Abstract
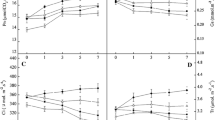
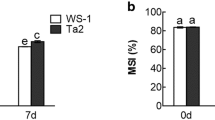
Over-expression of chloroplastic glycerol-3-phosphate acyltransferase gene (LeGPAT) increased unsaturated fatty acid contents in phosphatidylglycerol (PG) of thylakoid membrane in tomato. The effect of this increase on the xanthophyll cycle and chloroplast antioxidant enzymes was examined by comparing wild type (WT) tomato with the transgenic (TG) lines at chilling temperature (4 °C) under low irradiance (100 µmol m−2 s−1). Net photosynthetic rate and the maximal photochemical efficiency of photosystem (PS) 2 (Fv/Fm) in TG plants decreased more slowly during chilling stress and Fv/Fm recovered faster than that in WT plants under optimal conditions. The oxidizable P700 in both WT and TG plants decreased during chilling stress under low irradiance, but recovered faster in TG plants than in the WT ones. During chilling stress, non-photochemical quenching (NPQ) and the de-epoxidized ratio of xanthophyll cycle in WT plants were lower than those of TG tomatoes. The higher activities of superoxide dismutase (SOD) and ascorbate peroxidase (APX) in TG plants resulted in the reduction of O2 −· and H2O2 contents during chilling stress. Hence the increase in content of unsaturated fatty acids in PG by the over-expression of LeGPAT could alleviate photoinhibition of PS2 and PS1 by improving the de-epoxidized ratio of xanthophyll cycle and activities of SOD and APX in chloroplast.
Similar content being viewed by others
Abbreviations
- A:
-
antheraxanthin
- APX:
-
ascorbate peroxidase (EC 1.11.1.11)
- AsA:
-
ascorbate acid
- Fv/Fm :
-
maximal photochemical efficiency of PS2
- F0 :
-
initial fluorescence
- Fv :
-
variable fluorescence
- Fm :
-
maximum yield of fluorescence
- GPAT:
-
glycerol-3-phosphate acyltransferase
- LeGPAT:
-
Lycopersicon esculentum glycerol-3-phosphate acyltransferase gene
- H2O2 :
-
hydrogen peroxide
- NPQ:
-
non-photochemical quenching
- O2 −· :
-
superoxide radical
- PBS:
-
phosphate buffer solution
- PFD:
-
photon flux density
- PG:
-
phosphatidylglycerol
- P N :
-
net photosynthetic rate
- PS:
-
photosystem
- P700:
-
PS1 reaction centre
- RC:
-
reaction centre
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase (EC 1.15.1.1)
- TG:
-
transgenic
- V:
-
violaxanthin
- WT:
-
wild type
- Z:
-
zeaxanthin
References
Allen, D.J., Ort, D.R.: Impacts of chilling temperatures on photosynthesis in warm-climate plants.-Trends Plant Sci. 6: 36–42, 2001.
Apel, K., Hirt, H.: Reactive oxygen species: Metabolism, oxidative stress, and signal transduction.-Annu. Rev. Plant Biol. 55: 373–399, 2004.
Aro, E.-M., Virgin, I., Andersson, B.: Photoinhibition of Photosystem II. Inactivation, protein damage and turnover.-Biochim. biophys. Acta 1143: 113–134, 1993.
Asada, K.: Ascorbate peroxidase: a hydrogen peroxide-scavenging enzyme in plants.-Physiol. Plant. 85: 235–241, 1992.
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.-Anal. Biochem. 72: 248–254, 1976.
Chen, Z.Q., Xu, C.H., Chen, M.J., Xu, L., Wang, K.F., Lin, S.Q., Kuang, T.Y.: Effect of chilling acclimation on thylakoid membrane protein of wheat.-Acta bot. sin. 36: 423–429, 1994.
Demmig-Adams, B., Adams, W.W., III: The role of xanthophyll cycle carotenoids in the protection of photosynthesis.-Trends Plant Sci. 1: 21–26, 1996.
