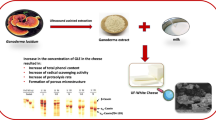
Abstract
A novel approach for enhancing the nutritional benefits and shelf-life of fresh cheese, while reducing food industry waste, is adding chestnut shell extract to the cheese. Chestnut shells are a great source of bioactive substances including phenolic compounds, with anti-inflammatory, antibacterial, and antioxidant activities. The chestnut shell extract exhibited interesting antioxidant properties, resulting from the presence of catechin and gallic acid, 2.59 and 0.97 mgcompound∙gsample−1, respectively, in its composition. The incorporation of the phenolic extract did not influence the pH value of the cheese, with values within the range of 6.2–6.5. Additionally, the extract decreased the syneresis which is important in terms of stability. Regarding the antioxidant characteristics, incorporating the extract improved this biological property. The results proved that an increase in the extract concentration allows the inhibition of higher percentages of this radical. Cheese with extract exhibited higher acceptance, but visual components need improvement. Hence, incorporating chestnut shell extract into fresh cheese is a promising approach to adding value to dairy products and promoting sustainable food production. This study can be considered innovative and relevant because it allows the utilisation of agro-industrial by-products to increase the shelf life of fresh cheese, an extremely relevant parameter for the producer, promoting the circular economy.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Castanea sativa Mill., commonly known as European chestnut, belongs to the Fagaceae family and the genus Castanea. Although this tree has several uses, for example, timber production, chestnuts are primarily grown for their fruit, which can be used as human and animal foods, due to their interesting nutritional value. During the processing of chestnuts, significant amounts of by-products, primarily shells, are created. Chestnut shells, mostly used as fuel, are a plentiful and underutilized by-product of the chestnut peeling process. Just the shell makes up about 20% of the chestnut’s total weight, with the outer shell making up 9–14% of that weight [1]. Chestnut shell (CS) extract is rich in bioactive components, such as phenolic compounds (PC), particularly gallic and ellagic acids, flavonoids (rutin, quercetin, and apigenin), and tannins (condensed and hydrolysable) [2, 3].
Phenolic compounds (PC) are secondary metabolites of plants, that are used as protective agents against external aggressions, such as parasite and pathogens infections, excess UV radiation, and air pollution, among others, to maintain plant health [4, 5]. Regarding their chemical structure, phenols display at least one aromatic ring with one or more hydroxyl groups; normally they are divided into two categories: simple phenols and polyphenols [6]. Chestnuts have been used for a long time in folk and traditional medicine, due to their proven health benefits, particularly anti-inflammatory, anti-microbial, and antioxidant actions. Considering CS, their biological activities have been associated with PC in their composition [1, 7]. Additionally, based on its biological activity and phytochemical profile, the use of CS in the prevention and treatment of chronic illnesses (such as oxidative stress-mediated disorders, inflammatory pathologies, cancer, and gastritis) has been suggested [7]. The biological activities of CS for human health are covered in Fig. 1.
Chestnut shells are gaining attention from the food industry since they display interesting and promising applications for the development of value-added foods and nutraceuticals. Additionally, this by-product is easy to obtain from industry, for example, the production of frozen chestnuts, which facilitates the management of CS. This industry has been focusing on the development of foods with “nature-based” ingredients, not only to increase their nutritional value and functional properties but also to increase the shelf-life of the products [8, 9]. The reuse of this underutilized agro-industrial by-product is supported by scientific evidence about the phytochemical composition, bioactivity, and safety, which were presented in several studies [7, 10]. Indeed, over the past years, consumers have grown interested in natural and functional foods, which increased the demand for new valuable food and the reformulation of others with new or improved functionalities [11, 12]. Functional foods are defined as those that go beyond simple nutritional requirements to provide particular physiologically beneficial effects and/or lower the risk of chronic illness [13]. There has been growing interest in incorporating natural extracts into food formulations to enhance their sensory characteristics and nutritional value [14,15,16,17]. One of those extracts is the CS extract, due to its rich composition in bioactive compounds, including phenolics and antioxidants [18]. Thereby, allying the new trends in the food industry with the line of thought of circular economy and sustainability, it arises the opportunity to incorporate natural phenolic extracts, from the chestnut shell, as a food preservative and as a nutritional supplement, beneficial to human health.
Cheese is one of the most produced dairy products worldwide and one of the most complex and diverse foods enjoyed globally. Indeed, its production has been increasing every year and is expected to keep growing at an annual rate of 6.9% [19]. Cheese is an extremely nutritious food, and it is the given name for a group of fermented milk-based food products [20]. For the production of cheese, three crucial factors must be taken into consideration: the coagulation method used to transform the milk; the acidification characteristics; extra phases in the cheesemaking process (e.g., cooking temperature and pressing and salting conditions) that regulate the moisture levels of the young cheese [21, 22]. The wide range of possible combinations in coagulation technique as well as in coagulum treatment combinations contributes to the diversity of cheese since it allows the development of cheeses with different characteristics, such as appearance, texture, flavour, odour, and shelf life.
Food storage stability depends on multiple factors that can be intrinsic – the quality of the raw material, pH, water content, redox potential – and extrinsic – processing conditions, hygiene, storage conditions, incorrect handling, and packaging material [23]. The influence of these factors can cause a decrease in the quality and safety of the food. To avoid this problem some strategies can be performed: increasing the shelf-life period, adding preservatives, modified atmospheres, selection of adequate packaging, high hydrostatic pressures, biopreservation, and the incorporation of essential oils and phenolic compounds [24,25,26]. Undeniably, some studies have been performed in order to prove that incorporating natural extracts (oils, phenolic and ethanolic extracts from plants, and by-products, among others) into cheese influences their performance and helps shelf-life. In Table 1, some of the mentioned studies are presented along with the main results for the literature research using the keywords: “cheese fortification”, “phenolic compounds” and “natural extracts”.
Fresh cheese is the result of the coagulation and draining of milk employing lactic fermentation, with added rennet, and not subjected to a ripening process. Typically, it displays a white colour and a soft texture. Regarding, the conservation mode, this product is perishable and presents a very limited self-preservation capacity, additionally, it must be stored at temperatures above 0 ºC and below 6 ºC. This cheese is a widely consumed dairy product known for its mild flavour and delicate texture, and it offers an ideal substrate for incorporating natural extracts [32]. The addition of chestnut shell extract to fresh cheese presents an opportunity to create a unique and potentially health-promoting product. However, before introducing such a product to the market, a comprehensive sensory analysis is essential to assess its sensory attributes and consumer acceptance.
