Abstract
Effect of Cu (0.1, 1, 10, and 100 μM) on the regeneration of carrot (Daucus carota L.) androgenic embryos of var. Feria and 1014 breeding line as well as on polyamines (PAs), proline contents, lipid peroxidation and Cu accumulation after 16 and 24 weeks was studied. Generally, growth of Feria rosettes was better than that of the 1014 line. Significant increase in Cu content in tissues was observed in both cultures grown at the highest Cu concentration (100 μM). The dose-dependent increase in proline in the 16-week-old culture of Feria was observed, while in 1014 its level increased only at the highest applied Cu concentration. On the contrary, in the 24-week-old culture, significant increase in the proline content were observed at 100 and 10 μM Cu in Feria and in 1014 breeding lines, respectively. The decline in proline content and decrease in embryogenic ability in the line 1014 grown in the presence of the highest Cu concentration for 24 weeks may indicate that a certain threshold of intracellular Cu was crossed. Both in Feria and 1014 line, putrescine and spermidine were the most abundant free PAs. The increased content of proline and higher contents of the constitutive free putrescine and spermidine in Feria cultivated for 24 weeks at the highest Cu concentration point to better protection of this cultivar. Thus, it seems that the higher tolerance of Feria to oxidative stress (characterized by lower thiobarbituric acid reactive substances value) may result from higher constitutive level of PAs. These data confirm the suggestion that variations in PA levels depend not only on the concentrations of metals tested, but also on plant species and cultivars. The role of PAs and proline in the carrot cultures treated with Cu is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased heavy metal concentration in the soils, up to toxic levels, has become an important environmental problem. They result from human activities such as industry, agriculture, and mining. Metals such as Ni, Cu, Zn, Cd, Pb, and Mn exhibit various phytotoxic effects; including reduction of growth, photosynthesis and chlorophyll content, inhibition of enzyme activities, and damage of chloroplasts and mitochondria (Maksymiec 2007). Various hypotheses have been proposed to explain the survival of plants in metal-contaminated environments, including the exclusion or avoidance of metal uptake as well as their compartmentalization.
Copper (Cu) is an essential microelement for growth and plant development; however, in higher doses it can become very toxic and disturb basic physiological processes such as photosynthesis and mitochondrial electron transport, nitrogen assimilation, cell wall metabolism and many others (Maksymiec 1997). This metal at higher concentrations induces oxidative stress due to overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which was observed in different plant species. On the other hand, it is suggested that ROS/RNS can serve as signal molecules for acclimation to environmental stresses such as those caused by heavy metals (Zhang et al. 2008).
Superoxide radicals are the most abundant ROS in the course of cellular metabolism. Their dismutation increases the amount of H2O2 rapidly converted to OH·, the most dangerous oxygen form that damages important cell compounds, such as nucleic acids, lipids, proteins, and others (Fernandes and Henriques 1991). One of the first symptoms of oxidative stress in plant tissues is lipid peroxidation of cell membranes. Plants possess weapon to protect themselves against heavy metals through various antioxidative strategies, e.g., by increasing antioxidative enzyme activities or by higher production of low molecular compounds such as proline, phenolic compounds, PAs, tocopherols, etc.
Proline (compatible osmolyte) that accumulates in response to various abiotic stresses, e.g., non-optimal temperatures, heavy metals, wounding (Hare and Cress 1997; Sharma and Dietz 2006), contributes to osmotic adjustment in cells, but it has been shown that this amino acid can be an effective antioxidant, electron sink, stabilizer of macromolecules, as well as a cell wall component (Matysik et al. 2002). Also, PAs play an important role in protecting plants against biotic and abiotic stresses (Bouchereau et al. 1999), and enhancement of their metabolism is one of the responses to heavy metal stress. The heavy metal-induced changes of the endogenous PAs have been reported in various plants (Wang et al. 2007); however, a specific role of these compounds is unknown. They may reduce the oxidative damage by increasing the activities of antioxidant enzymes (Zhang et al. 2009) and they have also been suggested to function as metal chelators (Lovaas 1996) and/or as direct or indirect free radical scavengers (Ha et al. 1998).
