Abstract
Consumers are making their lifestyle healthier by changing diet, so food producers are exploring the enrichment of daily products using natural additives with nutraceutical properties. The addition of bee products is a good example, enabling a positive input in nutrients at the same time that the phytochemical substances present on it, may add complementary bioactive functions. This study centred on formulating recipes for traditional bread, incorporating bee products such as bee pollen and bee bread. The fortified breads revealed a coherent increase on protein and lipid content, while the fibre value depends on the type of bee product added. The enrichment seems also to induce changes in the physical properties, with the fortified breads becoming yellow-brownish colour and firming, due to the increase in the protein content, at the same time that some reduction in elasticity is observed. The sensorial perception, tested to assess the market product acceptability, gave encouraging outcomes with high scores for bee bread enrichment. It is relevant to highlight that the recipes used can be classified as “low salt content” bread, with salt values below 130 mg/100 g.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
People awareness on the impact of the diet in the individual health status is becoming a crucial factor that induces changes in the consumption choices for functional foods [1]. Recent studies showed that consumers choose functional foods due to their desire for a healthy lifestyle, to protect themselves from diseases, to enhance their life span, overall, to increasing life quality. These principles stands on the knowledge that functional foods, enriched with specific compounds, provide high nutritional or physiological benefits to the consumers [2, 3].
Bread is an important part of the worldwide daily diet, made mainly from grain-based carbohydrates. The nutritional quality of this cereal-based food is often poor in protein, vitamins, minerals, but also limited in essential amino acids and fatty acids [4, 5]. Enrichment of wheat-based conventional breads with natural constituents is an appealing mechanism for improving the consumer’s health due to their daily and affordable consumption. [6]. Several examples of this approach were already explored, such as the enrichment of bread with fruits, nuts or seeds [7], incorporation of Brassica leaves to increase the amount of health-promoting secondary metabolites resultant from the degradation of flavonoids and chlorophyll [8], the addition of seaweeds to reduce the level of carbohydrate digestion [9] or the combination of wheat flour and grasshopper powder to enrich the protein content of bread and soften texture [10].
Bee products are also a potential source of natural ingredients for functional foods and for bread making: bee pollen and bee bread seems two excellent options due to their nutritional value [11]. Bee pollen results from the collection of flower pollen by honey bees, which they mix with their own secretions and nectar, and afterwards transport to the hive as agglutinated pellets. It has a composition rich in carbohydrates (40–85%), proteins and amino acids (14–30%) and lipids (1–10%) [12]. Other minor compounds such as vitamins, minerals, carotenoids and phenolic compounds are also present [13]. Once inside the beehive, bees pack the pollen pellets in the combs and preserve it with a small layer of honey, where it undergoes biochemical changes turning into bee bread [11]. Bee bread goes through an anaerobic fermentation process caused by the action of moisture, temperature (35 °C), different enzymes from bee glandular secretions and some microorganisms such as Pseudomonas, Lactobacillus, Saccharomyces, that are naturally present in pollen [14]. With this process, the outer layer of pollen breaks down, giving a greater bioavailability to bee bread [15]. When compared with pollen, the acidity is higher in bee bread due to the presence of lactic acid [11]. Also, bee bread has essential amino acids, minerals, fatty acids and carotenoids [15].
In previous studies, the anti-inflammatory, anticarcinogenic, anti-atherosclerosis and anti-allergic [11] activities of bee products, especially bee pollen and bee bread, have been confirmed, showing good results when these products are introduced in the daily consumption. Although the nutritional and bioactive properties of bee pollen and bee bread provide these bee products a great potential as ingredients for food functionalization, they have been neglected in bread making. Indeed, to our knowledge, there is only one reported study on gluten-free bread enriched with bee pollen, with positive outputs in technical, sensorial and nutritional characteristics [16]. Therefore, the objective of this work is to develop traditional wheat breads enriched with bee pollen and bee bread, and evaluate changes in the nutritional value, technological properties and microbial stability. The consumer’s preference was also evaluated by a sensory panel, in order to find which product have the highest potential to be introduced in the market.
