Abstract
Increased effectiveness and decreasing toxicity are prime objectives in drug research. Overwhelming evidence suggests the use of appropriate combination therapy for the better efficacy of drugs owing to their synergistic profile. Dietary active constituents play a major role in health outcomes. Therefore, it is possible to increase the effectiveness of the drug by combining contemporary medication with active natural/semi-synthetic constituents. One such dietary constituent, caffeic acid (CA), is a by-product of the shikimate pathway in plants and is a polyphenol of hydroxycinnamic acid class. Extensive research on CA has proposed its efficacy against inflammatory, neurodegenerative, oncologic, and metabolic disorders. The synergistic/additive effects of CA in combination with drugs like caffeine, metformin, pioglitazone, and quercetin have been reported in several experimental models and thus the present review is an attempt to consolidate outcomes of this research. Multi-target-based mechanistic studies will facilitate the development of effective combination regimens of CA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caffeic acid (CA) is a polyphenol and a secondary metabolite obtained from natural sources like olives, berries, potatoes, carrots but majorly from coffee beans (Silva et al. 2014; Monteiro Espíndola et al. 2019). It is a constituent of hydroxycinnamic acid, which is a common everyday dietary ingredient for humans (Agunloye et al. 2018). Various in vitro and in vivo experiments proved that CA has enormous biological activities like antibacterial activity (Pinho et al. 2015), antioxidant property (Mehrotra et al. 2011; Sato et al., 2011), anti-atherosclerotic activity (Norata et al. 2007), immunostimulant activity (Yilmaz et al. 2019), anti-inflammatory/ analgesic activity (Mehrotra et al. 2011), antiviral activity (Wu et al. 2017), cardioprotective (Agunloye et al 2019), and anti-proliferative active (Feriotto et al. 2021; Mirzaei et al. 2021). Thus, being a dietary component with promising multiple benefits, it is worth to explore the potentials of CA for combination therapy.
The combination therapy, in its simplest sense, is the treatment approach with two or more drugs with the intent of reaching the same efficacy levels with lower toxicities at doses lower than usual, have higher potency with additive/ synergistic effects (Bayat Mokhtari et al. 2017; Chen et al. 2016). Combined treatment strategy with natural products can prevent the cause of acquired drug resistance such as with chemotherapy. Successful combinations of therapeutic agents with natural products can achieve the desired outcome but with a lower toxicity profile (Bukowska et al. 2015; Das et al. 2019). The combination therapy started gaining recognition after the first US-FDA approval of four regimens in the 1940s and has gone up to 419 by 2018 (Bukowska et al. 2015). In the past 20 years, the drug discovery rate has reduced by half, which is an alarming call for the researchers of the healthcare industry. To break the walls of this decline in drug design and development, the focus is being shifted toward adapting new strategies like combination therapy.
Combination therapy has a remarkable effect in indications such as alimentary tract and metabolism, anti-infective, cardiovascular, dermatological, endocrine, genitourinary tract, musculoskeletal, nervous system, respiratory system, sensory organ, oncology, viral, and microbial infections, etc. (Das et al. 2019). Given the complexity of disease pathology, resistant pathogen strains, and patients’ genetic variation, combination therapy always supersedes the monotherapy (MacDonald et al. 2017; Garjón et al. 2020). However, combination therapy can still be unsuccessful if not planned and monitored carefully (Bang et al. 2010).
Emerging drug resistance against available therapy points toward the need to search the bioactive molecules, particularly from natural sources considering their therapeutic potential which is not fully explored (Loke et al. 2018). Traditional systems like Ayurveda and Siddha are the backbone of folklore medicine where combination therapies have been extensively practiced to cure the diseases (Ravishankar et al. 2007; Pandey et al. 2013; Cammisotto et al. 2021). A few clinical studies have also highlighted the role such combinations can play in better therapeutic outcomes. In one such study, three months of vitamin C, vitamin E, in combination with metformin, significantly improved the glycemic value, reduced fasting blood sugar (FBS), HbA1c, and insulin compared to metformin alone (Dakhale et al. 2011). In another study, a combination of Nigella sativa extract and Vitex agnus-castus extract along with citalopram increased the efficacy of citalopram in improving the symptoms of hot flashes in healthy postmenopausal women (Molaie et al. 2019). This substantiates that a combination of nutraceuticals/food items with desired chemical composition has the potential to be utilized in conjunction with modern-day medicine to enhance its therapeutic potential.
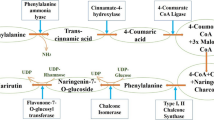
Although plant products like CA have a versatile and broad spread chemical composition (Touaibia et al. 2012), a few of these chemical constituents have outshone others. A systematic review highlighting the benefits of plant products along with prescription drugs in old adults found out that the most common class of compounds with which patients combined the plant products were anticoagulants, analgesics, antihistamines, antidiabetics, antidepressants, and statins (Agbabiaka et al. 2017). Phenolic acids are one such class of compounds encompassing compounds such as ferulic acid (FA), CA, chlorogenic acid (ChA), and cinnamic acid (CinA). The phenolic acids have been tested for their in vivo and in vitro effectiveness in widespread conditions (Kinra et al. 2019; Kinra et al. 2021), but CA has been widely studied in combination and reported to be synergistic/ additive with multiple regimens. Within plant phenolics, it belongs to the sub-class of cinnamic acids. It is synthesized in plants through the shikimic acid pathway in which its precursor is phenylalanine and/or tyrosine (Kumar et al. 2019).
CA, in both free and esterified form, is the most abundant hydroxycinnamic acid (Yang et al. 2001; Brglez Mojzer et al. 2016). It constitutes 75% of total polyphenols in most fruits (Murkovic et al. 2003; Pandey et al. 2009). The free form is easily absorbed through the stomach and reaches peak plasma levels within one hour (Stalmach et al. 2012; Del Rio et al. 2013). Esterified form of CA is not readily absorbed. When esterified CA reaches the stomach, only 5% of it is absorbed. The remaining passes to the intestine where sits colonial microflora produce esterases, which break down the ester part to CA for its remaining 95% absorption (Manach et al. 2004; Wojtunik-Kulesza et al. 2020). A high concentration of CA is absorbed in plasma as dihydrocaffeic acid which further undergoes metabolism and forms dihydrocaffeic acid-3-sulfate (Del Rio et al. 2010; Wojtunik-Kulesza et al. 2020). The peak plasma level is observed after one hour of absorption.
This review highlights the potential studies reporting the outcome of the selected combinations with CA for different indications.