PAs, low molecular polycationic compounds that are found in all organisms, play a role in a number of biochemical and physiological processes related to plant growth and development (Bouchereau et al. 1999; Baron and Stasolla 2008). In plants, PAs occur in a free form, but can also be bound to phenolic acids, mostly hydroxycinnamic acids (soluble conjugated PAs), as well as to high molecular compounds, e.g., hemicelluloses and lignins or proteins (insoluble conjugated PAs).
Carrot is one of the most widely cultivated root vegetables in the world, as well as one of the dominant plant species growing in Cu mine areas (Ke et al. 2007), and thus it is an object of intensive research to obtain new cultivars. The haploid technique has become a common tool to speed up breeding of many modern plant cultivars. Mutation and transformation of the androgenetic plant material cultured in the medium supplemented with particular toxins including heavy metals lead to the selection of resistant forms in a relatively short time (Henderson and Pauls 1992). Among the factors reported to affect haploid induction in an anther carrot culture, genotype as well as individual donor plants are among the most important (Górecka et al. 2005).
Although plants have evolved a variety of mechanisms for metal tolerance, species and cultivars vary widely in this respect, and closely related genotypes can be valuable tools in studying the mechanisms of toxicity/tolerance. Little information is available on the Cu accumulation capacity and the Cu resistance mechanism of Daucus carota. Therefore, this study was undertaken to test the responses of the plant material regenerated from the androgenic embryos obtained from the anther culture of carrot (D. carota L.) var. Feria and the 1014 breeding line cultivated in the presence of different Cu ion concentrations. The aims were to reveal changes in (1) growth parameters, (2) Cu accumulation, (3) contents of free PAs as well as the stress markers—lipid peroxidation and endogenous proline content in the carrot rosettes obtained from anther culture.
Materials and methods
Plant materials
Donor plants of D. carota L., which were used for culture, developed from the roots kept in a greenhouse at about 20°C. Detailed description of the anther culture procedure was previously provided by Górecka et al. (2005). The anther cultures were kept in darkness at the temperature of 27°C. After emergence of the embryos, the cultures were transferred to continuous light and the temperature was kept the same. When the embryos become green, they were transferred onto the regeneration B5 medium.
B5 medium (Gamborg et al. 1968) with 20 g dm−3 sucrose, 6.5 g dm−3 agar and without amino acids and growth regulators was supplemented with Cu, in the form of CuSO4·5H2O at concentrations of 1, 10, and 100 μM. The control medium contained 0.1 μM of Cu and its pH was set at 5.6. The embryos were incubated under light (30 μmol m−2 s−1, 20°C, photoperiod 16/8) for 16 and 24 weeks.
Extraction and determination of polyamines
The carrot tissue was ground in liquid nitrogen and extracted overnight at 4°C with 5% (v/v) perchloric acid (PCA) per 100 mg fresh weight tissue. 1,7-Diaminoheptane was added as an internal standard and the extracts were centrifuged at 21,000×g for 15 min. PCA-soluble free PAs were determined in one-half volume of the supernatant. The remaining supernatant and pellet were acid hydrolyzed in 6M HCl for 18h at 110°C to obtain PCA-soluble and PCA-insoluble conjugates of PAs as described by Slocum et al. (1989). The standards (Sigma-Aldrich, Prague, Czech Republic) the PCA-soluble free PAs, and the acid hydrolyzed PA conjugates were benzoylated according to the method of Slocum et al. (1989), and the resulting benzoyl-amines were analyzed by HPLC using a Beckman chromatographic system equipped with a 125S Gradient Solvent Delivery Module, 507 Variable Mode Injection Autosampler and 168 Diode Array Detector (Beckman Instruments, Inc., Fullerton, CA, USA). A Gold Nouveau software data system was used to collect, integrate and analyze the chromatographic data. A C18 column (Phenomenex Aqua, 5 µm, 125A, 250 × 4.6 mm, Phenomenex, Utrecht, NL) was used for the separation of polyamines. The analyzed samples (5 or 10 μl) were injected for each run. Elution was carried out at a flow rate of 0.4 ml min−1 at 45°C. The mobile phase consisted of solvent A (10% v/v methanol) and solvent B (80% v/v methanol). The gradient program (expressed as percentages of solvent A) was as follows: 0–10 min, 45% to 0%; 10–30 min, isocratic 0%; 30–40 min, 0% to 45%. The column was washed with 45% solvent A for 30 min between samples. Eluted polyamines were detected with a UV detector at 254 nm by comparing their retention time values with those of the standards (Sigma-Aldrich, Prague, Czech Republic).