Fresh cheese is a product whose shelf life is quite short, due not only to its high moisture content and low salt content but also to the fact that it is not a sterile product and is easily contaminated by bacteria; additionally, as a product containing some fat, it can also suffer oxidation. This hinders the whole process of preparing, shipping and selling the product. Therefore, it is essential to increase the durability of this product. To do so different preservatives, such as sorbic acid, can be added to avoid oxidation and microbial contamination of the product; however, some synthetic preservatives can be harmful to the consumer or decrease the product’s nutritional value. In addition, as previously stated, consumers have become increasingly concerned about consuming healthy, nutritious products with fewer synthetic compounds. Literature reveals that the incorporation of extracts from plants and ago-industrial by-products into cheese is an effective strategy to increase shelf-life. However, the number of studies considering the incorporation of these extracts into fresh cheese is scarce. In fact, this type of cheese is an extremely consumed product in Portugal and has a short shelf life. As such, it is extremely important and relevant to find strategies to extend the shelf life of fresh cheese. Thus, the incorporation of phenolic extracts of natural origin seems to be an interesting strategy to increase the antioxidant properties of these cheeses. increase the antioxidant properties of these cheeses, consequently prolonging their shelf life.
Hence, the present study intended to incorporate phenolic-rich extracts from agricultural by-products, chestnut shells, into fresh cheese and evaluate their potential to act as a natural preservative, assessing the physicochemical parameters and their antioxidant capacity. Furthermore, various sensory evaluation methods, including descriptive analysis and consumer testing, were employed to elucidate the impact of chestnut shell extract on the sensory characteristics of the cheese, contributing to a better understanding of the sensory profile of fresh cheese enriched with natural extracts and highlighting the potential applications of chestnut shell extract in the dairy industry. Additionally, it is intended to create a strategy that allows the interaction of companies responsible for the production of chestnut shells (an easily obtainable and economically important by-product), with dairy industries for the development of value-added products, reducing the environmental impact of this by-product and, at the same time, establishing a value chain.
Materials and methods
Samples and reagents
All chemical reagents, solvents, and standards were of analytical reagent grade and were purchased commercially. The company Agromontenegro, located in Serra da Padrela (Alto Trás-os-Montes, Portugal), kindly provided the chestnut shell samples. Ethanol, the extraction solvent, (Ref. 1.02371.1000, C2H6O, CAS 64-17-5) was purchased from VWR (Rosny-sous-Bois, France). The standards used for the characterization of the extract 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Ref. D9132, C18H12N5O6, CAS 1898-66-4), ABTS (Ref. A1888 C18H24N6O6S4, CAS 30931-67-0), Trolox (Ref. 238,813, C14H18O4, CAS 53188-07-1), Sorbic Acid (Ref. S1626, C6H8O2, CAS 110-44-1), (+)-catechin hydrate (Ref. C1251, C15H14O6, CAS 225,937- 10-0), gallic acid (Ref. 91,215, C7H6O5, CAS 149-91-7), resveratrol (Ref. R5010, C14H12O3, CAS 501-36-0) and rosmarinic acid (Ref. 00390580, C18H16O8, CAS 20283-92-5) were acquired from Sigma Aldrich (St. Louis, MO, USA). For the ultrapure water (UPW) a Merck Millipore Mill-Q purification equipment with 18.2 Ω of electric resistance (Billerica, MA, USA), was applied.
Methods
Extraction of the phenolic compounds from the chestnut shells
The phenolic extract was obtained from the CS using the solid-liquid extraction technique, with a Soxhlet apparatus. The sample was previously lyophilized to remove water. Ethanol was used as an extraction solvent due to its relatively low polarity and due to being a GRAS (Generally Recognized as Safe) solvent. The ratio between sample mass and solvent volume was 1:20 (m/V), the extraction temperature was 70 °C and the extraction time was 2 h. The solvent was evaporated with a rotary evaporator and full evaporation was achieved with a nitrogen stream.
Characterization of the phenolic extract
According to the literature, the Total Phenolic Content (TPC) determination was evaluated [33]. A 2 mL cuvette containing 20 µL of the sample solution, 100 µL of the Folin-Ciocalteu reagent, and 1580 µL of distilled water were filled. After that, 333.3 mg∙L-1 of saturated sodium carbonate solution was added, and the cuvette was left to incubate in the dark for 2 h. A spectrophotometer was used to test the absorbance at 750 nm (V-530, Jasco, OK, USA). TPC was calculated as Gallic Acid Equivalent (GAE) per g extract (mgGAE∙gextract-1). The concentration of gallic acid equivalents is obtained using the calibration curve of the absorbance against the concentration of gallic acid (Abs = 0.0748 × Cgallic acid).
Two methods - the assay with 2,2-diphenyl-1-picrylhydrazyl (DPPH) and the assay with 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), also known as Trolox Equivalent Antioxidant Capacity - were used to measure the antioxidant capacity of the phenolic extracts (TEAC). The assessment of this biological capacity was performed according to literature protocols [34]. The percentage of inhibition of the free radicals was determined and the IC50 values were determined for both assays. The results were expressed as Trolox Equivalent per g of extract (mgTrolox∙gextract-1).
Identification of the main phenolic compounds using HPLC-DAD
The phenolic compounds present in the extract were identified and quantified through high-performance liquid chromatography (HPLC) utilizing an Elite LaChrom HPLC system (Hitachi, Japan), outfitted with a diode array detector (DAD). The separation of the phenolic compounds was performed employing a Purospher STAR RP-18 endcapped (4.0 mm × 250 mm ID, particle size 5 μm) reverse phase chromatography column with a LiChrosphere 100 RP-18 (particle size 5 μm) pre-column. As a solvent, acetonitrile, water, and ethanol (2:1:1 V/V/V) were used to create standards and sample solutions. The extract obtained was reconstituted in 10 mL of the solvent to create the sample solution. For mobile phase A UPW was combined with 0.5% orthophosphoric acid, whereas mobile phase B was an 80:20 (V/V) mixture of methanol and acetonitrile. The gradient’s values were as follows: 0–10 min, 10% B; 10–25 min, 15% B; 25–40 min, 30% B; and 40–50 min. The flow rate was 0.8 mL and the injection volume was 40 µL. Phenolic compounds were identified and quantified using the external standard method. For that, the compounds were identified by their retention time and by comparing the chromatogram of the sample with the chromatogram of the standard alone.
Fresh cheese production
To produce the fresh cheese 1 L of pasteurised milk, bought in a local supermarket, was heated to 34 °C and a teaspoon (1 gram) of sodium chloride was added. Afterwards, when the milk reached 39 °C, 1 mL of rennet was added. The mixture was homogenised by continuously mixing with a glass stirrer, covered with a cloth, and left to rest for 30 min. Subsequently, the product obtained was strained to remove the serum and placed in a mould. Finally, it was placed in the refrigerator until the next day. Six cheeses were produced with slightly different changes in the additives as described in Table 2. The cheeses were stored in a fridge at 4 °C. Stability tests of the cheeses were performed at four different times: t0, t1, t2, and t3, over 2 weeks.