Literature reports on synergistic effects of CA with other pharmaceutical entities
Synergistic effect of CA with antioxidant agents
Caffeic acid and caffeine
Caffeine (CAF) an indispensable part of coffee beverages for avid consumers (Depaula et al. 2019), is a methylxanthine derivative and acts as a central nervous system (CNS) stimulant (Nehlig et al. 1992). It is the major functional food consumed worldwide (Reyes et al. 2018). CAF also has several reported pharmacological effects including memory enhancement, antioxidant, and anti-proliferative effects (Fredholm et al. 1999). The higher doses of CAF exert deleterious effects such as including insomnia, anxiety, agitation, and elevated heart rate (Temple et al. 2017; Vliegenthart et al. 2018; Wolk et al. 2012).
In a preclinical study, the effects of CA combined with CAF were analyzed for the markers of male reproductive system toxicity in rats. CA and CAF showed synergistic effect at 50 mg/kg in combination where they decreased testicular cholesterol and increased testicular steroidogenic enzyme activity, glycogen, and zinc levels. This ultimately led to an increase in follicular stimulating factor (FSH) and luteinizing hormone (LH). The combination therapy inhibited the formation of nitric oxide in testicular cells, which further synergistically, downregulated catalase (CAT) and superoxide anions responsible for lipid peroxidation. These synergistic effects substantiate the mechanism of the combination in preventing lipid peroxidation-mediated impairment of spermatozoa (Fig. 1). Therefore, the combination exerts a significant synergistic/additive effect to improve the male reproductive function (Akomolafe et al. 2018).
Synergistic activity of CA with other antioxidants. The combination therapy inhibits the reactive oxygen species (ROS) which is generated due to oxidative stress in the body. This ultimately stops the overexpression of superoxide dismutase (SOD), catalase (CAT), and other anions of oxygen species generated in the cells
A study conducted by Mudgal et al. (2020) on the combined effect of CA and CAF on the immune system and its effect on the systemic inflammatory pathways showed the beneficial effect of CA and CAF combination against sickness-like behavior in mice. The behavioral tests suggested that CA + CAF (10 mg/kg + 5 mg/kg) expressed anxiolytic-like properties in mice post systemic administration of LPS (1.5 mg/kg) which induced profound immobility, increased the systemic and brain cytokine (TNF-α and IL-6) levels and altered the host antioxidant defense. This study was conducted over a week where the pre-treatment with CA + CAF significantly subdued the effects of LPS suggesting that when CA was combined with CAF, the anxiolytic and antidepressant effects were synergized.
Further, coffee a major dietary source of CA and CAF was tested for its anti-genotoxic effect. Co-administration of 100 mg/kg coffee, which is equivalent to 3cups/person/day, with other dietary constituents including a combination of CA (20 mg/kg), ChA (20 mg/kg), FA (15 mg/kg) and, ellagic acid (15 mg/kg) showed significant protection against N-methyl-N’-nitro-N-nitrosoguanidine induced genotoxicity (Abraham et al. 1996). Based on these pieces of evidence from animal studies, the combination therapy of CA with CAF was found to exert a synergistic effect compared to when administered alone.
Caffeic acid and quercetin in genotoxicity
Quercetin, a natural flavonoid, is present in several vegetables and abundantly in onions. Over the years, quercetin alone and in combination has proven its efficacy against many disorders in pre-clinical setup. It mainly serves as an antioxidant agent (Zhang et al. 2011). Combination therapy of CA and quercetin was studied for its protective effect against lambda-cyhalothrin (LTC)-induced in vitro genotoxicity (Abdallah et al. 2012). This protective activity can be due to the finding of Abraham et al. 1996 where he explained the combination effect of coffee and its constituent produced anti-genotoxic effect due to synergestic action against N-methyl-N-nitro-N-nitrosoguanidine (MNNG) and urethane. The combination therapy showed a significant antioxidant effect, which reduced the rate of DNA damage in the cells caused by insecticides by LTC and thereby reduced the overall toxic effect (Fig. 1). However, future in vivo studies will provide more insight into the protective effect of CA and quercetin against genotoxicity.
Caffeic acid and rofecoxib in excitotoxicity and mitochondrial dysfunction
Rofecoxib is a cyclooxygenase-2 (COX-2) inhibitor used for therapeutic relief from inflammatory pain. A study analyzed the in vivo combined effect of CA with rofecoxib and compared it to their individual effects against quinolinic acid-induced mitochondrial dysfunction and associated neuronal death. Treatment of rats with CA + rofecoxib for 21 days exerted a significant synergistic effect against quinolinic acid (an NMDA receptor agonist)-mediated excitotoxicity compared to per se treatments (Kalonia et al. 2009) (Fig. 1). However, future studies can validate the synergistic effect of CA + rofecoxib on behavioral parameters as observed on biochemical markers.
Synergistic effect of CA with anti-cancer agents
Caffeic acid and metformin in cancer cells
Metformin (Met), a well-known biguanide oral anti-hyperglycemic drug (Nasri et al. 2014), has been reviewed for possible anti-cancer activity (Kasznicki et al. 2014). Many studies report a beneficial effect of Met against cancer in humans where it reduced the risk of cancers (Saini et al. 2018; Malek et al. 2013; Guo et al. 2016; Cauchy et al. 2017).
Met and CA have been reported to have synergistic/ additive effects when combined with traditional anti-cancer therapies particularly against HTB-34 cells of metastatic cervical cancer (Tyszka-Czochara et al. 2017a). In another study, CA exerted cytotoxicity via necrosis to SiHa cervical cancer cells but when combined with Met, the cytotoxicity mechanism shifted toward apoptosis without affecting normal human fibroblasts. The combination was also found to regulate mitochondrial metabolism and induced ROS formation in metastatic cervical carcinoma cells. In fact, the incubation of cancer cells with Met and CA caused a dramatic shift to the G0 phase from the G1 phase. Studies are still ongoing to investigate the mechanism of action of the anti-tumor property of the combination of Met and CA (Tyszka-Czochara et al. 2017b).
Several pieces of evidence indicated that the anti-cancer activity of Met could be due to cell cycle changes, especially in G0/G1 and G2/M phases (Wang et al. 2018). In a pre-clinical study, SiHa cells exposed to the combination of CA + Met had a dramatic drop in the number of G0-phase cells in the total population (Tyszka-Czochara et al. 2018).
Therefore, the combination of CA and Met shows a significant in vitro anti-tumor effect in cervical carcinoma cells compared to Met alone. This approach needs to be further investigated in the appropriate in vivo models to establish the effect of these combinations.
