Abstract
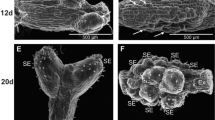
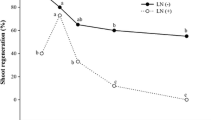
We examined the effects of cooling applied for 4 to 20 weeks on donor cultures of four dwarfing apple rootstocks (P16, P22, P59 and M26). Our aim includes increasing their competence for in vitro adventitious shoot regeneration from the leaves. Donor cultures were maintained on a shoot multiplication medium at 4°C in the dark for 4 months, followed by subculture on a fresh medium for 4 weeks. The cooling of the cultures caused an increase in the adventitious shoot number and a decrease in the starch content and an increase in the soluble sugar content (monosaccharides, raffinose and stachyose). The accumulation of stachyose in response to cold is a new observation, and it suggests that raffinose and stachyose play important role in the acclimation of dwarf apple rootstocks to low temperatures.



Similar content being viewed by others
Abbreviations
- BAP:
-
6-Benzylaminopurine
- IAA:
-
Indole-3-acetic acid
- MS:
-
Murashige and Skoog medium
- NAA:
-
Alfa-napthaleneacetic acid
- TDZ:
-
Thidiazuron
- WPM:
-
Woody plant medium
References
Bachmann M, Keller F (1995) Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Plant Physiol 109:991–998
Benson EE (2000) In vitro plant recalcitrance: an introduction. In Vitro Cell Dev Biol Plant 36:141–148
Caboni E, Lauri P, D’Angeli S (2000) In vitro plant regeneration from callus of shoot apices in apple shoot culture. Plant Cell Rep 19:755–760
Chen J, Ziv M (2005) The effects of storage on starch metabolism and regeneration potentials of twin scales and inflorescence stem explants of Narcissus tazetta. In Vitro Cell Dev Biol Plant 41:816–821
Chevreau E, Leblay C (1993) The effect of mother plant pretreatment and explant choise on regeneration from in vitro pear leaves. Acta Hortic 336:263–268
De Klerk G-J, Arnhold-Schmitt B, Lieberai R, Neumann K-H (1997) Regeneration of roots, shoots and embryos: physiological, biochemical and molecular aspects. Biol Plant 39:53–66
Diaz-Sala C, Rey M, Rodriguez R (1990) In vitro establishment of a cycloclonal chain from nodal segments and apical buds of adult hazel (Corylus avellana L.). Plant Cell Tissue Organ Cult 23:151–157
Dobránszki J, Magyar-Tábori K, Jámbor-Benczúr E, Kiss E, Lazányi J, Bubán T (2002) Effect of conditioning apple shoots with meta-topolin on the morphogenic activity of in vitro leaves. Acta Agron Hung 50:117–126
Famiani F, Ferradini N, Staffolani P, Standardi A (1994) Effect of leaf excision time and age BA concentration and dark treatments on in vitro shoot regeneration of M.26 apple rootstock. J Hortic Sci 69:679–685
Ferradini N, Famiani F, Proietti P, Stanica F (1996) Influence of growth regulators and light on in vitro shoot regeneration in M.26 apple rootstock. J Hortic Sci 71:859–865
Fortes AM, Pais MS (2000) Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): histological studies and changes in the starch content. Am J Bot 87:971–979
Gentile A, Monticelli S, Damiano C (2002) Adventitious shoot regeneration in peach [Prunus persica (L.) Batsch]. Plant Cell Rep 20:1011–1016
Hanke V, Rohde A, Grafe C (1991) Untersuchungen zur Regeneration an somatischem Gewebe in vitro I. Zur Adventivsprossbildung an Plattexplantaten bei Apfel (Malus domestica Borckh.). Gartenbauwissenschaft 56:214–220
Imanishi HT, Suzuki T, Maruda K, Harada T (1998) Accumulation of raffinose and stachyose in shoot apices of Lonicera caerulea L. during cold acclimation. Sci Hortic 72:255–263
James DJ, Passey AJ, Rugini E (1988) Factors affecting high frequency plant regeneration from apple leaf tissue cultured in vitro. J Plant Physiol 132:148–154
Klotke J, Kopka J, Gatzke N, Heyer AG (2004) Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation—evidence for a role of raffinose in cold acclimation. Plant Cell Environ 27:1395–1404
Kucharska D, Orlikowska T (2005) Genetic variability in rose responses to in vitro factors. In: Book of abstracts COST 843 final conference, June 28–July 3, Stara Lesna, pp 34–36
Lahuta LB (2006) Biosynthesis of raffinose family oligosaccharides and galactosyl pinitols in developing and maturing seeds of winter vetch (Vicia villosa Roth.). Acta Soc Bot Pol 75:219–227
Lane WD, Iketami H, Hayashi T (1998) Shoot regeneration from cultured leaves of Japanese pear (Pyrus pyrifolia). Plant Cell Tissue Organ Cult 54:9–14
Langens-Gerrits MM, Miller WBM, Croes AF, de Klerk G-J (2003) Effect of low temperature on dormancy breaking and growth after planting in lily bulblets regenerated in vitro. Plant Growth Regul 40:267–275
Li M, Leung DWM (2000) Starch accumulation is associated with adventitious root formation in hypocotyls cuttings of Pinus radiate. J Plant Growth Regul 19:423–428
Liu J-JJ, Krenz DC, Galvez AF, de Lumen BD (1998a) Galactinol synthase (GS): increased enzyme activity and levels of mRNA due to cold and desiccation. Plant Sci 134:11–20