Domonkos, I., Malec, P., Sallai, A., Kovacs, L., Itoh, K., Shen, G., Ughy, B., Bogos, B., Sakurai, I., Kis, M., Strzalka, K., Wada, H., Itoh, S., Farkas, T., Gombos, Z.: Phosphatidylglycerol is essential for oligomerization of photosystem I reaction center.-Plant Physiol. 134: 1471–1478, 2004.
Foyer, C.H., Halliwell, B.: The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism.-Planta 133: 21–25, 1976.
Foyer, C.H., Lelandais, M., Kunert, K.J.: Photooxidative stress in plants.-Physiol. Plant. 92: 696–717, 1994.
Giannopolitis, C.N., Ries, S.K.: Superoxide dismutases. I. Occurrence in higher plants.-Plant Physiol. 59: 309–314, 1977.
Gray, G.R., Savitch, L.V., Ivanov, A.G., Huner, N.P.A.: Photosystem II excitation pressure and development of resistance to photoinhibition.-Plant Physiol. 110: 61–71, 1996.
Hagio, M., Sakurai, I., Sato, S., Kato, T., Tabata, S., Wada, H.: Phosphatidylglycerol is essential for the development of thylakoid membranes in Arabidopsis thaliana.-Plant Cell Physiol. 43: 1456–1464, 2002.
Havaux, M., Davaud, A.: Photoinhibition of photosynthesis in chilled potato leaves is not correlated with loss of Photosystem-II activity. Preferential inactivation of Photosystem I.-Photosynth. Res. 40: 75–92, 1994.
Hieber, A.D., Kawabata, O., Yamamoto, H.Y.: Significance of the lipid phase in the dynamics and functions of the xanthophyll cycle as revealed by PsbS overexpression in tobacco and in-vitro de-epoxidation in monogalactosyldiacylglycerol micelles.-Plant Cell Physiol. 45: 92–102, 2004.
Holsters, M., De Waele, D., Depicker, A.: Transfection and transformation of Agrobacterium tumefaciens.-Mol. gen. Genet. 163: 181–187, 1978.
Horsch, R.B., Fry, J.E., Hoffmann, N.L., Eichholtz, D., Rogers, S.G., Fraley, R.T.: A simple and general method for transferring gene into plants.-Science 227: 1229–1231, 1985.
Horton, P., Ruban, A., Walters, R.G.: Regulation of light harvesting in green plants. Indication by nonphotochemical quenching of chlorophyll fluorescence.-Plant Physiol. 106: 415–420, 1994.
Huner, N.P.A., Öquist, G., Sarhan, F.: Energy balance and acclimation to light and cold.-Trends Plant Sci. 3: 224–230, 1998.
Inoue, K., Fujii, T., Yokoyama, E., Matsuura, K., Hiyama, T., Sakurai, H.: The photoinhibition site of photosystem I in isolated chloroplasts under extremely reducing conditions.-Plant Cell Physiol. 30: 65–71, 1989.
Jakob, B., Heber, U.: Photoproduction and detoxification of hydroxyl radicals in chloroplasts and leaves and relation to photoinactivation of photosystem I and II.-Plant Cell Physiol. 37: 629–635, 1996.
Jimenez, A., Hernandez, J.A., del Rio, L.A., Sevilla, F.: Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves.-Plant Physiol. 114: 272–284, 1997.
Kratsch, H.A., Wise, R.R.: The ultrastructure of chilling stress.-Plant Cell Environ. 23: 337–350, 2000.
Li, X.-G., Bi, Y.-P., Zhao, S.-J., Meng, Q.-W., Zou, Q., He, Q.-W.: Cooperation of xanthophyll cycle with water-water cycle in the protection of photosystems 1 and 2 against inactivation during chilling stress under low irradiance.-Photosynthetica 43: 261–266, 2005.
Li, X.G., Duan, W., Meng, Q.W., Zou, Q., Zhao, S.J.: The function of chloroplastic NAD(P)H dehydrogenase in tobacco during chilling stress under low irradiance.-Plant Cell Physiol. 45: 103–108, 2004a.