Caffeic acid and all-trans-retinoic acid (ATRA) in cancer
CA and ATRA combination was studied for the anti-neoplastic effect in Saos-2 and OSA-01, osteosarcoma cell lines. Treated cell populations were observed for any changes in matrix mineralization, cell proliferation, and occurrence of differentiation markers at fixed intervals. The results showed the potential of CA in enhancing the anti-proliferative effect of ATRA in both cell lines (Veselska et al. 2014). The combination index (CI) for the Saos-2 cell line indicated synergism at 0.1 µM ATRA + 13 µM CA. For OSA-01 cell lines, synergism was observed with 0.1 µM ATRA + 13 µM CA. The mechanism target by this novel combination was cell proliferation, angiogenesis, and apoptosis for its anti-neoplastic effect (Paukovcekova et al. 2020). The mechanism for the anti-neoplastic effect was further studied and even reported the synergistic effect of CA on ATRA in medulloblastoma cell lines (Chlapek et al. 2014). These results warrant the scope of testing such a combination in the preclinical and clinical setup (Martinez-outschoorn et al. 2017).
Caffeic acid and cisplatin in cancer therapy
Cisplatin is a well-known chemotherapeutic agent with a promising effect on cervical (Aggarwal et al. 2017), ovarian (Helm et al. 2009), and testicular cancer (de Vries et al. 2020). Despite the rich therapeutic value, cisplatin shows tumor resistance development (Dasari et al. 2014). It is reported that resistance development is either intrinsic or acquired due to a decrease in cisplatin influx in the cell through CRT1 copper transporter. This drop-in influx of cisplatin in the cell imbalances the cell proliferation factors like anti-apoptotic factors, pro-apoptotic factors, DNA repair mechanism and influences DNA damage tolerance mechanism (Zhou et al. 2020) (Fig. 2).
CA and cisplatin showed promising anti-cancer activity in ovarian cancer. Cisplatin sensitive cells when exposed to combination therapy of 5 µM cisplatin and 50 µM CA, they showed rapid increase in the activity of the apoptotic cascade by increased caspase activity (1.7 folds) compared to single administration of 5 µM cisplatin. Further study on A2780cisR tumor cells showed that the combination of 5: 50 µM (cisplatin/CA) rise the caspase activity by 4:3 folds with 60% cell viability (Sirota et al. 2017).
A study was conducted in which the combination of cisplatin with CA was tested to stop the problem of resistance development in cancer treatment. The study was supported by the fact that CA is an inhibitor of glutathione (GSH) S-transferase and GSH reductase, which are the catalytic enzymes of glucopyranosylidene-spiro-thiohydantoin (GTH) (Fig. 2) (Siddik et al., 2003; Islam et al. 2017). This dual enzyme inhibitory property of CA was utilized, and combination therapy of the CA and cisplatin was tested. When 5 µM cisplatin and 50 µM CA were co-administered, the caspase 3 activity was reported to increase 1.7 fold in cancerous cells and 4.3 fold in resistant cells. Sirota et al. (2017) also studied the effect of CA + cisplatin on DNA platination. They found a 2.2 folds increase in the DNA –platinum when cisplatin (20 µM) was co-administered with CA (50 µM) but not with pre-treatment of CA. Therefore, it was concluded that CA and cisplatin, when administered as combination therapy, shows synergistic cytotoxicity to chemo-resistant cancer cells compared to cisplatin alone. Authors suggested that the timing of administering the CA + Cisplatin combination is vital to the outcome of treatment as synergism is seen when both the treatments were administered simultaneously, thus highlighting the importance of developing a new “platinum-CA compound” for the treatment of resistant cancer (Sirota et al. 2017; Mirzaei et al., 2021).
Caffeic acid and caffeine in breast cancer patients
A translational cohort study involving women with primary invasive breast cancer reported a decrease in metastatic tumor size [estrogen receptor-positive, (ER+)] with high coffee (more or equal to 5 cups/day) consumption. This was further confirmed with an experimental trial using ER+ and ER− cell lines. The two major constituents of coffee, i.e., CAF and CA showed better efficacy against ER+ tumors (Rosendahl et al. 2015). However, the combined effect of these constituents was not evaluated. Thus, it can be stated that there is a scope of testing the combined effect of CA and CAF to reveal their potential against breast cancer and to evaluate the molecular mechanisms of combination with a multi-target approach (Fig. 2).
Synergistic effect of CA with anti-neurodegenerative agents
Caffeic acid with chlorogenic acid in Alzheimer’s disease
ChA is isolated from green coffee seeds, which radially decomposes to form quinic acid and CA. It also exists in the leaves and seeds of many dicotyledonous plants (Sharma et al. 2020). It accounts for a 5–10% chemical constitution of coffee beans and is reported to influence the astringent and sour taste of coffee (Izawa et al. 2010). ChA is reported to have free radical scavenging capability, hence it is a well-known antioxidant agent (Xi et al. 2017). It is also reported that ChA has wide range of biological effect on the neurological system like Alzheimer’s disease (Mancini et al. 2018). So ChA is an ideal candidate in combination with CA against neurodegenerative disease.
The combined effect of CA and ChA against Alzheimer’s disease was conducted to find its synergestic or additive effect on the biological system. Even though the etiologic reason for Alzheimer’s disease is unknown (Jellinger et al. 2007); it is well accepted by the researchers that the strategy targeting inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) have good effect against Alzheimer’s disease (Zhao et al. 2013) (Fig. 3). The study was conducted using male Wistar rats with three combination groups, where the first group treated with 50% of CA and 50% of chlorogenic acid, the second group by 25% of CA and 75% of ChA and the third group content was 75% CA and 25% ChA (Oboh et al. 2013).
The results reflected that the combination of CA and ChA showed inhibition of AChE and BChE activities irreversibly as compared to ChA alone. The highest percentage of inhibition was recorded when 75% of CA was incorporated in the combination therapy, i.e., when the ratio of CA is higher in the combination. Also, pro-oxidants such as, FeSO4, sodium nitroprusside and quinolinic acid caused an increase in the malondialdehyde (MDA) contents of the brain which was significantly decreased dose-dependently due to the administration of the combination of CA and ChA solution. Inhibition of AChE and BChE activities slow down acetylcholine and butyrylcholine breakdown in the brain. This combination therapy of CA and ChA showed a synergistic activity. Therefore, the mechanism through which the combination therapy of CA and ChA exert their neuroprotective properties is by inhibiting AChE and BChE activities as well as preventing oxidative stress-induced neurodegeneration (Oboh et al. 2013).
