Foundation of China) (31300205), Natural Science Research Foundation of Shandong (ZR2016JL028, ZR2013CQ009), the Science and Technology Development Projects of Shandong Province (2014GNC113005), the Opening Foundation of the State Key Laboratory of Crop Biology, China (2015KF01), the Opening Foundation of Shandong Provincial Key Laboratory of Crop Genetic Improvement, Ecology and Physiology and the Program For Scientific research innovation team in colleges and universities of Shandong Province.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by O. Ferrarese-Filho.
Rights and permissions
About this article
Cite this article
Liu, S., Wang, W., Li, M. et al. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol Plant 39, 207 (2017). https://doi.org/10.1007/s11738-017-2501-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2501-y