Physical-chemical characterization of the cheeses
To determine the pH value, 9 mL of water was added to 1 g of sample, homogenized for 1 min in a high-performance homogeniser (IKA T18 digital Ultra Turrax), and, using the digital pH meter (XS pH 50+), the pH value of the different samples was measured.
Syneresis allows the evaluation of cheese moisture and is defined as the whey that the cheese releases after it is ready [35]. In order to evaluate this parameter, 2 g of each sample was weighed and centrifuged for 20 min at 1100 rpm (700×g). Using the final serum mass, it was possible to determine the percentage of syneresis using Eq. 1, where m represents the mass.
Also to determine moisture, the Water Holding Capacity (WHC), which indicates the total water that remains bound to the cheese matrix after the application of an external force, was determined. Each sample was weighed 0.25 g and centrifuged for 20 min at 3000 rpm (1510×g). Subsequently, the obtained product was placed in the oven at 105 °C until completely dry [36]. By comparing the final mass of the sample with the mass weighed initially, it was possible to determine its total water content, using Eq. 2.
Evaluation of the antioxidant properties of the cheeses
To determine the TPC and antioxidant capacity of the samples, the phenolic compounds were first extracted. For this, 2 g of sample were diluted in 4 mL of ethanol (extraction solvent) with the help of a vortex (VWR VV3) for 1 min. Afterwards, the solution was subjected to a 5-minute sonication treatment in an ultrasonic bath, followed by centrifugation at 1100 rpm (203×g) for 5 min. Subsequently, the supernatant was collected and mixed with 4 mL of ethanol. The solution was vortexed for 1 min and placed in the ultrasonic bath for 5 min. These steps were repeated. The solution was centrifuged for 10 min at 1100 rpm (203×g). The supernatant was removed and stored for total phenolic content (TPC) analyses and antioxidant capacity assays, which were performed according to literature protocols [34].
Sensorial analysis
Sensory panellists who expressed consistent willingness to participate were internally recruited from a pool of students, researchers, and technicians. Subsequently, the volunteers underwent a screening process to assess their sensory acuity and familiarity with the specific product category under evaluation. In the initial phase of the study a comprehensive characterization of the panel, consisting of 24 individuals, was conducted. The subjects underwent a series of tests to evaluate their sensory capabilities. These tests included a basic taste identification assessment, an odour recognition test, a colour identification test utilizing the triangular method, and the Ishihara colour blindness test. To identify fundamental tastes, including sweet, sour, salty, bitter, and umami, the guidelines outlined in ISO 3972:2011 were followed. Solutions adhering to the concentrations specified in the standard were prepared to evaluate subjects’ ability to recognize and differentiate these tastes. The concentrations used corresponded to the detection threshold, ensuring that subjects were exposed to reference samples with known intensities for precise taste evaluation. ISO 5496:2006 guidelines were employed to assess odour characteristics. Subjects described a range of odours using standardized odour reference materials in covered test tubes. To assess the participants’ sensitivity to subtle variations in colouration, the methodology outlined by Chaves and Sproesser (1993) was adopted. This methodology involved conducting a triangular test, wherein one diluted sample was deliberately different from the others [37]. The Ishihara test was also utilized to evaluate the subjects’ visual characteristics.
Following the characterization assessment of the panel, two types of tests were conducted: the control difference test and the affective test. For the control difference test, three samples for comparative analysis were utilized. The control sample consisted of fresh cheese containing sorbic acid (synthetic preservative), while the second sample involved fresh cheese with the chestnut extract. The third sample mirrored the control but incorporated pigment, to replicate the colour of the cheese with the extract. The subjects were tasked with determining which sample closely resembled the control.
In the affective test, the same three samples of fresh cheese were presented to the subjects. They were requested to express their preference among the samples by assigning numerical values ranging from 1 to 9 to each attribute, including appearance, colour, odour, taste, and texture. This rating scale aimed to capture the extent of influence each attribute had on their selection, with 1 denoting “no influence” and 9 denoting “total influence.”
One week later, the control difference test was replicated using fresh cheese containing sorbic acid as the control. However, this time, the other samples comprised fresh cheese with CS extract and fresh cheese with CS extract combined with pigment. The volunteers were once again tasked with identifying the sample that closely resembled the control. Additionally, in the descriptive analysis tests, the subjects.
evaluated the previous samples regarding appearance, colour, odour, taste, texture, and overall evaluation. The evaluative scale ranged from 1, signifying “complete dislike,” to 9, signifying “complete preference.”
Statistical analysis
The study of variance (ANOVA) was applied for the statistical study. The null hypothesis is correct when all the sample values are the same or do not significantly differ from one another. The values were considered statistically significant for p < 0.05 (95% confidence interval), using the GraphPad software.
For the sensorial analysis, the t-test for dependent samples and cluster analysis were used to analyse the results. The t-test for dependent samples was chosen because it allows to compare the means of two related groups, which is essential in our study design. On the other hand, cluster analysis was selected as a multivariate exploratory technique because it helps to identify patterns and groupings within the study data, providing a comprehensive understanding of the underlying structure. The t-test for dependent samples and cluster analysis were conducted using Statistica for Windows software, version 7.0.
Results and discussion
Chestnut shell extract characterization
Extraction is one of the most critical phases in the process of isolation of bioactive compounds from natural food matrices. The PC present in chestnut shells were extracted using a solid-liquid extraction technique with a Soxhlet apparatus. Soxhlet extraction offers some attractive advantages over other extraction methods. It is a simple procedure that requires low-cost equipment and enables the extraction of a large amount of material without requiring filtration afterwards. Despite its drawbacks, such as the duration of the extraction and the amount of solvent utilized, this approach is nevertheless widely employed in many laboratories [38]. Ethanol was selected as the solvent since it is generally recognized as safe (GRAS) and has a low boiling point, thereby lowering energy expenditures and simplifying the solvent removal procedure. The obtained results for the characterization of the extract – its total phenolic content, antioxidant capacity, and chemical composition – are present in Table 3.
The CS extract displays a TPC of 510.8 ± 29.9 mgGAE∙gextract−1. The extraction method applied, along with factors, such as light, temperature, geographical location, water availability, and nutrients can affect this property. Additionally, the drying pre-treatment applied (lyophilization) and the selected extraction method can also impact this value. Literature reports values between the range of 111–806 mgGAE∙gextract−1, so, the achieved results are within literature values [7, 38, 39]. Due to its high TPC, it is expected that the CS extract displays high antioxidant capacity.