Caffeic acid and CAF in Alzheimer’s disease
Studies have shown the effect of CA and its combinations with CAF (25:75, 50:50, 75:25) on various enzymes such as AChE and monoamine oxidase (MAO) activity. CA and CAF combination at 25:75 ratio, CAF significantly increased the AChE and MAO inhibitory effect of CA as compared to the 75:25 ratio (CA: CAF). The observed synergistic effect of combination against these enzymes can contribute to the improved efficacy and safety of these constituents against neurological conditions such as Parkinson’s and Alzheimer’s disease (Fig. 3). It suggest that CA and CAF combination therapy at specific ratio could be a better choice for further evaluation against Alzheimer’s disease. Therefore, the food constituents are present in combination and thus have the potential to exert a better neuroprotective effect (Akomolafe et al. 2017).
Synergistic effect of CA with anti-hyperglycemic agents
Caffeic acid and pioglitazone in chronic fatigue
Pioglitazone is an anti-hyperglycemic thiazolidinedione derivative prescribed for the treatment of type II diabetes (Desouza et al. 2010). Pioglitazone was reported to have antioxidant and neuroprotective actions (Medhi et al. 2010). Another study conducted by Kumar et al. (2010) was focused on evaluating the possible synergistic effect of the combination against stress-induced behavioral, biochemical, and cellular alterations in the chronic fatigue model in rats. In this study, CA and pioglitazone-altered mitochondrial enzymes complex activities and mitochondrial redox activity. Further, the combination of the lower dose of pioglitazone (5 mg/kg) and CA (5 mg/kg) showed a significant protective effect suggesting a good synergistic activity compared to their higher dose levels, i.e., 10 mg/kg (Kumar et al. 2010). Pioglitazone and CA are well-known anti-inflammatory and antioxidant compounds, individually (Hasegawa et al. 2011). In the study, the combination showed the synergistic effect on improving the antioxidant enzymes, i.e., reduced glutathione (GSH) and CAT. Oxidative stress being a major pathological factor in fatigue and the combination with its synergistic neuroprotective and antioxidant effects exerted a beneficial effect on fatigue. These findings can pave the way for developing a hypothesis for testing these two molecules in combination against neurodegenerative conditions, where oxidative stress is the major pathomechanism (Kumar et al. 2010).
Caffeic acid and 18β-glycyrrhetinic acid in type II diabetes
18β-glycyrrhetinic acid is a metabolite of liquorice and glycyrrhizin (Tabuchi et al. 2012). It is an aglycone absorbed in the intestine by aerobic microbes. The 18β-glycyrrhetinic acid has been reported to have a wide range of pharmacological activities like anti-inflammatory (Quan et al. 2021), anti-ulcerative, anti-hepatotoxic, anti-tumorigenic, and anti-viral activity (Hendricks et al. 2012).
Mohammed et al. (2015) conducted a study to compare the combined effect of CA and 18β-glycyrrhetinic acid against type II diabetes. The treatment of diabetic rats with 18β-glycyrrhetinic acid and CA combination showed a reduction in fasting blood glucose levels. Also, circulating insulin levels were reversed in diabetic animals. Thus, the combined treatment with 18β-glycyrrhetinic acid and CA exerted an overall promising effect against oxidative stress as compared to 18β-glycyrrhetinic acid alone.
Synergistic effect of CA against metabolic disorders
Caffeic acid and ferulic acid in metabolic disorder
Ferulic acid is a phytoconstituent obtained from many plants especially from the genus Ferula (Kumar et al. 2014). FA was majorly explored for its pharmacological effects in the 1970s when FA sterol esters were extracted from rice oil and tested by Japanese researchers for their antioxidant potential (Truong et al. 2017; Vissers et al. 2000).
A recent study by Bocco et al. 2016, evaluated the beneficial effect of the FA combined with CA when administered to mice with metabolic disorders. CA (0.9 mg/kg, s.c.) and FA (50 mg/kg, s.c.), when administered in combination, they reversed the high fat diet (7.52 kcal/g) -induced hyperglycemia, hypercholesterolemia, and hypertriglyceridemia. This synergistic effect was confirmed with improved glucose tolerance and decreased white adipose tissue size. The report suggested that the combination increased the uptake as well as the export of hepatic cholesterol by inducing mRNA expression of LDL receptor (LDL-R), sterol regulatory element-binding protein 2 (SREBP-2), and liver X receptor alpha (LXRa) in the mouse. These effects corroborate the therapeutic role of the combination in the amelioration of metabolic disorder as compared to respective molecules alone.
Caffeic acid and curcumin in liver injury
Curcumin is a diketone extracted from the rhizomes of Curcuma longa (Amalraj et al. 2017). Curcumin has been reported to have diverse biological effects such as hepato-protective (Farzaei et al. 2018), anti-inflammatory, and tumor growth inhibition potential (Fadus et al. 2017). Combination therapy of CA (20 mg/kg) with curcumin (50 mg/kg) was explored on nicotine-induced lung injury in rats. The oral treatment of this combination showed normalized butyrylcholinesterase (BChE), plasma alanine aminotransferase (ALT), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), lactate dehydrogenase (LDH) and well as plasma and lung thiobarbituric acid reactive substances (TBARS), nitric oxide (NO) and Tumor Necrosis Factor (TNF-α). The therapy also showed a significant decline in non-enzymatic antioxidants like reduced GSH and enzymatic antioxidants such as catalase (CAT) and superoxide dismutase (SOD) as well as high-density lipoprotein cholesterol (HDL-C), which are the important markers of liver injury (Boshra et al. 2016).
Therefore, it can be concluded that CA and curcumin in combination exert a better antioxidant and lung-protective activity compared to per se treatment, against nicotine-induced liver toxicity which can be attributed to their superior free radical scavenging properties.
Synergistic effect of CA with anti-microbial agents
In a study by Lima et al. (2016), the synergistic antimicrobial effects of compounds such as gallic acid, CA, and pyrogallol were evaluated with standard antibiotics such as norfloxacin/ imipenem/ gentamicin against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. CA exerted significant synergistic action compared to other test molecules. It reduced the minimum inhibitory concentration (MIC) of norfloxacin (from 156.3 to 15.5 mg/mL, against S. aureus), imipenem (from 2500 to 1574 mg/mL, against E. coli), and gentamicin (from 625 to 24.61 mg/mL, against P. aeruginosa). CA presented the best synergistic effect with imipenem against P. aeruginosa, where MIC of imipenem was reduced from 1250 to 78.13 mg/mL. This points toward the potential of functional food components like CA as promising potentiating agents for antimicrobial drugs and may offer newer approaches toward better efficacy of antibiotics against resistant bacteria owing to their synergistic/ additive effect (Lima et al. 2016). One of the study based on combined effect of CA with fosfomycin to inhibit growth of a resistant Listeria monocytogenes strain; it was observed that CA enhanced the antimicrobial activity of fosfomycin against Listeria monocytogenes from 5% of fosfomycin alone to 82% of the fosfomycin and CA combination (Zhang et al. 2020).