Li, X.-G., Meng, Q.-W., Jiang, G.-Q., Zou, Q.: The susceptibility of cucumber and sweet pepper to chilling under low irradiance is related to energy dissipation and water-water cycle.-Photosynthetica 41: 259–265, 2003.
Li, X.-G., Wang, X.-M., Meng, Q.-W., Zou, Q.: Factors limiting photosynthetic recovery in sweet pepper leaves after shortterm chilling stress under low irradiance.-Photosynthetica 42: 257–262, 2004b.
Lyons, J.M., Raison, J.K.: A temperature-induced transition in mitochondrial oxidation: contrasts between cold and warmblooded animals.-Comp. Biochem. Physiol. 37: 405–411, 1970.
Müller, P., Li, X.P., Niyogi, K.K.: Non-photochemical quenching. A response to excess light energy.-Plant Physiol. 125: 1558–1566, 2001.
Murata, N., Los, D.A.: Membrane fluidity and temperature perception.-Plant Physiol. 115: 875–879, 1997.
Nishida, I., Murata, N.: Chilling sensitivity in plants and cyanobacteria: The crucial contribution of membrane lipids.-Plant mol. Biol. 47: 541–568, 1996.
Niyogi, K.K.: Photoprotection revisited: Genetic and molecular approaches.-Annu. Rev. Plant Physiol. Plant mol. Biol. 50: 333–359, 1999.
Noctor, G., Foyer, C.H.: Ascorbate and glutathione: Keeping active oxygen under control.-Annu. Rev. Plant Physiol. Plant mol. Biol. 49: 249–279, 1998.
Orvar, B.L., Sangwan, V., Omann, F., Dhindsa, R.S.: Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity.-Plant J. 23: 785–794, 2000.
Osmond, C.B., Grace, S.C.: Perspectives on photoinhibition and photorespiration in the field: Quintessential inefficiencies of the light and dark reactions of photosynthesis?-J. exp. Bot. 46: 1351–1362, 1995.
Pang, C.H., Zhang, S.J., Gong, Z.Z., Wang, B.S.: NaCl treatment markedly enhances H2O2-scavenging system in leaves of halophyte Suaeda salsa.-Physiol. Plant. 125: 490–499, 2005.
Robinson, S.P., Downton, W.J.S., Millhouse, J.A.: Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach.-Plant Physiol. 73: 238–242, 1983.
Roughan, P.G., Slack, C.R.: Cellular organization of glycerolipid metabolism.-Annu. Rev. Plant Physiol. 33: 97–132, 1982.
Sairam, P.K., Srivastava, G.C.: Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress.-Plant Sci. 162: 897–904, 2002.
Savitch, L.V., Massacci, A., Gray, G.R., Huner, N.P.A.: Acclimation to low temperature or high light mitigates sensitivity to photoinhibition: roles of the Calvin cycle and the Mehler reaction.-Aust. J. Plant Physiol. 27: 253–264, 2000.
Schansker, G., Srivastava, A., Govindjee, Strasser, R.J.: Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves.-Funct. Plant Biol. 30: 785–96, 2003.
Schreiber, U., Bilger, W., Neubauer, C.: Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis.-In: Schulze, E.-D., Caldwell, M.M. (ed.): Ecophysiology of Photosynthesis. Pp. 49–70. Springer-Verlag, Berlin 1994.
Siegenthaler, P.A., Eichenberger, W.: Structure, function and metabolism of plant lipids.-Pp. 485–488. Elsevier Science Publishers, Amsterdam 1984.
Somerville, C.: Direct tests of the role of membrane lipid composition in low-temperature-induced photoinhibition and chilling sensitivity in plants and cyanobacteria.-Proc. nat. Acad. Sci. USA 92: 6215–6218, 1995.
Somerville, C., Browse, J.: Plant lipids: metabolism, mutants and membranes.-Science 252: 80–87, 1991.