To evaluate the antioxidant capacity of the CS extract, the assays with the DPPH and ABTS radicals were conducted. The results in Table 3 reveal that the IC50 – the necessary concentration to inhibit 50% of the DPPH radical – is 9.4 ± 0.3 mg∙L− 1. Literature reports that the IC50 value of the CS extract is between 2.7 and 101.0 3 mg∙mL− 1 [38, 40, 41]. The value obtained in the present study is within the values reported in the literature and is considerably lower than the maximum limit. Additionally, the literature reports that an IC50 < 50 mg∙L− 1 indicates a very strong antioxidant capacity [42]. This extract demonstrates high antioxidant capacity as lower IC50 values indicate greater capacity. For both assays, the antioxidant capacity of the extract was compared to the antioxidant capacity of the standard antioxidant Trolox, a vitamin E analogue with strong antioxidant properties. The achieved values were 471.23 ± 8.75 mgtrolox∙gsample−1 and 780.60 ± 23.55 mgtrolox∙gsample−1 for the ABTS radical and the assay with DPPH, respectively. The present results allowed to verify that the chestnut shell extract exhibited a very strong antioxidant capacity.
An HPLC-DAD analysis was carried out to identify and quantify the phenolic compounds contained in the CS extract, to gain a better understanding of its biological characteristics. According to Table 3, the main phenolics present in the CS extract (based on the standards used in this study) were catechin and gallic acid. Previous literature studies have revealed that these two compounds, along with ellagic acid, are commonly found [10, 43, 44].
However, the concentration of these compounds can vary depending on the origin and cultivar circumstances of chestnuts, as well as the extraction technique used. These variations can be observed in the literature values. Due to their molecular structure, both these compounds have the capacity to perform as antioxidant agents since they display scavenging capability. This ability comes from the possibility of these compounds donating hydrogen atoms in the presence of free radicals and, subsequently, delocalizing electrons to stabilize the structure of the compounds. Therefore, the strong antioxidant capacity of the CS extract may be due to the presence of catechin and gallic acid (as well as other CFs not analysed) in the extract composition. Overall, these findings suggest that the CS extract may have potential health benefits due to its antioxidant properties.
Characterization of the fortified cheeses
The present study intended to evaluate the effect of the incorporation of a phenolic-rich extract, from chestnut shells, into fresh cheese. For that, six cheese formulations were studied: NC (Negative Control) without additives, PC (Positive Control) with sorbic acid as a synthetic preservative, CS 1 (Chestnut Shell 1) with 1 gram of CS extract, CS 2 (Chestnut Shell 2) with 2 g of CS extract, MIX 1 (Mixture 1) with 0.5 g of sorbic acid and 0.5 g of CS extract and MIX 2 (Mixture 2) with 1 gram of sorbic acid and 1 gram of CS extract. An initial sensorial analysis revealed that all cheeses displayed the appearance and smell of fresh cheese. However, the cheeses supplemented with CS extract had a slight aroma of the extract. Moreover, the incorporation of CS extract caused the colour of the cheeses to become slightly brownish/greyish, and the addition of more extract resulted in a more potent colour change. The produced cheeses are displayed in Fig. 2.
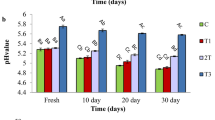
To evaluate the physical and chemical stability of the produced cheeses, the determination of pH value, syneresis, and water holding capacity (WHC) of each sample were determined in four different analysis times. The pH value and, consequently, acidity, are parameters that play a pivotal role in influencing the flavour of the cheese. Furthermore, these parameters are fundamental indicators of the chemical and microbiological safety measures for foods. Syneresis and water holding capacity (WHC) are two important factors regarding the performance of the cheese. Syneresis is a parameter that denotes the separation of the serum from the cheese, primarily occurring at the top of the product and it can be a result of the gel matrix contracting and sedimenting during storage [43]. This problem can be overcome by incorporating additives. The WHC is inversely correlated to syneresis since it measures the ability of the cheese to retain water. The pH values for each sample throughout the study period are depicted in Fig. 3(a), while the achieved values for WHC and syneresis can be found in Fig. 3(b).
From the results presented in Fig. 3(a), it is possible to observe that all the cheeses exhibited pH values within the usual range of 6.2–6.5. Additionally, the incorporation of the CS extract allowed for a slight decrease in the pH value of the cheese, when compared to the NC sample. Comparing the samples with extract to the PC sample, it is observable that the behaviour of the pH of the cheese samples is similar; it decreases with time. Additionally, the results show that the pH of the samples with extract is, overall, significantly different from the PC, displaying a lower pH. It has been reported in the literature that the addition of phenolic extracts to foods often results in a decrease in the pH value when compared to the control [44, 45]. The acidic nature associated with PC might explain the decrease in the cheese’s pH. On the other hand, it is also noticeable that the pH decreases with time. This reduction may indicate a change in the texture of the food, for example, due to processes of proteolysis and lipolysis that originate amino acids and free fatty acids, leading to a reduction in the pH [46]. The results, therefore, demonstrate that it is feasible to include CS extracts in fresh cheese while maintaining a pH that is safe for consumption.
Characterization of the cheeses throughout the study time: pH values for the six produced cheeses (a) and variation of the water holding capacity and the syneresis (b) NC - Negative Control, PC - Positive Control, CS 1 - Chestnut Shell with 1 gram of CS extract, CS 2 - Chestnut Shell with 2 g of CS extract, MIX 1 - Mixture 1 with 0.5 g of sorbic acid and 0.5 g of CS extract and MIX 2 - Mixture 2 with 1 gram of sorbic acid and 1 gram of CS extract. The results are expressed as means ± standard deviations of n = 3 independent measurements. The different capital letters represent significantly different values (p < 0.05) for the same sample, at different times. Different small letters represent significantly different values (p < 0.05) for the same time between samples
Based on the observation from Fig. 3(b), it is noticeable that all the samples displayed a similar ability to retain water throughout the study. This indicates that the cheeses produced had the capacity to sustain elevated levels of moisture. Regarding the syneresis, it is noticeable that the values had slight oscillations among the samples and during the analysis. The obtained data reveals that the incorporation of the CS extract allowed to reduction of this phenomenon, as well as to decrease its variations during storage. Throughout the study period, sample PC and sample CS 1 exhibited similar behaviour for both parameters. However, when the amount of extract in the cheese increased (sample CS 2), the syneresis decreased within the given period. It is noteworthy that the combination of sorbic acid and phenolic extract (samples Mix 1 and Mix 2) helped to reduce this phenomenon in the cheese. This suggests that there may be a synergistic interaction between the positive control and the phenolic extract. A possible explanation for these results is the interaction between phenolic compounds present in the extract with casein. These interactions can form stable complexes, due to strong internal bonds, that can prevent protein rearrangement during storage, maintaining casein networks [35, 47, 48]. Additionally, the literature suggests that PC can bond to water, decreasing whey release and increasing water holding capacity [47]. This may benefit cheese stability during storage.
Consequently, the cheese is able to retain the water, reducing the syneresis. Overall, the present results seem to indicate that the incorporation of CS phenolic-rich extract does not influence the WHC of the cheese, while it reduces the syneresis of the product. This demonstrates that the addition of CS extract could serve as a promising ingredient to enhance the performance of fresh cheese.