Lipid peroxidation
Lipid peroxidation in carrot rosettes was evaluated by spectrophotometric measurements of thiobarbituric acid reactive substances (TBARS) levels, inter alia malondialdehyde (MDA), according to the modified Heath and Packer (1968) method. Lyophilized tissue (45 mg) was homogenized in 5 ml of 1% trichloroacetic acid (TCA). The homogenate was centrifuged at 15,000×g for 15 min at 4°C. To 1 ml of the aliquot of the supernatant, 4 ml of 0.5% thiobarbituric acid (TBA) in 20% TCA was added. The mixture was heated at 95°C for 30 min in a water bath and then cooled in an ice bath. After centrifugation, the absorbance was measured at 532 nm with a UV/Vis spectrophotometer (Hitachi U-2001, Hitachi Instruments Inc, Japan). The value for non-specific absorption at 600 nm was subtracted. The TBARS content was calculated according to MDA extinction coefficient of 155 mM−1 cm−1 and expressed as micromole of MDA per gram of DW. The results are the mean values of two independent experiments in two replications ± SD.
Endogenous proline determination
The proline content was estimated by the method of Bates et al. (1973). Lyophilized plant material (45 mg) was homogenized in 4 ml of 3% aqueous sulfosalicylic acid and the homogenate was centrifuged at 15,000×g for 15 min at 4°C. The supernatant was used for the estimation of proline content. The reaction mixture consisted of 1 ml of supernatant, 1 ml of glacial acetic acid and 1 ml of acid ninhydrin, which was boiled at 100°C for 1 h. After termination of the reaction in an ice bath, the reaction mixture was extracted with 3 ml of toluene and absorbance was measured at 520 nm. The results were expressed as milligrams of proline per gram of DW.
Analyses of copper content
Lyophilized carrot tissue was placed in Teflon vessels and 5 ml of 65% nitric acid and 1 ml of 30% H2O2 was added. The samples were digested in a closed system by Milestone Inc. microwave oven model Ethos-1 (Milestone, USA) at 200°C for 20 min (Borowski 2003). The solutions after digestion were analyzed for Cu content with Perkin-Elmer ICP sequential spectrometer model Optima 2000 DV (Perkin-Elmer, Norwalk, CT, USA). The element was detected at the 327.393-nm wavelength. The Merck ICP multi-element standard solution (Merck, Darmstadt, Germany) was used to prepare the calibration curve. The results are the mean values of three measurements ± SD.
Statistical analyses
Statistical tests were analyzed using the Student’s t test distribution criteria.