Conclusion
Combination therapy is becoming a choice of drug development in the pharmaceutical industries for solving drug resistance, reducing the adverse drug reactions and increasing the efficacy of drugs. Several disease conditions predominantly require combination therapy due to their complex pathophysiology and progression. CA is the major constituent of dietary polyphenols, and its consumption has been indicated even in traditional systems of medicine. CA with compounds of different classes has been tested in combination extensively for various types of disorders. These outcomes have been listed in Table 1.
CA, owing to its free radical scavenging property (Kadoma et al. 2008), was able to increase the antioxidant efficacy of agents such as CAF, quercetin and rofecoxib for better treatment outcomes. As reviewed, CA was reported to be synergistic with Met, ATRA an cisplatin for anti-neoplastic effects. With ChA, CA could synergize for neuroprotective activity in a dose-dependent manner. CA was also reported to have synergistic effect with CAF and Pioglitazone for MAO, AChE inhibition and against stress-induced changes in a chronic fatigue in rats, respectively. Although CA has proved its effectiveness against a multitude of pathological mechanisms, the majority of studies have been conducted in either in vitro or in vivo pre-clinical setups. This opens a significant scope for future exploration of CA in combination with drugs/ active chemical moieties in validated animal models. Such future studies can lead to the development of CA as a promising candidate clinically in different disease conditions as combination therapy.
Though there is a success of the combination therapy of CA with other therapeutic agents in pre-clinical studies; the transition of these therapy have a void to pass into clinical studies due to failure in efficacy and safety in humans. This is due to difference in predictive value of human specific pharmacodynamic rate compared to animals and further lack of methodologies of superior predictive analysis for human adverse effect (Martin et al. 2012). Many studies also suggested that failure in anticipating the outcome of cytokine release rate and immunosuppressant activity is one of the major reason for discordance between the preclinical and clinical safety studies (Polson et al. 2012). The drug development studies should gather more information on the appropriate efficacy outcome for a better translational effect in clinical trials.
References
Abdallah FB, Fetoui H, Fakhfakh F, Keskes L (2012) Caffeic acid and quercetin protect erythrocytes against the oxidative stress and the genotoxic effects of lambda-cyhalothrin in vitro. Hum Exp Toxicol 31:92–100. https://doi.org/10.1177/0960327111424303
Abraham SK (1996) Anti-genotoxic effects in mice after the interaction between coffee and dietary constituents. Food Chem Toxicol 34:15–20. https://doi.org/10.1016/0278-6915(95)00085-2
Agbabiaka TB et al (2017) Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drug Aging Spring Int Publ 34(12):891–905. https://doi.org/10.1007/s40266-017-0501-7
Aggarwal U, Goyal AK, Rath G (2017) Development and characterization of the cisplatin loaded nanofibers for the treatment of cervical cancer. Mater Sci Eng C 75:125–132. https://doi.org/10.1016/j.msec.2017.02.013
Agunloye OM, Oboh G (2018) Caffeic acid and chlorogenic acid: evaluation of antioxidant effect and inhibition of key enzymes linked with hypertension. J Food Biochem 42(4):1–10. https://doi.org/10.1111/jfbc.12541
Agunloye OM et al (2019) Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats’. Biomed Pharmacother 109:450–458. https://doi.org/10.1016/j.biopha.2018.10.044
Akomolafe SF et al (2017) Effect of caffeine, caffeic acid and their various combinations on enzymes of cholinergic, monoaminergic and purinergic systems critical to neurodegeneration in rat brain—In vitro. NeuroToxicol 62:6–13. https://doi.org/10.1016/j.neuro.2017.04.008
Akomolafe SF et al (2018) Co-administration of caffeine and caffeic acid alters some key enzymes linked with reproductive function in male rats. Andrologia 50(2):1–10. https://doi.org/10.1111/and.12839
Amalraj A et al (2017) Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives – a review. J Tradit Complement Med 7(2):205–233. https://doi.org/10.1016/j.jtcme.2016.05.005
Bang YJ et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697. https://doi.org/10.1016/S0140-6736(10)61121-X
Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H (2017) Combination therapy in combating cancer. Oncotarget 8(23):38022–38043. https://doi.org/10.18632/oncotarget.16723
Bocco BM et al (2016) Combined treatment with caffeic and ferulic acid from Baccharis uncinella C. DC. (Asteraceae) protects against metabolic syndrome in mice. Braz J Med Biol Res 49(3):3–9. https://doi.org/10.1590/1414-431X20155003
Boshra SA (2016) The protective effects of curcumin and caffeic acid alone or in combination on nicotine-induced lung injury in rats. Int J Phytomed 8(2):238–248
Brglez Mojzer E. et al. (2016) Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules (Basel, Switzerland). doi: https://doi.org/10.3390/molecules21070901.