Materials and methods
Chemicals and reagents
Ethanol, methanol, sodium phosphate, potassium phosphate, potassium ferrocyanide, trichloroacetic acid, acetonitrile, formic acid, sulphuric acid, diethyl ether, sodium hydroxide, petroleum ether, and gallic acid were purchased from Fisher Scientific (Pittsburgh, PA, USA). Megazyme TDF kit (Megazyme K-TDFR-200 A, Wicklow, Ireland) was used for total dietary fibre (TDF) quantification. Peptone water (PW), Dichloran Rose Bengal Chloramphenicol (DRBC), Iron Sulphite Agar (ISA) and Plate Count Agar (PCA) were purchased from HiMedia (Mumbai, India). Water was subjected to a treatment in a Milli-Q water purification system (TGI pure system, Houston, TX, USA).
Bee pollen and bee bread samples
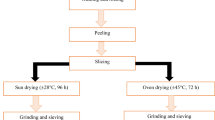
Bee pollen (BP) and bee bread (BB) samples were obtained from Apis mellifera iberiensis hives located in Trás-os-Montes region, in the north of Portugal, during the spring-summer season of 2019. Bee pollen was collected with pollen traps placed in front of the beehive entrance, while bee bread was extracted from frames inside the beehive. The separation of bee bread from the wax comb required a previous freezing procedure of the frames (24 h) following a mechanical crush of the wax. After the collection process, both bee pollen and bee bread were cleaned manually from debris, wax and dead bee parts, and then grounded, homogenized, lyophilized, and kept in the freezer (-20 ºC) until use in bread making.
For botanical origin assessment of the bee products, about one gram of BP and BB samples, previously mixed and homogenized, were placed in a vial with distilled water, allowed to soften slightly and vigorously vortexed. Then, 200 µL of the mixture were removed from the mixture and centrifuged for 15 min at 1000 g. After the centrifugation, the pellet was subjected to the acetolysis procedure in accordance to Louveaux et al. [17] with the recommended modifications from Von der Ohe et al. [18]. Counts and identifications were achieved using an optical microscope (Nikon Microphot – FXA, Melville, NY, USA). More than 1,200 grains per preparation were counted.
Bread making
Breads were prepared using a home-making bread machine (Tefal bread maker XXL, Windsor, Berkshire, UK). The selected bread-making protocol included dough preparation (140 min) and baking (20 min). The wheat flour and yeast used were purchase from Lallemand, Setúbal, Portugal, through a local bakery, Nopabril LDA, from Bragança, Portugal. The breads were prepared following the bakery own recipe, using the quantities of each ingredient as listed in Table 1. For enriched bread the standard formulation was modified: a proportion of the flour (1%, 3% or 5%, flour basis) was replaced by bee pollen or bee bread. Bread prepared without addition of bee products was used as control.
Nutritional analysis
The nutritional composition of the raw ingredients and breads were analysed following the official AOAC methods [19]. The water content was evaluated with 2 g of the sample on a PMB Moisture Analyser (Kingston, Milton Keynes, U.K.). Ash content was estimated through the incineration of samples (2 g) in a muffle (Biotech - Optic Ivymen System, Madrid, Spain), at 550 ± 5 °C, according to the AOAC 942.05 procedure. The protein content was evaluated through Macro-Kjeldahl nitrogen determination using a copper catalyst in 1 g of sample [20], using a Kjeldahl steam distillation unit (Pro-Nitro A, Selecta, Barcelona, Spain) following the AOAC 920.87 method and applying N x 6.25 as the conversion factor.
For lipids, 2 g of sample was mixed with petroleum ether in a Soxhlet apparatus for 4 h. The resulting extracts were then evaporated to dryness, under reduced pressure, and the residues were weighed, expressing the lipid content, as dry weight, in mass percentage [21].