Results and discussion
Effect of Cu on growth of carrot cultures
Usually, plant tissue culture media contain low concentrations of Cu and the adjustment of Cu level appears to be a key point in promoting plant regeneration (Dahleen 1995), but it depends on the type of in vitro culture and the cultivars tested. Some Cu concentrations have a beneficial effect on anther cultures, improving both quantitative and qualitative yield of androgenesis (Wojnarowicz et al. 2002). Table 1 presents the number of rosettes regenerated from embryos obtained in the anther carrot culture of var. Feria and the 1014 breeding line analyzed after 16 and 24 weeks of cultivation on the medium containing 0.1 (control), 1, 10, and 100 μM Cu. Growth of the var. Feria rosettes was better than that of the 1014 line, both after 16 and 24 weeks of cultivation. The only exception was the number of rosettes of the 1014 line treated with 10 μM Cu after 24 weeks of cultivation, which was 2.4 times higher than that in var. Feria. The highest Cu concentration (100 μM) decreased the organogenic ability of embryos in both cultures, but was stronger in the 1014 line than in var. Feria especially after 16 weeks of cultivation (Table 1). These results agree partially with the observations of Gori et al. (1998), who found that lower Cu concentration (0.1 and 50 μM) did not inhibit Nicotiana tabacum callus growth, while higher concentrations (100–200 μM) were toxic for this culture. However, the 10 μM concentration of this metal stimulated morphogenetic processes during micropropagation of Dendrobium kingianum Bidwill (Prażak 2000) and was optimal for Hordeum vulgare L. androgenesis (Caredda et al. 2000). The results presented above do not agree with our earlier study where Cu at 1 and 10 μM caused strong growth reduction of the carrot culture var. Narbonne (Górecka et al. 2007) similarly to radish seedlings (Chen et al. 2002). Thus, it seems that these contrasting effects of Cu on carrot regeneration may be due to genotypic differences of individual carrot plants, which were used to establish androgenic cultures (Ke et al. 2007).
Effect of Cu on endogenous copper content and lipid peroxidation
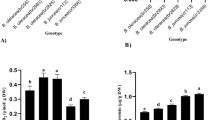
When the carrot rosettes were grown at the highest Cu concentration (100 μM), significant increase in Cu content in tissues was observed both in var. Feria and the 1014 cultures (Fig. 1a, b). The Cu level in the rosettes growing at the highest Cu concentration was higher after 24 weeks than after 16 weeks in var. Feria (Fig. 1a). The opposite trend was observed in the 1014 line where 24-week exposure to 100 μM Cu caused slight statistically insignificant decrease in Cu accumulation in comparison to the culture after 16 weeks of cultivation (Fig. 1b).
Effect of Cu2+ on TBARS contents and accumulation of Cu in the plant material regenerated from the embryos obtained in the anther culture of two carrot varieties (Daucus carota L.): var. Feria (a) and 1014 breeding line (b) after 16 and 24 weeks of cultivation on B5 medium (Gamborg et al. 1968). Control 0.1 μM of Cu2+. The symbols (*, **, ***) show that results obtained from Cu2+-treated carrot cultures are significantly different from controls (0.1 μM) at 0.05 and 0.01 level of Student’s t test. Bars represent standard deviations
The lipid peroxidation, evaluated as TBARS level, was much higher in the rosettes of the 1014 line than of var. Feria cultivated on the control medium. The highest Cu concentration (100 μM) enhanced Cu accumulation in the rosettes of var. Feria, but an expected massive increase in the TBARS level in both 16- and 24-week-old rosettes was not observed (Fig. 1a). After 16 weeks of cultivation, the carrot rosettes of the 1014 line accumulated higher amount of Cu, which induced oxidative stress evidenced by slight increase in TBARS content in comparison to the control. The amount of lipid peroxidation products was almost twofold higher in these rosettes after 16 weeks of Cu exposure in comparison to the culture grown under Cu stress conditions for 24 weeks (Fig. 1b). A decrease in TBARS level after 24 weeks of cultivation was seen only in the 1014 breeding line, which seemed to be a more sensitive carrot culture. Thus, this phenomenon can be linked to an overall inhibition of plant metabolism similar to that in salt-sensitive rice cultivars (Lutts et al. 1996).
The obtained results suggest that the carrot culture of var. Feria was able to develop more effective protection system/strategy against Cu excess in comparison to the 1014 line.
Effect of Cu on proline content
One of the most commonly induced adaptive responses of plants to heavy metal is the accumulation of proline (Balestrasse et al. 2005). Based on its known properties, proline may be involved in the reduction of plant heavy metal stress by different mechanisms, i.e., osmo- and redox-regulation, metal chelation and scavenging of free radicals. Accumulation of proline in plant response to excess of Cu has been reported in wheat seedlings (Bassi and Sharma 1993a), lentil roots (Janas et al. 2010), Lemna minor (Bassi and Sharma 1993b), Silene vulgaris (Schat et al. 1997), and leaves of rice (Oryza sativa) (Chen et al. 2001).