Antioxidant capacities of the fortified cheeses
The antioxidant properties of the produced cheeses were analysed to assess the potential of the phenolic extract from CS to act as an antioxidant agent. For that, the total phenolic content (TPC) and the assay with the DPPH radical were performed. The achieved results are presented in Table 4.
Upon analysing Table 4, it is noticeable that all samples exhibited significant increases in TPC over time. The data shows that the TPC of the fresh cheese was greatly improved by the addition of CS extract. The CS 2 and Mix 2 samples, in particular, demonstrated significantly higher values for all the time points assessed, compared to the negative control sample. This indicates that the addition of CS extract had a positive impact on the overall phenolic content of the fresh cheese, enhancing its nutritional value and potentially increasing its health benefits. According to the literature, milk proteins have been observed to interact with phenolic and polyphenolic compounds. These interactions, coupled with the interaction of other peptides found in cheese, have been shown to result in a reduction of the antioxidant activity and total phenolic content [48,49,50]. Therefore, milk proteins and peptides in cheese can potentially affect the bioavailability of these compounds in the cheese. This is one possible reason why the difference with the control is only noticeable for higher extract concentrations since smaller amounts of the extract can cause PC to bind more easily to milk proteins. Over time, it is possible to observe that the TPC of the cheeses increases. During cheese storing, the release of phenolic compounds from the cheese network can occur due to the release of chains of peptides and amino acids resulting in changes in the protein structure subsequent to microbial activity. Additionally, some of those products, such as tyrosine, have a similar structure to phenolic, which can interfere with the Folin-Ciocalteu reagent [49]. Over time, this phenomenon can cause an increase in the TPC of cheeses.
Considering the antioxidant capacity, this biological property was determined through the assay with the DPPH radical. It is noticeable, from Table 4, that the antioxidant capacity of the samples remained constant throughout the study period since none of the samples exhibited significantly different values. This result shows that an increase in TPC does not necessarily lead to an increase in antioxidant capacity, indicating that this increase may be due to the metabolism of microorganisms as mentioned previously [49]. It is noteworthy that, the negative control cheese (NC) displayed some antioxidant capacity; this can be the result of the presence of bioactive peptides, that display some biological activity, such as antioxidant [30]. Furthermore, cheeses also present compounds, such as vitamin E, β-carotene among others, that display antioxidant capacity, enhancing the antioxidant capacity of the final product [50]. It is observable that the incorporation of CS extract results in a higher DPPH inhibition compared to both negative and positive controls. However, the increase is only significant for higher extract concentrations, resulting in greater inhibition percentages. Based on the data, it appears that the sample containing 2 g of the extract (CS 2) and the one with 1 gram of sorbic acid and 1 gram of extract (Mix 2) have comparable inhibition values over time. The findings suggest that the CS extract may enhance the performance of the positive control and indicate that the extract has the potential to replace it if used in larger amounts. The increase in the antioxidant capacity of the cheeses is a consequence of phenolic compounds (PC) being excellent hydrogen donors, which help to stabilize free radicals. Furthermore, PC are able to create covalent bonds with the nucleophilic chains of proteins, originating a protein-phenol derivative, that also exhibits antioxidant potential [51,52,53]. As a result of these interactions, it appears that the protein-phenolic complexes can reduce DPPH radicals by becoming free radicals themselves. Some of the samples show a slight decrease in the percentage of DPPH inhibition over time. This could be due to a decrease in the bioavailability of the phenolic compounds, as explained earlier. Moreover, the instability of PC could also be a reason for this decrease, since they can be sensitive to various factors such as pH, light, and enzymatic activity. Therefore, to address this issue, a possible solution would be to microencapsulate the phenolic extract from CS, which would increase its stability and protect it from external factors. This will also allow for a controlled release of the bioactive compounds.
Even so, the results of the study indicate that the addition of phenolic extract from chestnut shells can enhance the antioxidant properties of fresh cheese and create fortified foods with high antioxidant potential. Additionally, the study revealed that incorporating the extract can increase the shelf life of the food by improving its physical stability over time when compared to the control samples.
Sensorial analysis
Sensory analysis plays a crucial role in evaluating food products, providing valuable insights into their organoleptic properties. The organoleptic properties, including taste, aroma, texture, and appearance, contribute significantly to consumer acceptance and overall product quality.
Control difference tests are important in sensory analysis as they help compare and identify samples that closely resemble a control, providing valuable insights into the characteristics and quality of the tested products. In the first phase of the sensory study, the sensory panellists rated the cheese with extract as more similar to the control, with a score of 54.2%, while the control with pigment scored 45.8%. This result was largely influenced by the pink colour induced by the pigment, which contributed to a higher score in the “appearance” component for the cheese with the extract. However, it is noteworthy that the control with pigment received higher scores in terms of the “odour” and “taste” attributes, indicating the panel’s discerning sensory evaluation capabilities in perceiving this sample as closer to the control. Regarding preference, the results show a tendency of the panel towards the control with pigment, with a score of 41.7%, scoring the control and the cheese with extract with the same score, 29.2%. Once again, colour played an important role in the choice made by the subjects. To determine the weight of other attributes in the subjects’ preference, the results were analysed without considering the colour attribute. Thus, it was found that the preference predominantly focuses on the cheese with extract, with a score of 34.6%, followed by the control with 33.7%, and then the control with pigment with 31.7%.
During the second phase, one week later, the tasters re-evaluated the cheese samples. They found the cheese with extract to be more similar to the control, scoring it at 85.7%. Meanwhile, the cheese sample with extract and pigment received a score of only 14.3%. As for preference, the sample with pigment was again the most favoured, with a score of 52.4%, followed by the cheese with extract alone with 28.6%, and the control with 19%. The same analysis of the results without considering the colour attribute was conducted, and this time it revealed a preference that was distributed without significant differences among the three samples.
Descriptive tests were conducted alongside the affective testing to evaluate the overall consumer acceptance of the fresh cheese enriched with CS extract. These tests were crucial in determining the market potential and consumer perception of the novel cheese product. The same panel was used for both types of testing. Descriptive analysis, a widely known sensory evaluation method, was used to identify and quantify the specific sensory attributes influenced by the addition of CS extract. Subjects evaluated the samples based on predetermined sensory descriptors, allowing for a detailed characterization of the sensory profile. This analysis provided valuable information on the flavour, taste, texture, and appearance of the cheese, facilitating a better understanding of the impact of chestnut peel extract on these attributes. After evaluating the attributes of appearance, colour, odour, taste, texture, and overall evaluation of the cheese with extract and the control, it was found that there were significant differences in appearance and colour (p < 0.001) due to the darker hue resultant of adding the extract to the cheese. Additionally, there were differences in odour and taste (p = 0.05). When comparing the cheese with extract to the cheese with extract plus pigment, significant differences were observed only in appearance and colour attributes (p < 0.05), due to the evident differences between a cheese with a pink hue and another with a whiter hue. Since the base cheese was the same for both, the results did not show any statistically significant differences in the remaining attributes.