Total dietary fibre (TDF) was quantified following the AOAC 985.29 method [22]. Briefly, 1 g of dried sample was mixed with 12.5 mL of phosphate buffer followed by 12.5 µL of α-amylase solution, and kept at 100 °C for 30 min. Subsequently, 25 µL of protease solution was added and incubated for 30 min at 60 °C. Finally, a new incubation under the same conditions was performed after the addition of 50 µL of amyloglucosidase solution. The total available carbohydrates (without fibre) and energy were obtained by calculation using the following expressions [21, 23, 24]: Total available carbohydrates (%) = 100 − (g ashes + g proteins + g lipids + dietary fibre); Energy (kcal) = 4 × (g protein + g carbohydrate) + 2 × (g dietary fibre) + 9 × (g lipids).
Mineral profile
The mineral composition was evaluated by flame ionization atomic absorption spectroscopy (FAAS) using a Perkin Elmer PinAAcle 900T Spectrometer (Waltham, MA, USA).
The sample preparation was carried out through microwave-assisted extraction, using a MARS 5 Digestion Microwave System (CEM Corporation, Matthews, NC, USA). Approximately 0.5 g of the sample was weighed into a PTFE digestion tube followed by the addition of 10 mL of concentrated nitric acid. The digestion was performed by setting the ramp temperature program: 15 min until 200 ºC with a power of 1200 W, followed by an additional 15 min at the same temperature and power conditions. Finally, once cold, the digested mixture was quantitatively transfer to a 50 mL flask, diluted with deionized water and analysed by FAAS, with prior treatment for specific elements. For the determination of potassium and sodium, the sample was diluted in a cesium chloride solution (1 g.L− 1); for calcium and magnesium, the sample was diluted in a lanthanum chloride solution (1 g.L− 1); while iron, manganese and copper were directly analysed. The quantification of the elements was achieved by comparing the absorbance responses for pure analytical solutions.
Physical characterization
Specific volume
The specific volume of bread samples was measured according to the American Association of Cereal Chemists (AACC) with some modifications, following the rapeseed displacement method [25] and applying the following equation:
Volume (bread) = Volume (PP) × ((weight of the rapeseed “inside” the PP) / (weight of the rapeseed that enters the PP with bread on it)). PP refers to the parallelepiped form used on the test.
Colour
The colour was analysed with a portable colorimeter CR400 from Konica Minolta (Chiyoda, Tokyo, Japan). The measurements were performed in three different points on the bread crumb and crust. The colour was recorded in the CIELAB system, which consists of a lightness component (L*) and two chromatic components, a* value representing green (− a) to red (+ a) and b* value representing blue (− b) to yellow (+ b) colours [23]. The equipment was calibrated using a standard white plate (L* = 93.3, a* = −0.53 and b* = 4.13). The colour difference (ΔE*) was calculated as described by the following equation [26]:
Texture profile analysis (TPA)
The texture was evaluated with a Stable Micro Systems TA.XT Plus Texture Analyser (Vienna Court, Godalming, UK) applying the method described in the literature [23] with a 5 kg load cell. A P/35 aluminium cylinder probe (35 mm diameter) was used to for the TPA which is a test that imitates the chewing action by performing two compressions of the matrix. Results from the analysis were processed via a macro in order to reach the different parameters of texture, such as hardness, cohesiveness, springiness, adhesiveness, chewiness, and resilience.
Sensory evaluation
The consumer acceptability was assessed with the application of an attribute sensory test using a hedonic rating, allowing to identify the reasons for the preference and rejection of the bread samples through the individual attributes. A panel composed of 15 volunteers (with ages ranging from 20 to 50 years) was asked to evaluate the samples and rate their preferences using a 9-point hedonic scale (1 = extremely dislike; 9 = extremely like) [16]. The breads were rated based on the acceptance of their colour, taste, smell, texture, mouthfeel, and overall acceptance. Breads were prepared on the same day of the sensory evaluation.