Bukowska B, Gajek A, Marczak A (2015) Two drugs are better than one. A short history of combined therapy of ovarian cancer. Wspolcz Onkol 19(5):350–353. https://doi.org/10.5114/wo.2014.43975
Cammisotto V et al (2021) The role of antioxidants supplementation in clinical practice: focus on cardiovascular risk factors. Antioxidants 10(2):1–32. https://doi.org/10.3390/antiox10020146
Cauchy F et al (2017) Strong antineoplastic effects of metformin in preclinical models of liver carcinogenesis. Clin Sci 131(1):27–36. https://doi.org/10.1042/CS20160438
Chen X et al (2016) NLLSS: predicting synergistic drug combinations based on semi-supervised learning. PLoS Comput Biol 12(7):1–23. https://doi.org/10.1371/journal.pcbi.1004975
Chlapek P, Neradil J, Redova M, Zitterbart K, Sterba J, Veselska R (2014) The ATRA-induced differentiation of medulloblastoma cells is enhanced with LOX/COX inhibitors: an analysis of gene expression. Cancer Cell Int 13(14):51. https://doi.org/10.1186/1475-2867-14-51 (PMID:24959102;PMCID:PMC4066709)
Dakhale GN, Chaudhari HV, Shrivastava M (2011) Supplementation of vitamin C reduces blood glucose and improves glycosylated hemoglobin in type 2 diabetes mellitus: a randomized, double-blind study. Adv Pharmacol Sci 2011:195271. https://doi.org/10.1155/2011/195271
Das P et al (2019) A survey of the structures of US FDA approved combination drugs. J Med Chem 62(9):4265–4311. https://doi.org/10.1021/acs.jmedchem.8b01610
Dasari S, Bernard Tchounwou P (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. https://doi.org/10.1016/j.ejphar.2014.07.025
de Vries G et al (2020) Testicular cancer: determinants of cisplatin sensitivity and novel therapeutic opportunities. Cancer Treat Rev. https://doi.org/10.1016/j.ctrv.2020.102054
Del Rio D et al (2010) Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients 2(8):820–833. https://doi.org/10.3390/nu2080820
Del Rio D et al (2013) Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18(14):1818–1892. https://doi.org/10.1089/ars.2012.4581
Depaula J, Farah A (2019) Caffeine consumption through coffee: content in the beverage, metabolism, health benefits and risks. Beverages. https://doi.org/10.3390/beverages5020037
Desouza CV, Shivaswamy V (2010) Pioglitazone in the treatment of type 2 diabetes: safety and efficacy review. Clinical Medicine Insights: Endocrinology and Diabetes, 3, p. CMED.S5372. doi: https://doi.org/10.4137/cmed.s5372
Fadus MC et al (2017) Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. J Tradit Complement Med 7(3):339–346. https://doi.org/10.1016/j.jtcme.2016.08.002
Farzaei MH et al (2018) Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients. https://doi.org/10.3390/nu10070855
Feriotto G et al (2021) Caffeic acid enhances the anti-leukemic effect of imatinib on chronic myeloid leukemia cells and triggers apoptosis in cells sensitive and resistant to imatinib. Int J Mol Sci 22(4):1–12. https://doi.org/10.3390/ijms22041644
Fredholm BB et al (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51(1):83–133
Garjón J et al (2020) First-line combination therapy versus first-line monotherapy for primary hypertension. Cochrane Database Systematic Rev. https://doi.org/10.1002/14651858.CD010316.pub3
Guo Q et al (2016) Metformin inhibits growth of human non-small cell lung cancer cells via liver kinase B-1-independent activation of adenosine monophosphate-activated protein kinase. Mol Med Rep 13(3):2590–2596. https://doi.org/10.3892/mmr.2016.4830
Hasegawa T et al (2011) Antioxidant properties of pioglitazone limit nicotinamide adenine dinucleotide phosphate hydrogen oxidase and augment superoxide dismutase activity in cardiac allotransplantation. J Heart Lung Transplant 30(10):1186–1196. https://doi.org/10.1016/j.healun.2011.07.006
Helm CW, States JC (2009) Enhancing the efficacy of cisplatin in ovarian cancer treatment - could arsenic have a role. J Ovarian Res 2(1):1–7. https://doi.org/10.1186/1757-2215-2-2
Hendricks JM et al (2012) 18β-Glycyrrhetinic acid delivered orally induces isolated lymphoid follicle maturation at the intestinal mucosa and attenuates rotavirus shedding. PLoS ONE. https://doi.org/10.1371/journal.pone.0049491
Islam S et al (2017) Genome-wide identification and expression analysis of glutathione S-transferase gene family in tomato: gaining an insight to their physiological and stress-specific roles. PLoS ONE 12(11):1–28. https://doi.org/10.1371/journal.pone.0187504
Izawa K, Amino Y, Kohmura M, Ueda Y, Kuroda M (2010) 4.16 - Human–Environment Interactions – Taste. In: Liu H-W, Mander L (eds) Comprehensive Natural Products II, Elsevier, pp 631–671.https://doi.org/10.1016/B978-008045382-8.00108-8
Jellinger K A (2007) 6 - Alzheimer's Disease. In: Gilman S (ed) Neurobiology of disease, Academic Press, Elsevier, Cambridge, Massachusetts, pp 69–82.https://doi.org/10.1016/B978-012088592-3/50008-6
Kadoma Y, Fujisawa S (2008) A comparative study of the radical-scavenging activity of the phenolcarboxylic acids caffeic acid, p-coumaric acid, chlorogenic acid and ferulic acid, with or without 2-mercaptoethanol, a thiol, using the induction period method. Molecules 13(10):2488–2499. https://doi.org/10.3390/molecules13102488
Kalonia H et al (2009) Effect of caffeic acid and rofecoxib and their combination against intrastriatal quinolinic acid induced oxidative damage, mitochondrial and histological alterations in rats. Inflammopharmacology 17(4):211–219. https://doi.org/10.1007/s10787-009-0012-1
Kasznicki J, Sliwinska A, Drzewoski J (2014) Metformin in cancer prevention and therapy. Ann Transl Med 2(6):1–11. https://doi.org/10.3978/j.issn.2305-5839.2014.06.01
Kinra M et al (2019) Effect of caffeic acid on ischemia-reperfusion-induced acute renal failure in rats. Pharmacology 103(5–6):315–319. https://doi.org/10.1159/000497474
Kinra M et al (2021) Inhibition of NLRP3-inflammasome mediated IL-1β release by phenylpropanoic acid derivatives: in-silico and in-vitro approach. Eur J Pharm Sci 157:105637. https://doi.org/10.1016/j.ejps.2020.105637
Kumar N, Goel N (2019) ‘Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep 24:e00370. https://doi.org/10.1016/j.btre.2019.e00370
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep 4(1):86–93. https://doi.org/10.1016/j.btre.2014.09.002
Kumar A, Vashist A, Kumar P (2010) Potential role of pioglitazone, caffeic acid and their combination against fatigue syndrome-induced behavioural, biochemical and mitochondrial alterations in mice. Inflammopharmacology 18(5):241–251. https://doi.org/10.1007/s10787-010-0048-2