Based on the results presented in Fig. 4(a), it is noticeable that cheeses fortified with CS extract scored higher in terms of odour and flavour attributes compared to the control sample. However, the control sample received better ratings from the panellists for visual components such as appearance and colour. Concerning the texture and overall evaluation, no significant differences were observed among the three samples. Figure 4(b) depicts the cluster analysis, illustrating how the key variables assessed by the panel manifest individually and cooperatively. Odour is considered an individually expressed variable, demonstrating strong discriminatory power in assessing the sensory characteristics of the cheese samples. On the other hand, taste and texture are expressed cooperatively in cheese evaluation, along with colour and appearance.
Conclusion
The present study intended to evaluate the possibility of increasing the nutritional value and shelf-life period of fresh cheese through the incorporation of chestnut shell extract, as a strategy to overcome the problems caused by agro-industrial by-products, which follow the principles of sustainability and circular economy. The characterization of the extract displayed interesting antioxidant properties, exhibiting a total phenolic content of 510.8 ± 29.9 mgGAE∙gextract−1 and 471.23 ± 8.75 mgtrolox∙gsample−1 and 780.60 ± 23.55 mgtrolox∙gsample−1 for the assay with the ABTS radical and the assay with DPPH, respectively. The incorporation of the extract did not affect the stability of the fresh cheese, throughout the storage period. It played a pivotal role in reducing cheese syneresis, thus significantly enhancing its stability, which is particularly vital for a product with a limited shelf life. The evaluation of the antioxidant capacity of the cheeses demonstrated that the extract increased the total phenolic content and the cheese’s overall antioxidant properties of the cheese. This improvement might be related to the presence of catechin and gallic acid in the extract composition, along with other beneficial phenolic compounds. A sensorial analysis revealed that the cheese with CS extract demonstrated superior odour and taste attributes compared to the control, while the control received higher ratings in visual components such as appearance and colour. These findings contribute to a better understanding of the sensory characteristics of the cheese enriched with CS extract. They highlight the importance of further research to optimize its sensory attributes and consumer acceptance. Therefore, the achieved results provide valuable information on the potential for agricultural by-product extracts to be used to enrich dairy products, such as fresh cheese. Furthermore, this strategy is a key step in the creation of innovative, health-conscious, and consumer-oriented dairy products that align with evolving consumer preferences, while allowing reducing the waste from agricultural industries.
References
N. Echegaray, B. Gómez, F.J. Barba, D. Franco, M. Estévez, J. Carballo, K. Marszałek, J.M. Lorenzo, Chestnuts and By-Products as source of natural antioxidants in Meat and Meat products: a review. Trends Food Sci. Technol. 82(April), 110–121 (2018). https://doi.org/10.1016/j.tifs.2018.10.005
N.A. Cacciola, G. Squillaci, M. D’Apolito, O. Petillo, F. Veraldi, F. La Cara, G. Peluso, S. Margarucci, A. Morana, Castanea Sativa Mill. Shells aqueous extract exhibits Anticancer Properties Inducing Cytotoxic and Pro-apoptotic effects. Molecules. 24(18) (2019). https://doi.org/10.3390/molecules24183401
de M.C.B.M. Vasconcelos, R.N. Bennett, E.A.S. Rosa, J.V. Ferreira-Cardoso, Composition of European Chestnut (Castanea Sativa Mill.) And Association with Health effects: Fresh and Processed products. J. Sci. Food Agric. 90(10), 1578–1589 (2010). https://doi.org/10.1002/jsfa.4016
M. Anastasiadi, H. Pratsinis, D. Kletsas, A.-L. Skaltsounis, S.A. Haroutounian, Grape stem extracts: Polyphenolic Content and Assessment of their in Vitro antioxidant Properties. LWT - Food Sci. Technol. 48(2), 316–322 (2012). https://doi.org/10.1016/j.lwt.2012.04.006
A.M. Višnjevec, P. Baker, A. Charlton, D. Preskett, K. Peeters, C. Tavzes, K. Kramberger, M. Schwarzkopf, Developing an Olive Biorefinery in Slovenia: analysis of Phenolic compounds found in Olive Mill Pomace and Wastewater. Molecules. 26, 7 (2021). https://doi.org/10.3390/molecules26010007
M.L. Soto, E. Falqué, H. Domínguez, Relevance of natural phenolics from grape and Derivative products in the Formulation of cosmetics. Cosmetics. 2(3), 259–276 (2015). https://doi.org/10.3390/cosmetics2030259
D. Pinto, M. Cádiz-Gurrea, de la L. Vallverdú-Queralt, A. Delerue-Matos, C. Rodrigues, Castanea Sativa shells: a review on Phytochemical Composition, Bioactivity and Waste Management Approaches for Industrial Valorization. Food Res. Int. 144(April) (2021). https://doi.org/10.1016/j.foodres.2021.110364
C.R.M. Rudke, C. Camelo-Silva, A.R. Rudke, E.S. Prudencio, de C.J. Andrade, Trends in dairy products: New ingredients and Ultrasound-based Processing. Food Bioprocess. Technol. (2023). https://doi.org/10.1007/s11947-023-03153-7
C.C. Chu, S.C. Chew, W.C. Liew, K.L. Nyam, Review article vitamin E: a multi-functional ingredient for Health Enhancement and Food Preservation. J. Food Meas. Charact. (2023). https://doi.org/10.1007/s11694-023-02042-z
F. Lameirão, D. Pinto, E.F. Vieira, A.F. Peixoto, C. Freire, F. Rodrigues, Green-sustainable recovery of phenolic and antioxidant compounds from Industrial Chestnut shells using Ultrasound-assisted extraction: optimization and evaluation of Biological activities in Vitro. Antioxidants. 9, 267 (2020)
F. Chamorro, M. Carpena, M. Fraga-Corral, J. Echave, M.S. Riaz Rajoka, F.J. Barba, H. Cao, J. Xiao, M.A. Prieto, J. Simal-Gandara, Valorization of Kiwi Agricultural Waste and Industry By-Products by recovering bioactive compounds and applications as Food additives: a circular economy model. Food Chem. 370, 131315 (2022). https://doi.org/10.1016/j.foodchem.2021.131315