As shown in Fig. 2a in the carrot culture of var. Feria, the dose-dependent increase in proline was observed after 16 weeks of cultivation (except the 1 μM Cu) in comparison to the control, whereas in the 1014 line the proline level significantly increased only in the rosettes grown at the highest Cu concentration (100 μM). Prolongation of the incubation time (24 weeks) caused slight decrease in the proline content in var. Feria grown at 1 and 10 μM as compared to the control and almost doubled in the rosettes treated with 100 μM Cu. A dramatic increase in proline content and concomitant lower level of endogenous Cu were observed in the 1014 line treated with 10 μM Cu. However, a significant decrease in proline (Fig. 2b) was observed, together with higher endogenous Cu level in the 1014 line at 100 μM Cu after 24 weeks of cultivation (Fig. 1b). Similar results were observed in Chlorella vulgaris, in which proline content increased up to a certain intracellular Cu level, beyond which it declined (Metha and Gaur 1999). High level of endogenous proline and lower Cu content in the 1014 line rosettes resulted in better growth regeneration capacities in the presence of 10 μM Cu. In this context, suggestions have been made that proline provides protection by (a) maintaining the water balance, (b) chelating Cu in cytoplasm, or (c) reducing metal uptake (Wu et al. 1998). Decline in proline content in the 1014 line grown in the presence of 100 μM Cu for 24 weeks may indicate that a certain threshold of intracellular Cu was crossed, or antioxidative systems were unable to cope with the oxidative stress induced by Cu excess. However, the increased content of this amino acid in the rosettes of var. Feria cultivated for 24 weeks at the highest Cu concentration points to a better protection of this cultivar against Cu toxicity (Fig. 2a).
Effect of Cu2+ on proline contents in the plant material regenerated from the embryos obtained in the anther culture of two carrot varieties (Daucus carota L.): var. Feria (a) and 1014 breeding line (b) after 16 and 24 weeks of cultivation on B5 medium (Gamborg et al. 1968). Control 0.1 μM of Cu2+. The symbols (*, **, ***) show that results obtained from Cu2+-treated carrot cultures are significantly different from controls (0.1 μM) at 0.05 and 0.01 level of Student’s t test. Bars represent standard deviations
Generally, there is a strong positive relationship between stress tolerance and proline accumulation in higher plants (Ali et al. 1998; Ashraf and Fooland 2007), and the accumulation of proline might be related to a tolerance mechanism dealing with Cu stress.
Effect of Cu on polyamines content
On exposure to metals, plants often synthesize diverse metabolites, particularly, specific amino acids such as proline and histidine, peptides such as glutathione and phytochelatins, and PAs as spermine, spermidine, and putrescine (Sharma and Dietz 2006; Górecka et al. 2007). The relationship between PAs and proline might be of a substrate–product nature (Galston et al. 1997).
The content of free PAs in var. Feria and the 1014 breeding line after 16 and 24 weeks of cultivation are summarized in Table 2 and Fig. 3. In addition to Put, Spd, and Spm, the typical representatives of the PAs pool, low concentrations of cadaverine and 1,3-diaminopropane were identified in both carrot cultures; however, they are not presented in Table 2. In both var. Feria and 1014 line cultures, Put and Spd were the most abundant free PAs. Contents of the constitutive free Put and Spd were higher in var. Feria than in the 1014 line, which, on the other hand, contained higher constitutive level of proline. It seems that the higher tolerance of var. Feria to oxidative stress (characterized by lower TBARS value) may result from higher constitutive level of PAs, similarly as in Conyza bonariensis (Ye et al. 1997).