Lima VN et al (2016) Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: gallic acid, caffeic acid and pyrogallol. Microb Pathog 99:56–61. https://doi.org/10.1016/j.micpath.2016.08.004
Loke MF, Hanafi A (2018) Molecular mechanisms responsible for drug resistance. Encycl Bioinform Comput Biol ABC of Bioinform 1–3(1):926–931. https://doi.org/10.1016/B978-0-12-809633-8.20467-6
MacDonald TM et al (2017) Combination therapy is superior to sequential monotherapy for the initial treatment of hypertension: a double-blind randomized controlled trial. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.006986
Malek M et al (2013) Risk of cancer in diabetes: the effect of metformin. ISRN Endocrinol 2013:1–9. https://doi.org/10.1155/2013/636927
Manach C et al (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79(5):727–747. https://doi.org/10.1093/ajcn/79.5.727
Mancini RS, Wang Y, Weaver DF (2018) Phenylindanes in brewed coffee inhibit amyloid-beta and tau aggregation. Front Neurosci 12:1–14. https://doi.org/10.3389/fnins.2018.00735
Martin PL, Bugelski PJ (2012) Concordance of preclinical and clinical pharmacology and toxicology of monoclonal antibodies and fusion proteins: soluble targets. Br J Pharmacol 166(3):806–822. https://doi.org/10.1111/j.1476-5381.2011.01812.x
Martinez-outschoorn UE et al (2017) ‘Erratum: cancer metabolism: a therapeutic perspective (nature reviews clinical oncology (2017) 14 (11–31))’, nature reviews clinical oncology. Nat Publ Group 14(2):113. https://doi.org/10.1038/nrclinonc.2017.1
Medhi B, Aggarwal R, Chakrabarti A (2010) Neuroprotective effect of pioglitazone on acute phase changes induced by partial global cerebral ischemia in mice. Indian J Exp Biol 48(8):793–799 (PMID: 21341537)
Mehrotra A, Shanbhag R, Chamallamudi MR, Singh VP, Mudgal J (2011) Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur J Pharmacol 666(1–3):80–86. https://doi.org/10.1016/j.ejphar.2011.05.039 (Epub 2011 May 30 PMID: 21645514)
Mirzaei S, Gholami MH, Zabolian A, Saleki H, Farahani MV, Hamzehlou S, Far FB, Sharifzadeh SO, Samarghandian S, Khan H, Aref AR, Ashrafizadeh M, Zarrabi A, Sethi G (2021) Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: new hope in the fight against cancer. Pharmacol Res 171:105759. https://doi.org/10.1016/j.phrs.2021.105759
Mohammed FZ, Al-Hussaini ASED, El-Shehabi MES (2015) Antidiabetic activity of caffeic acid and 18β-glycyrrhetinic acid and its relationship with the antioxidant property. Asian J Pharm Clin Res 8(5):255–260
Molaie M et al (2019) Effects of a combination of nigella sativa and Vitex agnus-castus with citalopram on healthy menopausal women with hot flashes: results from a subpopulation analysis. Gynecol Endocrinol 35(1):58–61. https://doi.org/10.1080/09513590.2018.1499086
Molaie M, Darvishi B, Jafari Azar Z, Shirazi M, Amin G, Afshar S (2019) Effects of a combination of Nigella sativa and Vitex agnus-castus with citalopram on healthy menopausal women with hot flashes: results from a subpopulation analysis. Gynecol Endocrinol 35(1):58–61. https://doi.org/10.1080/09513590.2018.1499086 (Epub 2018 Aug 21 PMID: 30129806)
Monteiro Espíndola KM et al (2019) ‘Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol 9:3–5. https://doi.org/10.3389/fonc.2019.00541
Mudgal J et al (2020) Effect of coffee constituents, caffeine and caffeic acid on anxiety and lipopolysaccharide-induced sickness behavior in mice. J Funct Foods 64(May):103638. https://doi.org/10.1016/j.jff.2019.103638
Murkovic M (2003) Phenolic compounds. In: Caballero B (ed) Encyclopedia of food sciences and nutrition (Second Edition). Academic Press, Cambridge, pp 4507–4514. https://doi.org/10.1016/B0-12-227055-X/00914-7
Nasri H, Rafieian-Kopaei M (2014) Metformin: current knowledge. J Res Med Sci 19(7):658–664
Nehlig A, Daval JL, Debry G (1992) Caffeine and the central nervous system: mechanisms of action. Brain Res Rev 17(2):139–170
Norata GD et al (2007) Anti-inflammatory and anti-atherogenic effects of catechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis 191(2):265–271. https://doi.org/10.1016/j.atherosclerosis.2006.05.047
Oboh G et al (2013) Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem Res 38(2):413–419. https://doi.org/10.1007/s11064-012-0935-6
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5):270–278. https://doi.org/10.4161/oxim.2.5.9498
Pandey MM, Rastogi S, Rawat AK (2013) Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid-Based Complement Altern Med ecam 2013:376327. https://doi.org/10.1155/2013/376327
Paukovcekova S et al (2020) Enhanced antiproliferative effect of combined treatment with calcitriol and all-trans retinoic acid in relation to vitamin d receptor and retinoic acid receptor α expression in osteosarcoma cell lines. Int J Mol Sci 21(18):1–19. https://doi.org/10.3390/ijms21186591
Pinho E, Soares G, Henriques M (2015) Evaluation of antibacterial activity of caffeic acid encapsulated by β-cyclodextrins. J Microencapsul 32(8):804–810. https://doi.org/10.3109/02652048.2015.1094531
Polson AG, Fuji RN (2012) The successes and limitations of preclinical studies in predicting the pharmacodynamics and safety of cell-surface-targeted biological agents in patients. Br J Pharmacol 166(5):1600–1602. https://doi.org/10.1111/j.1476-5381.2012.01916.x
Quan W et al (2021) Use of 18β-glycyrrhetinic acid nanocrystals to enhance anti-inflammatory activity by improving topical delivery. Colloid Surf B: Biointerfaces 205:111791. https://doi.org/10.1016/j.colsurfb.2021.111791
Ravishankar B, Shukla VJ (2007) Indian systems of medicine: a brief profile. Afr J Tradit Complement Altern Med AJTCAM 4(3):319–337. https://doi.org/10.4314/ajtcam.v4i3.31226
Reyes CM, Cornelis MC (2018) Caffeine in the diet: country-level consumption and guidelines. Nutrients. https://doi.org/10.3390/nu10111772
Rosendahl AH et al (2015) Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor i receptor levels in human breast cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-14-1748
Saini N, Yang X (2018) Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin 50(2):133–143. https://doi.org/10.1093/abbs/gmx106
Sato Y et al (2011) In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid’. Int J Pharm 403(1–2):136–138. https://doi.org/10.1016/j.ijpharm.2010.09.035
Sharma H (2020) A Detail Chemistry of Coffee and Its Analysis. In: Castanheira D T (ed) Coffee - Production and Research, IntechOpen, London, https://doi.org/10.5772/intechopen.91725.