Microbial analysis
Sampling
The microbial analysis of the breads was performed in the raw dough and in cooked breads at two specific time periods: immediately after baking and 3 days after. The preparation followed the procedure described in ISO 6887-1:1999 [27]. Samples were taken from 3 different parts of the dough and breads, under sterile conditions. Ten g of sample were weighed into a stomacher bag, mixed with 90 mL of peptone water (PW) and homogenized using a Stomacher 400 (Seward type 400, Liofilchem, Teramo, Italy) for 60 s. The obtained suspensions were further diluted to concentrations from 10− 1 to 10− 5.
Total viable count
Total viable counts (TVC) were determined according to the procedure described in ISO 4833-1:2013 [28]. Suspensions were inoculated in PCA by the pour plate technique: 1 mL of each suspension was pipetted into a 9-cm diameter Petri plate and mixed with 15 mL of melted PCA. Plates were then incubated at 30 °C for 72 h, in reversed position, and the colonies counted (plates having between 15 and 300 colonies). The results were expressed as log10 colony-forming units per gram of sample (log10 CFU/g), as described in ISO 7218:2007 [29]. The limit of quantification (LOQ) was 1.0 log10 CFU/g.
Yeasts and moulds
Yeasts and moulds were counted using the procedure described in ISO 21527-1:2008 [30]. Dilutions were inoculated in DRBC using the spread plate technique: 0.2 mL of each suspension were pipetted onto a plate containing 15 mL of the DRBC medium, spread with a disposable loop, and incubated at 25 °C, for 5 days, in the upright position. Yeast and mould colonies were counted separately after 2 and 5 days of incubation, respectively, in plates having less than 150 colonies. The results were expressed as log10 CFU/g. The LOQ was 1.7 log10 CFU/g.
Spores of sulphite-reducing clostridia
The determination of spores of sulphite-reducing clostridia (SRC) followed the procedure described in ISO 15213:2003 [31], with some modifications. Five mL of the initial suspension (10− 1) were transferred into a 50 mL Falcon tube and dipped in a water bath at 80 °C for 10 min. After the heat-treatment, the suspension was immediately cooled in a water bath and mixed with 25 mL of iron sulphite agar (ISA). After solidification of the medium, the tube was filled with ISA to create anaerobiosis and incubated at 30 °C for 24 h to 48 h (final counting at 48 h). The number of black spots obtained in the tubes was counted. The results were expressed as CFU/g. The LOQ was 2 CFU/g.
Statistical analysis
All analyses were performed in triplicate and the data were denoted as mean ± standard deviation (SD). The results were analysed using GraphPad Prism version 8 (San Diego, CA, USA). One-way analysis of variance and Tukey’s multiple comparison test was conducted to see whether there was a statistical significance. p < 0.05 was considered significant.
Results and discussion
Palynological analysis
The chemical composition of pollen is highly dependent on its botanical origin, so it is important to evaluate the floral sources that may contribute to the bee products used as functional ingredients. A total of 11 pollen types, above 3%, were identified in the bee pollen and bee bread samples. Figure 1 shows the most representative, with the detailed relative predominance of the pollen types available in the supplementary material, Table S1. All samples were classified as multifloral due to the absence of any taxa with a relative frequency greater than 80% [32]. Indeed, Cytisus striatus and Crepis capillaris were detected as accompanying pollen for bee pollen samples, with a relative predominance of 21% and 17%, respectively, while Castanea sativa and Rubus sp., also as accompanying pollen, were found in the bee bread samples at 39% and 18%, respectively. The observed botanical profile disclose the plants’ diversity around the apiaries where the bee products were collected and are common of the north-eastern Portugal [21].