Total contents of free polyamines (represented by the sum of putrescine, spermidine and spermine) in the plant material regenerated from the embryos obtained in the anther culture of two carrot varieties (Daucus carota L.): var. Feria (a) and 1014 breeding line (b) after 16 and 24 weeks of cultivation on B5 medium (Gamborg et al. 1968). Control 0.1 μM of Cu2+. The symbols (*, **) show that that results obtained from Cu2+-treated carrot cultures are significantly different from controls (0.1 μM) at 0.05 and 0.01 level of Student’s t test
After 16 and 24 weeks of cultivation, PA contents were differently affected according to the Cu concentration used. The increase in Put, Spd, and Spm both in Feria and 1014 carrot cultures cultivated for 16 weeks in presence of 1 μM Cu well correlated with the decrease in proline content (owing to the common precursor in the biosynthesis) observed in these cultures (Figs. 2, 3; Table 2). However, there were quantitative differences: the PA values determined in the stressed 1014 rosettes did not even reach the values found in the control rosettes of Feria (cultured at 0.1 μM Cu) (Fig. 3). A slight rise in the proline content in Feria cultivated at 10 μM Cu and predominately marked proline increase in both cultures cultivated at 100 μM Cu were accompanied by the decrease in Put and Spd. It is well known that proline can be synthesized by an alternative path from ornithine via ornithine aminotransferase, while this pathway competes for the substrate (ornithine) with pathway for synthesis of PAs. Thus, it is possible that there is competition between the two pathways for ornithine use, and less ornithine may become available to decarboxylase ornithine for Put production when proline production is high, which occurred under 100 μM Cu in Feria and 10 μM Cu in the 1014 line. The level of Spm remained high even in rosettes cultivated at the highest Cu concentration (compared with the control) (Table 2). Lower level of PAs in both controls after 24 weeks (high Spm content in all treated tissues was the exception) might coincide with the higher content of proline in these 24-week-old cultures (compared with 16-week-old ones). These results do not agree with our earlier study with the carrot cultures of var. Narbonne where free Spd and Spm levels did not change significantly, but this cultivar was much more sensitive to Cu and its growth was strongly inhibited by 10 μM of this metal (Górecka et al. 2007). It is known that Spd and Spm play a key role in preserving the integrity of thylakoid membranes of osmotically stressed oat leaves (Basford et al. 1993), whereas Put has been reported to cause depolarization of membranes and increase potassium leakage (Tiburcio et al. 1990).
In the present study the highest (Spd + Spm)/Put ratio in the free fraction of PAs was observed after 24 weeks in rosettes of the 1014 line treated with 10 μM Cu (Table 2). It was shown that the elevation of (Spd + Spm)/Put reduced the accumulation of Cu in the leaves of Nymphoides peltatum, markedly reversed negative Cu-induced effects and improved Cu tolerance in these plants similar to that in the 1014 line by maintaining the structure and function of membranes (Wang et al. 2007). Taking into consideration the elevated level of proline in this culture (Fig. 2b), relatively low lipid peroxidation as well as Cu accumulation in tissues (Fig. 1b), it can be assumed that this Cu concentration (10 μM) induced effective defense mechanisms, which were reflected in a very high regenerative capacity of carrot rosettes (Table 1). It was shown that aliphatic PAs that have been classified as growth factors in plant cells were implicated in the control of important developmental processes, including cell growth and morphogenesis (Theiss et al. 2002). Our results suggest that PAs might be implicated also in the increase of the regenerative capacity of carrot rosettes. This hypothesis is supported by the results of Kurosaki et al. (1992), who found out that Spm and/or Spd, but not free Put, plays an important role in the growth of cultured carrot cells.
There was no uniform response of the tissues to metal stressors (Sharma and Dietz 2006). Groppa et al. (2001) were unable to detect any increase in PAs content in the response of sunflower leaf discs to metal-induced oxidative stress, even under 0.1 mM of Cu. In the leaves of N. peltatum, Cu stress disturbed PAs homeostasis, resulting in increased Put level and significant decreases in Spd and Spm levels (Wang et al. 2007). In sunflower leaves, Spd, but not Spm, content decreased under Cu and Cd stress (Groppa et al. 2003). Thus, these data confirm the suggestion that variations in PA levels depend not only on the concentrations of metals tested, but also on plant species and cultivars.