Siddik ZH (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22(47):7265–7279. https://doi.org/10.1038/sj.onc.1206933
Silva T, Oliveira C, Borges F (2014) Caffeic acid derivatives, analogs and applications: a patent review (2009–2013). Expert Opin Ther Pat 24(11):1257–1270. https://doi.org/10.1517/13543776.2014.959492 (Epub 2014 Oct 4 PMID: 25284760)
Sirota R, Gibson D, Kohen R (2017) The timing of caffeic acid treatment with cisplatin determines sensitization or resistance of ovarian carcinoma cell lines. Redox Biolo 11:170–175. https://doi.org/10.1016/j.redox.2016.12.006
Stalmach A, Edwards CA, Wightman JD, Crozier A (2012) Gastrointestinal stability and bioavailability of (poly)phenolic compounds following ingestion of concord grape juice by humans. Mol Nutr Food Res 56(3):497–509. https://doi.org/10.1002/mnfr.201100566 (Epub 2012 Feb 14 PMID: 22331633)
Tabuchi M et al (2012) The blood-brain barrier permeability of 18b-glycyrrhetinic acid, a major metabolite of glycyrrhizin in glycyrrhiza root, a constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol 32(7):1139–1146. https://doi.org/10.1007/s10571-012-9839-x
Temple JL et al (2017) The Safety of Ingested caffeine: a comprehensive review. Front Psych 8(May):1–19. https://doi.org/10.3389/fpsyt.2017.00080
Touaibia M, Jean-Francois J, Doiron J (2012) Caffeic acid, a versatile pharmacophore: an overview. Mini Rev Med Chem 11:695–713. https://doi.org/10.2174/138955711796268750
Truong HT et al (2017) A method for ferulic acid production from rice bran oil soapstock using a homogenous system. Appl Sci (Switzerland). https://doi.org/10.3390/app7080796
Tyszka-Czochara M, Konieczny P, Majka M (2017a) Caffeic acid expands anti-tumor effect of metformin in human metastatic cervical carcinoma HTB-34 cells: implications of AMPK activation and impairment of fatty acids de novo biosynthesis. Int J Mol Sci 18:462. https://doi.org/10.3390/ijms18020462
Tyszka-Czochara M, Bukowska-Strakova K, Majka M (2017b) Metformin and caffeic acid regulate metabolic reprogramming in human cervical carcinoma SiHa/HTB-35 cells and augment anticancer activity of Cisplatin via cell cycle regulation. Food Chem Toxicol 106:260–272. https://doi.org/10.1016/j.fct.2017.05.065
Tyszka-Czochara M, Bukowska-Strakova K, Kocemba-Pilarczyk KA, Majka M (2018) Caffeic acid targets AMPK signaling and regulates tricarboxylic acid cycle anaplerosis while metformin downregulates HIF-1α-induced glycolytic enzymes in human cervical squamous cell carcinoma lines. Nutrients 10:841. https://doi.org/10.3390/nu10070841
Veselska R et al (2014) The power of natural phenolic compounds: caffeic acid is able to enhance the retinoid-induced differentiation of tumor cells. Cancer Metab 2(S1):P81. https://doi.org/10.1186/2049-3002-2-s1-p81
Vissers MN et al (2000) Effect of plant sterols from rice bran oil and triterpene alcohols from sheanut oil on serum lipoprotein concentrations in humans. Am J Clin Nutr 72:1510–1515. https://doi.org/10.1093/ajcn/72.6.1510
Vliegenthart R et al (2018) High versus standard dose caffeine for apnoea: a systematic review. Arch Dis Child Fetal Neonatal Ed 103:F523–F529. https://doi.org/10.1136/archdischild-2017-313556
Wang Y et al (2018) Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res 37:1–12. https://doi.org/10.1186/s13046-018-0731-5
Wojtunik-Kulesza K et al (2020) Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—a non-systematic review. Nutrients 12:1401. https://doi.org/10.3390/nu12051401
Wolk BJ, Ganetsky M, Babu KM (2012) Toxicity of energy drinks. Curr Opin Pediatr 24:243–251. https://doi.org/10.1097/MOP.0b013e3283506827
Wu ZM et al (2017) In vitro antiviral efficacy of caffeic acid against canine distemper virus. Microb Pathog 110:240–244. https://doi.org/10.1016/j.micpath.2017.07.006
Xi Y et al (2017) Effects of chlorogenic acid on capacity of free radicals scavenging and proteomic changes in postharvest fruit of nectarine. PLoS ONE 12:1–14. https://doi.org/10.1371/journal.pone.0182494
Yang CS, Landau JM, Huang MT, Newmark HL (2001) Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr 21:381–406. https://doi.org/10.1146/annurev.nutr.21.1.381
Yilmaz S (2019) Effects of dietary caffeic acid supplement on antioxidant, immunological and liver gene expression responses, and resistance of nile tilapia, oreochromis niloticus to aeromonas veronii. Fish Shellfish Immunol 86:384–392. https://doi.org/10.1016/j.fsi.2018.11.068
Zhang M, Swarts SG, Yin L, Liu C, Tian Y, Cao Y, Swarts M, Yang S, Zhang SB, Zhang K, Ju S, Olek DJ Jr, Schwartz L, Keng PC, Howell R, Zhang L, Okunieff P (2011) Antioxidant properties of quercetin. Adv Exp Med Biol 701:283–289. https://doi.org/10.1007/978-1-4419-7756-4_38
Zhang F et al (2020) Synergistic effect of chlorogenic acid and caffeic acid with fosfomycin on growth inhibition of a resistant listeria monocytogenes strain. ACS Omega 5(13):7537–7544. https://doi.org/10.1021/acsomega.0c00352
Zhao T et al (2013) Acetylcholinesterase and butyrylcholinesterase inhibitory activities of β -carboline and quinoline alkaloids derivatives from the plants of genus peganum. J Chem. https://doi.org/10.1155/2013/717232
Zhou J et al (2020) The drug-resistance mechanisms of five platinum-based antitumor agents. Front Pharmacol 11(March):1–17. https://doi.org/10.3389/fphar.2020.00343
Acknowledgements
Authors would like to express their gratitude toward Manipal Academy of Higher Education for providing infrastructural support for drafting of this manuscript and collaboration opportunity with Griffith University, Gold Coast, QLD, Australia.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maity, S., Kinra, M., Nampoothiri, M. et al. Caffeic acid, a dietary polyphenol, as a promising candidate for combination therapy. Chem. Pap. 76, 1271–1283 (2022). https://doi.org/10.1007/s11696-021-01947-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01947-7