A. Grobelna, S. Kalisz, M. Kieliszek, The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the selected quality characteristics, Anthocyanin Stability, and antioxidant properties. Biomolecules. 9(11) (2019). https://doi.org/10.3390/biom9110744
D. Granato, M. Carocho, L. Barros, I. Zabetakis, A. Mocan, A. Tsoupras, A.G. Cruz, T.C. Pimentel, Implementation of Sustainable Development Goals in the dairy Sector: perspectives on the Use of Agro-industrial Side-streams to Design Functional Foods. Trends Food Sci. Technol. 124, 128–139 (2022). https://doi.org/10.1016/j.tifs.2022.04.009
S. Rout, S. Tambe, R.K. Deshmukh, S. Mali, J. Cruz, P.P. Srivastav, P.D. Amin, K.K. Gaikwad, ; de Aguiar Andrade, E. H.; de Oliveira, M. S. Recent Trends in the Application of Essential Oils: The next Generation of Food Preservation and Food Packaging. Trends Food Sci. Technol, 2022, 129, 421–439. https://doi.org/10.1016/j.tifs.2022.10.012
M. Pateiro, F. Barba, R. Domínguez, A. Sant’Ana, M. Gavahian, B. Gómez, J.M. Lorenzo, Essential oils as natural additives to prevent oxidation reactions in meat and Meat products: a review. Food Res. Int. 113, 156–166 (2018). https://doi.org/10.1016/j.foodres.2018.07.014
E. Messinese, O. Pitirollo, M. Grimaldi, D. Milanese, C. Sciancalepore, A. Cavazza, By-Products as sustainable source of Bioactive compounds for potential application in the field of Food and New materials for Packaging Development. Food Bioprocess. Technol. (2023). https://doi.org/10.1007/s11947-023-03158-2
A. Shenbagam, N. Kumar, K. Rahul, A. Upadhyay, M. Gniewosz, M. Kieliszek, Characterization of Aloe Vera Gel-based Edible Coating with Orange Peel Essential Oil and its Preservation effects on Button mushroom (Agaricus Bisporus). Food Bioprocess. Technol. 1–21 (2023). https://doi.org/10.1007/s11947-023-03107-z
C. Gowe, Review on potential use of fruit and vegetables By-Products as a Valuable source of Natural Food Additives. Food Sci. 45, 47–61 (2015)
M. Shahbandeh, Global Cheese Market - Statistics & Facts; 2022
P.F. Fox, L.H. Paul, McSweeney, Cheese: An Overview. In Cheese; 2017; pp 5–21. https://doi.org/10.1016/B978-0-12-417012-4.00001-6
M. Almena-Aliste, B. Mietton, Cheese classification, characterization, and categorization: A Global Perspective. Microbiol. Spectr. 2(1) (2014). https://doi.org/10.1128/microbiolspec.cm-0003-2012
X. Zheng, X. Shi, B.A. Wang, Review on the General Cheese Processing Technology, Flavor biochemical pathways and the influence of yeasts in cheese. Front. Microbiol. 12 (2021). https://doi.org/10.3389/fmicb.2021.703284
R.A. Wilbey, Estimating Shelf-Life. Int. J. Dairy. Technol. 50(2), 64–67 (1997)
C.A. Wallace, Safety in Food Processing. In Food Processing Handbook; Brennan, J. G., Grandison, A. S., Eds.; 2011; pp 491–513. https://doi.org/10.1002/9783527634361.ch15
T. Hasting, The Hygienic Design of Food Processing Plant. In Food Processing Handbook; Brennan, J. G., Grandison, A. S., Eds.; 2011; pp 533–558. https://doi.org/10.1002/9783527634361.ch17
N. Gautam, N. Sharma, Bacteriocin, Safest Approach to Preserve Food products. Indian J. Microbiol. 204–211 (2009). https://doi.org/10.1007/s12088-009-0048-3
M. Masmoudi, I. Ammar, H. Ghribi, H. Attia, Physicochemical, Radical scavenging activity and sensory properties of a soft cheese Forti Fi Ed with Arbutus Unedo L. Extract. Food Biosci. 35, 100579 (2020). https://doi.org/10.1016/j.fbio.2020.100579
M. Ávila, J. Calzada, N. Muñoz-Tébar, C. Sánchez, G. de Ortiz, M. Carmona, A. Molina, M.I. Berruga, S. Garde, Inhibitory activity of aromatic plant extracts against dairy-related Clostridium species and their use to prevent the late blowing defect of cheese. Food Microbiol. 110, 104185 (2023). https://doi.org/10.1016/j.fm.2022.104185
C.T. Pasini Deolindo, P.I. Monteiro, J.S. Santos, A.G. Cruz, M. Cristina da Silva, D. Granato, Phenolic-Rich Petit Suisse Cheese Manufactured with Organic Bordeaux grape juice, skin, and seed extract: Technological, sensory, and Functional properties. Lwt. 115(August), 108493 (2019). https://doi.org/10.1016/j.lwt.2019.108493
D. Vukić, B. Pavlić, V. Vukić, M. Iličić, K. Kanurić, M. Bjekić, Z. Zeković, Antioxidative capacity of fresh kombucha cheese fortified with sage herbal dust and its preparations. J. Food Sci. Technol. 59(6), 2274–2283 (2022). https://doi.org/10.1007/s13197-021-05241-y
N. Lee, R.K.C. Jeewanthi, E. Park, H. Paik, Short communication: Physicochemical and antioxidant properties of Cheddar-Type cheese fortified with Inula Britannica Extract. J. Dairy. Sci. 99(1), 83–88 (2016). https://doi.org/10.3168/jds.2015-9935
A.A. Saparbekova, G.O. Kantureyeva, D.E. Kudasova, Z.K. Konarbayeva, A.S. Latif, Potential of Phenolic compounds from Pomegranate (Punica Granatum L.) by-product with significant antioxidant and therapeutic effects: a narrative review. Saudi J. Biol. Sci. 30(2), 103553 (2023). https://doi.org/10.1016/j.sjbs.2022.103553
A.M.T. Silva, E. Nouli, N.P. Xekoukoulotakis, D. Mantzavinos, Effect of key operating parameters on Phenols Degradation during H2O2-Assisted TiO2 photocatalytic treatment of simulated and actual Olive Mill Wastewaters. Appl. Catal. B Environ. 73(1), 11–22 (2007). https://doi.org/10.1016/j.apcatb.2006.12.007
S.M. Ferreira, S.M. Gomes, L.A. Santos, Novel Approach in skin care: By-Product extracts as natural UV filters and an alternative to synthetic ones. Molecules. 28(5) (2023). https://doi.org/10.3390/molecules28052037
S.M. Ferreira, L. Santos, Incorporation of phenolic extracts from different By-Products in yoghurts to create fortified and Sustainable Foods. Food Biosci. 51, 102293 (2023). https://doi.org/10.1016/j.fbio.2022.102293