In conclusion, the study showed that, depending on cultivars, carrot cultures differently react to Cu treatment. The results indicate that both, increased proline content and higher level of PAs, might participate in the alleviation of toxic effect of Cu accumulation.
Abbreviations
- DW:
-
Dry weight
- FW:
-
Fresh weight
- H2O2 :
-
Hydrogen peroxide
- OH·:
-
Hydroxyl radical
- MDA:
-
Malondialdehyde
- PAs:
-
Polyamines
- PCA:
-
Perchloric acid
- Put:
-
Putrescine
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- Spd:
-
Spermidine
- Spm:
-
Spermine
- TBA:
-
Thiobarbituric acid
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroacetic acid
References
Ali G, Srivastava PS, Iqbal M (1998) Morphogenic response and proline content in Bacopa monniera cultures grown under copper stress. Plant Sci 138:191–195
Ashraf M, Fooland MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 9:206–216
Balestrasse KB, Gallego SM, Benavides MP, Tomaro ML (2005) Polyamines and proline are affected by cadmium stress in nodules and roots of soybean plants. Plant Soil 270:343–353
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. In Vitro Cell Dev Biol Plant 44:384–395
Basford RT, Richardson CM, Campos JL, Tiburcio AF (1993) Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta 89:201–206
Bassi R, Sharma SS (1993a) Proline accumulation in wheat seedlings exposed to zinc and copper. Phytochemistry 33:1339–1342
Bassi R, Sharma SS (1993b) Changes in proline content accompanying the uptake of zinc and copper by Lemna minor. Ann Bot 72:151–154
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 9:205–207
Borowski K (2003) Tips and techniques for milestone microwave lab station. An operations overview and practical guide, 1.2i edn Milestone Inc
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Caredda S, Doncoeur C, Devaux P, Sangwan RS, Clement C (2000) Plastid differentiation during androgenesis in albino and non-albino producing cultivars of barley (Hordeum vulgare L.). Sex Plant Reprod 13:95–104
Chen CT, Chen LM, Lin CC, Kao CH (2001) Regulation of proline accumulation in detached rice leaves exposed to excess copper. Plant Sci 160:283–290
Chen EL, Chen YA, Chen LM, Liu ZH (2002) Effect of copper on peroxidase activity and lignin content in Raphanus sativus. Plant Physiol Biochem 40:439–444
Dahleen L (1995) Improved plant regeneration from barley callus cultures by increased copper levels. Plant Cell Tiss Org Cult 43:267–269
Fernandes JC, Henriques FS (1991) Biochemical, physiological and structural effect of excess copper in plants. Bot Rev 57:246–273
Galston AW, Kaur-Sawhney R, Atabella T, Tiburcio AF (1997) Plant polyamines in reproductive activity and response to abiotic stress. Bot Acta 110:197–207
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Górecka K, Krzyżanowska D, Górecki R (2005) The influence of several factors on the efficiency of androgenesis in carrot. J Appl Genet 46:265–269
Górecka K, Cvikrová M, Kowalska U, Eder J, Szafrańska K, Górecki R, Janas KM (2007) The impact of Cu treatment on phenolic and polyamine levels in plant material regenerated from embryos obtained in anther culture of carrot. Plant Physiol Biochem 45:54–61
Gori P, Schiff S, Bennici S, Bennici A (1998) Response of in vitro cultures of Nicotiana tabacum L. to copper stress and selection of plants from Cu-tolerant callus. Plant Cell Tissue Org Cult 53:161–169
Groppa MD, Tomaro ML, Benavides MP (2001) Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci 161:481–488
Groppa MD, Benavides MP, Tomaro ML (2003) Polyamine metabolism in sunflower and wheat leaf discs under cadmium or copper stress. Plant Sci 164:294–299
Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95:11140–11145
Hare P, Cress W (1997) Metabolic implications of stress-induced proline accumulation in plant. Plant Growth Regul 21:79–102