Song, X.S., Hu, W.H., Mao, W.H., Ogweno, J.O., Zhou, Y.H., Yu, J.Q.: Response of ascorbate peroxidase isoenzymes and ascorbate regeneration system to abiotic stresses in Cucumis sativus L.-Plant Physiol. Biochem. 43: 1082–1088, 2005.
Sonoike, K.: Photoinhibition of photosystem I: its physiological significance in the chilling sensitivity of plants.-Plant Cell Physiol. 37: 239–247, 1996.
Sonoike, K., Kamo, M., Hihara, Y., Hiyama, T., Enami, I.: The mechanism of the degradation of psaB gene product, one of the photosynthetic reaction center subunits of Photosystem I, upon photoinhibition.-Photosynth. Res. 53: 55–63, 1997.
Sonoike, K., Terashima, I., Iwaki, M., Itoh, S.: Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures.-FEBS Lett. 362: 235–238, 1995.
Sung, D.Y., Kaplan, F., Lee, K.J., Guy, C.L.: Acquired tolerance to temperature extremes.-Trends Plant Sci. 8: 179–187, 2003.
Terashima, I., Funayama, S., Sonoike, K.: The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II.-Planta 193: 300–306, 1994.
Tjus, S.E., Möller, B.L., Scheller, H.V.: Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures.-Plant Physiol. 116: 755–764, 1998.
van Kooten, O., Snel, J.F.H.: The use of chlorophyll fluorescence nomenclature in plant stress physiology.-Photosynth. Res. 25: 147–150, 1990.
Wada, H., Murata, N.: Membrane lipids in cyanobacteria.-In: Siegenthaler, P.-A., Murata, N. (ed.): Lipids in Photosynthesis: Structure, Function and Genetics. Pp. 65–81. Kluwer Academic Publ., Dordrecht-Boston-London 1998.
Wang, A.G., Luo, G.H.: Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants.-Plant Physiol. Commun. 6: 55–57, 1990.
Xu, C.C., Jeon, Y.A., Lee, C.H.: Relative contributions of photochemical and non-photochemical routes to excitation energy dissipation in rice and barley illuminated at a chilling temperature.-Physiol. Plant. 107: 447–453, 1999.
Xu, C., Hartel, H., Wada, H., Hagio, M., Yu, B., Eakin, C., Benning, C.: The pgp1 mutant locus of Arabidopsis encodes a phosphatidylglycerolphosphate synthase with impaired activity.-Plant Physiol. 129: 594–604, 2002.
Xu, Y.N., Siegenthaler, P.A.: Low temperature treatments induce and increase in the relative content of both linolenic and 3-trans-hexadecenoic acids in thylakoid membrane phosphatidylglycerol of squash cotyledons.-Plant Cell Physiol. 38: 611–618, 1997.
Yamamoto, H.Y.: Functional roles of the major chloroplast lipids in the violaxanthin cycle.-Planta 224: 719–724, 2006.
Yamamoto, H.Y., Bangham, A.D.: Carotenoid organization in membranes. Thermal transition and spectral properties of carotenoid-containing liposomes.-Biochim. biophys. Acta 507: 119–129, 1978.
Yamamoto, H.Y., Chenchin, E.E., Yamada, D.K.: Effect of chloroplast lipids on violaxanthin de-epoxidase activity.-In: Avron, M. (ed.): Proceedings of the Third International Congress on Photosynthesis. Vol. III. Pp. 1999–2006. Elsevier, Amsterdam-Oxford-New York 1974.
Yu, C.W., Murphy, T.M., Sung, W.W., Lin, C.H.: H2O2 treatment induces glutathione accumulation and chilling tolerance in mung bean.-Funct. Plant Biol. 29: 1081–1087, 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sui, N., Li, M., Liu, XY. et al. Response of xanthophyll cycle and chloroplastic antioxidant enzymes to chilling stress in tomato over-expressing glycerol-3-phosphate acyltransferase gene. Photosynthetica 45, 447–454 (2007). https://doi.org/10.1007/s11099-007-0074-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11099-007-0074-5