Liu Q, Salih S, Hammerschlag F (1998b) Etiolation of ‘Royal Gala’ apple (Malus x domestica Borkh.) shoots promotes high-frequency shoot-organogenesis and enhanced beta-glucuronidase expression from stem internodes. Plant Cell Rep 18:32–36
Liu C, Xia X, Yin W, Huang L, Zhou J (2006) Shoot regeneration and somatic embryogenesis from needles of redwood (Sequoia sempervirens (D. Don.) Endl.). Plant Cell Rep 25:621–628
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb Proc Int Plant Prop Soc 30:421–427
Loescher WH (1987) Physiology and metabolism of sugar alcohols in higher plants. Physiol Plant 70:553–557
Loescher WH, Everard JD (1996) Sugar alcohol metabolism in sinks and sources. In: Zamski E, Schaffer AA (eds) Photoassimilate distribution in plants and crops: source-sink relationships. Marcel Dekker, New York, pp 185–207
Mangat BS, Pelekis MK, Cassels AC (1990) Changes in the starch content during organogenesis in vitro cultured Begonia rex stem explants. Physiol Plant 79:267–275
McCown BH (2000) Recalcitrance of woody and herbaceous perennial plants: dealing with genetic predeterminism. In Vitro Cell Dev Biol Plant 36:148–154
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Orlikowska T (1992) Effect of in vitro storage at 4°C on survival and proliferation of two apple rootstocks. Plant Cell Tissue Organ Cult 31:1–7
Orlikowska T, Nowak E, Marasek A, Kucharska D (1999) Effects of growth regulators and incubation period on in vitro regeneration of adventitious shoots from gerbera petioles. Plant Cell Tissue Organ Cult 59:95–102
Palonen P (1999) Relationship of seasonal changes in carbohydrates and cold hardiness in canes and buds of three red raspberry cultivars. J Am Soc Hortic Sci 124:458–563
Pawlicki N, Welander M (1994) Adventitious shoot regeneration from leaf segments of in vitro cultured shoots of the apple rootstock Jork 9. J Hortic Sci 69:687–696
Pennycook JC, Jones ML, Stushnoff C (2003) Down regulating α-galactosidase enhances freezing tolerance in transgenic petunia. Plant Physiol 133:901–909
Pommerrenig B, Papini-Terzi FS, Sauer N (2007) Differential regulation of sorbitol and sucrose loading into the phloem of Plantago major in response to salt stress. Plant Physiol 144:1029–1038
Predieri S, Malavasi FF (1989) High-frequency shoot regeneration from leaves of the apple rootstock M26 (Malus pumila Mill.). Plant Cell Tissue Organ Cult 17:133–142
Rugini E, Muganu M (1998) A novel strategy for the induction and maintenance of shoot regeneration from callus derived from established shoots of apple [Malus x domestica Borkh.] cv. Golden Delicious. Plant Cell Rep 17:581–585
Sorvari S, Ulvinen S, Hietaranta T, Hiirsalmi H (1993) Preculture medium promotes direct shoot regeneration from micropropagated strawberry leaf discs. HortSci 28:55–57
Sriskandarajah S, Goodwin P (1998) Conditioning promotes regeneration and transformation in apple leaf explants. Plant Cell Tissue Organ Cult 53:1–11
Swartz HJ, Bors R, Mohamed F, Naess SK (1990) The effect of in vitro pretreatments on subsequent shoot organogenesis from excised Rubus and Malus leaves. Plant Cell Tissue Organ Cult 21:179–184
Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29:417–426
Theiler-Hedtrich C, Theiler-Hedtrich R (1990) Influence of TDZ and BA on adventitious shoot regeneration from apple leaves. Acta Hortic 280:195–199
Thorpe TA, Murashige T (1970) Some histochemical changes underlying shoot initiation in tobacco callus culture. Can J Bot 48:277–285
Thorpe TA, Joy RW, Leung DWM (1986) Starch turnover in shoot-forming tobacco callus. Physiol Plant 66:58–62
Trinder P (1969) Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol 22:246
Webster CA, Jones OP (1991) Micropropagation of some cold-hardy dwarfing rootstocks for apple. J Hortic Sci 66:1–6
Welander M (1988) Plant regeneration from leaf and stem segments of shoots raised in vitro from mature apple trees. J Plant Physiol 132:738–744
Wiejacha K, Orlikowska T (2004) The effect of concentration of mineral salts, kind of solidifying agent, cytokinin and TIBA on adventitious regeneration of apple rootstock P.59]. Biotechnologia 2(65):191–198 (in Polish, English Abstract)
Yepes LM, Aldwinckle HS (1994) Factors that affect leaf regeneration efficiency in apple and effect of antibiotics in morphogenesis. Plant Cell Tissue Organ Cult 37:257–269
Zuther E, Büchel K, Hundertmark M, Stitt M, Hincha DK, Heyer AG (2004) The role of raffinose in the cold acclimation response of Arabidopsis thaliana. FEBS Lett 576:169–173
Acknowledgments
Authors are grateful to Mrs. Lucyna Ogórek for technical help. This study was financed by grant 580/E-177/SPB of the Polish Ministry of Sciences and High Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Pauk.
Rights and permissions
About this article
Cite this article
Orlikowska, T., Zawadzka, M., Kucharska, D. et al. The influence of the cooling of donor cultures on the in vitro adventitious regeneration and carbohydrate metabolism of four dwarfing apple rootstocks. Acta Physiol Plant 32, 333–340 (2010). https://doi.org/10.1007/s11738-009-0411-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0411-3