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Henderson CAP, Pauls KP (1992) The use of haploidy to develop plants that express several recessive traits using light-seeded canola (Brassica napus) as an example. Theor Appl Genet 83:476–479
Janas KM, Zielińska-Tomaszewska J, Rybaczek D, Maszewski J, Posmyk MM, Amarowicz R, Kosińska A (2010) The impact of copper ions on growth, lipid peroxidation as well as phenolic compounds accumulation and localization in lentil (Lens culinaris Medic.) seedlings. J Plant Physiol 167:270–276
Ke W, Xiong Z, Xie M, Luo Q (2007) Accumulation, subcellular location and ecophysiological responses to copper stress in two D. carota L. populations. Plant Soil 292:291–304
Kurosaki F, Matsushita M, Niski A (1992) Essential role of polyamines in growth of cultured carrot cells. Phytochemistry 31:3889–3892
Lovaas E (1996) Antioxidant and metal-chelating effects of polyamines. In: Sies H (ed) Advances in pharmacology. Antioxidants in disease mechanisms and therapy, vol 38. Academic Press, New York, pp 119–149
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Maksymiec W (1997) Effect of copper on cellular processes in higher plants. Photosynthetica 34:321–342
Maksymiec W (2007) Signalling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187
Matysik J, Alia BhaluB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Metha SK, Gaur JP (1999) Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol 143:253–259
Prażak R (2000) Effect of iodine, cobalt, copper and molybdenum on growth and development of Dendrobium kingianum Bidwill in in vitro culture. Adv Agric Sci Prob Iss 471:261–265
Schat H, Sharma SS, Vooijs R (1997) Heavy metal-induced accumulation of free proline in a metal-tolerant and nontolerant ecotype of Silene vulgaris. Physiol Plant 101:477–482
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Slocum RD, Flores HE, Galston AW, Weinstein LH (1989) Improved method for HPLC analysis of polyamines, agmatine and aromatic monoamines in plant tissue. Plant Physiol 89:512–517
Theiss C, Bohley P, Voigt J (2002) Regulation by polyamines of ornithine decarboxylase activity and cell division in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol 128:1470–1479
Tiburcio AF, Kaur-Sawhney R, Galston AW (1990) Polyamine metabolism. In: Miflin BJ, Lea PJ (eds) The biochemistry of plants intermediary nitrogen fixation. Academic Press, New York, pp 283–325
Wang X, Shi G, Xu Q, Hu J (2007) Exogenous polyamines enhance copper tolerance of Nymphoides peltatum. J Plant Physiol 164:1062–1070
Wojnarowicz G, Jacquard C, Devaux P, Sangwan RS, Clément C (2002) Influence of copper sulphate on anther culture in barley (Hordeum vulgare L.). Plant Sci 162:843–847
Wu JT, Hsieh MT, Know LC (1998) Role of proline accumulation in response to toxic copper in Chlorella sp. (Chlorophyceae) cells. J Phycol 34:113–117
Ye B, Müller H, Zhang J, Gressel J (1997) Constitutively elevated levels of putrescine and generating enzymes correlated with oxidant stress resistance in Coniza bonariensis and wheat. Plant Physiol 115:1443–1451
Zhang IP, Mehta SK, Liu ZP, Yang ZM (2008) Copper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. Plant Cell Physiol 49:411–419
Zhang W, Jiang B, Li W, Song H, Yu Y, Chen J (2009) Polyamines enhance chilling tolerance of cucumber (Cucumis sativus L.) through modulating antioxidative system. Acta Hortic 122:200–208
Acknowledgments
The scientific work was supported by the Ministry of Science and Higher Education in 2006–2009, as a research project No. N305 040 31/1576, and by the Ministry of Education of the Czech Republic, project No. OC08013.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klobus.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Szafrańska, K., Cvikrová, M., Kowalska, U. et al. Influence of copper ions on growth, lipid peroxidation, and proline and polyamines content in carrot rosettes obtained from anther culture. Acta Physiol Plant 33, 851–859 (2011). https://doi.org/10.1007/s11738-010-0610-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0610-y