S. Bakirci, E. Dagdemir, O.S. Boran, A.A. Hayaloglu, The Effect of Pumpkin Fibre on Quality and Storage Stability of reduced-Fat Set-Type Yogurt. Int. J. Food Sci. Technol. 52(1), 180–187 (2017). https://doi.org/10.1111/ijfs.13264
J.B. Chaves, R. Sproesser, Práticas De Laboratório De Análise Sensorial De Alimentos E Bebidas (UFV, Ed., 2013)
D. Pinto, A.M. Silva, V. Freitas, A. Vallverdú-Queralt, C. Delerue-Matos, F. Rodrigues, Microwave-assisted extraction as a Green Technology Approach to recover polyphenols from Castanea Sativa Shells. ACS Food Sci. Technol. 1(2), 229–241 (2021). https://doi.org/10.1021/acsfoodscitech.0c00055
G. Vázquez, E. Fontenla, J. Santos, M.S. Freire, J. González-Álvarez, G. Antorrena, Antioxidant activity and phenolic content of Chestnut (Castanea Sativa) Shell and Eucalyptus (Eucalyptus Globulus) bark extracts. Ind. Crops Prod. 28(3), 279–285 (2008). https://doi.org/10.1016/j.indcrop.2008.03.003
C. Agarwal, T. Hofmann, M. Vršanská, N. Schlosserová, E. Visi-Rajczi, S. Voběrková, Z. Pásztory, In Vitro antioxidant and antibacterial activities with polyphenolic profiling of Wild Cherry, the European Larch and Sweet Chestnut Tree Bark. Eur. Food Res. Technol. 247(9), 2355–2370 (2021). https://doi.org/10.1007/s00217-021-03796-w
I.F. Almeida, P. Valentão, P.B. Andrade, R.M. Seabra, T.M. Pereira, M.H. Amaral, P.C. Costa, M.F. Bahia, (Chestnut) Leaf Extract, a putative natural antioxidant for topical application. Basic. Clin. Pharmacol. Toxicol. 103(5), 461–467 (2008). https://doi.org/10.1111/j.1742-7843.2008.00301.x. Vivo Skin Irritation Potential of a Castanea Sativa
S.I. Fitrasyah, A. Ariani, N. Rahman, N. Nurulfuadi, U. Aiman, D. Nadila, F. Pradana, A. Rakhman, D.A. Hartini, Analysis of Chemical Properties and antioxidant activity of Sambiloto (Andrographis Paniculata nees.) Leaf Tea Formula as a functional drink in preventing Coronavirus diseases and degenerative diseases. Open. Access. Maced J. Med. Sci. 9, 196–201 (2021). https://doi.org/10.3889/oamjms.2021.5872
R. Hinrichs, J. Götz, M. Noll, A. Wolfschoon, H. Eibel, H. Weisser, Characterisation of the Water-Holding Capacity of Fresh cheese samples by means of low Resolution Nuclear magnetic resonance. Food Res. Int. 37(7), 667–676 (2004). https://doi.org/10.1016/j.foodres.2004.02.005
T.N. Soliman, D.M. Mohammed, T.M. El-Messery, M. Elaaser, A.A. Zaky, J.B. Eun, J.H. Shim, M.M. El-Said, Microencapsulation of Plant Phenolic Extracts Using Complex Coacervation Incorporated in Ultrafiltered cheese against AlCl3-Induced Neuroinflammation in rats. Front. Nutr. 9(June), 1–14 (2022). https://doi.org/10.3389/fnut.2022.929977
H. Jeong, Y. Lee, P. Ganesan, H. Kwak, Physicochemical, Microbial, and Sensory Properties of Queso Blanco cheese supplemented with Powdered microcapsules of Tomato extracts. Korean J. Food Sci. Anim. Resour. 37(3), 342–350 (2017)
A.N. Rinaldoni, D.R. Palatnik, N. Zaritzky, M.E. Campderrós, Soft cheese-like product development enriched with soy protein concentrates. Lwt. 55(1), 139–147 (2014). https://doi.org/10.1016/j.lwt.2013.09.003
H.S. Abdelmontaleb, F.A. Othman, M.A. Degheidi, K.A. Abbas, The Influence of Quinoa Flour Addition on the Physicochemical, antioxidant activity, Textural, and sensory characteristics of UF-Soft cheese during refrigerated storage. J. Food Process. Preserv. 45(5), e15494 (2021). https://doi.org/10.1111/jfpp.15494
M.H.F. Henriques, D.M.G.S. Gomes, C.J.D. Pereira, M.H.M. Gil, Effects of Liquid Whey Protein Concentrate on Functional and Sensorial properties of set yogurts and fresh cheese. Food Bioprocess. Technol. 6(4), 952–963 (2013). https://doi.org/10.1007/s11947-012-0778-9
A. Rashidinejad, E.J. Birch, D. Sun-Waterhouse, D.W. Everett, Total phenolic content and antioxidant properties of Hard Low-Fat cheese fortified with Catechin as affected by Invitro gastrointestinal digestion. Lwt. 62(1), 393–399 (2015). https://doi.org/10.1016/j.lwt.2014.12.058
M. Stobiecka, J. Król, A. Brodziak, Antioxidant activity of milk and dairy products. Animals. 12(3) (2022). https://doi.org/10.3390/ani12030245
S. Rohn, H.M. Rawel, J. Kroll, Antioxidant activity of protein-bound quercetin. J. Agric. Food Chem. 52(15), 4725–4729 (2004). https://doi.org/10.1021/jf0496797
A.S. Gad, M.H.A. El-Salam, The antioxidant properties of Skim milk supplemented with Rosemary and Green Tea extracts in response to Pasteurisation, Homogenisation and the addition of salts. Int. J. Dairy. Technol. 63(3), 349–355 (2010). https://doi.org/10.1111/j.1471-0307.2010.00585.x
P. Bourassa, R. Côté, S. Hutchandani, G. Samson, H.A. Tajmir-Riahi, The effect of milk alpha-casein on the antioxidant activity of tea polyphenols. J. Photochem. Photobiol B Biol. 128, 43–49 (2013). https://doi.org/10.1016/j.jphotobiol.2013.07.021
Acknowledgements
The authors would like to thank the company “Queijaria Licínia, Lda” for all the collaboration in the project.
Funding
This work was financially supported by: LA/P/0045/2020 (ALiCE), UIDB/00511/2020, and UIDP/00511/2020 (LEPABE), funded by national funds through FCT/MCTES (PIDDAC).
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Sara M. Ferreira: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft. Luís Carlos Matos: Methodology, Formal analysis, Writing - original draft. Lúcia Santos: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing - review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no conflict of interest.
Financial acknowledgements
Sara M. Ferreira would like to thank the Portuguese Foundation for Science and Technology (FCT) for her PhD grant (2022.10910.BD).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferreira, S.M., Matos, L.C. & Santos, L. Harnessing the potential of chestnut shell extract to enhance fresh cheese: a sustainable approach for nutritional enrichment and shelf-life extension. Food Measure 18, 1559–1573 (2024). https://doi.org/10.1007/s11694-023-02260-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02260-5