Nutritional analysis
The nutritional value for the individual ingredients and for the breads was assessed through the analysis of water, ash, lipid, protein and fibre contents, and the outputs can be found in Table 2. The water content was similar for BP and BB, with values in the range of 13–15%. In general, these bee products, after harvesting, can show a water content up to 30%, which may favour the development of undesired yeast and moulds, so its storage requires the removal of humidity to values below 4–8% [11, 32]. Our values revealed relatively low water contents which is consistent with the climatic conditions during the production season of the samples (summer). For the fermenting yeast and flour, the moisture content was lower, 4% and 9%, respectively, which are common values for these types of ingredients [33, 34]. The ash content for the ingredients varied between the minimum of 0.7% for the flour, to the 6% observed for the yeast, with the bee products revealing an intermediate value, around 3%, consistent with the typical values observed for these bee products [32]. The agreement between the obtained nutritional parameters for BP/BB and the results described on previous studies was also observed [21, 32, 35, 36], with values around 5% for lipids, 22% for proteins and 4–6% for fibres, leading to a total available carbohydrates close to 65% and an energy value slightly above 400 kcal/100 g. The higher carbohydrate content in pollen is justified mainly due to the polysaccharides cell wall material, while for bee bread, there is an extra source due to the addition of honey by the honeybee during the storage process [11, 21].
Comparing the nutritional value of bee products with the other ingredients, the flour is significantly poorer in proteins and lipids, while the yeast stands out for the rich content in proteins and fibres, leading to an energy reduction of 10%.
Through the analysis of the fortified breads results, Table 2, it is possible to observe a small but not statistically significant increase in the water and ash content when compared with the unfortified breads. Concerning the lipid content, enrichment with BP and BB has shown a significant increasing trend, with BB5% revealing the highest value. Also, the increase in the protein content of the functional breads was proportional to the added percentage of bee products, with the BP5% and BB5% breads presenting the highest values. A similar behaviour was described by Conte et al. [5], with the improvement in the protein content in gluten-free breads fortified with bee pollen. The increasing percentage of added BP lead also to a gain in the fibre content of the enriched breads, however, for BB-added breads the behaviour was the opposite. These different patterns are justified by the higher fibre content in flour, when compared to the BB, but lower if compared to BP.
The global trends on the individual nutritional parameters resulted in a statistically significant decrease of the total available carbohydrate value for the enriched breads, with BP5% and BB5% showing the lowest amount. This variation, however, did not return a statistical significance in the energy value.
Mineral profile
Pollen is a natural product with a rich mineral composition, which is connected to the geographical and botanical origin through the plant species, the soil type, and the climatic conditions [37]. To quantify the mineral content, we explored atomic absorption methods with microwave assistance, and the results are expressed in Table 3. BP and BB showed similar profiles, understandable since they share a common origin, with potassium as the most relevant macronutrient, above 4 g/kg, but also with a significant amount of calcium and magnesium, 400–600 mg/kg. Comparable studies on Serbian and Greek BP [38, 39] and Malaysian BB [40], showed similar levels for those macronutrients, with some variability resulting from the botanical and geographical origin. A closer comparison between the two bee products only detected statistical differences for iron, with bee bread presenting the double amount, around 225 mg/kg.
For the enriched bread, potassium was also observed as the major macronutrient, but now in the same range as sodium, 1–2 g/kg (100–200 mg/100 g). Indeed, sodium was the sole mineral that increased significantly, by a factor of 20 times, in comparison to the bee products, which is due to the baking soda (NaCO3) used on the bread doughs, but mainly due to the added salt (NaCl) used to improve flavour [41]. The amount of added salt in bread is an issue of current debate in many countries due to its excessive use in food processing, particularly in bakery, and its impact in heart diseases [42, 43]. The Portuguese legislation set a maximum value of 550 mg/100 g of sodium in bread [44], but there are several initiatives at the national and international levels to promote the reduction of salt intake and set maximum values for bread at around 400 mg/100 g [43, 45]. In comparison, the breads under study, with salt values lower than 130 mg/ 100 g, can be classified as “low salt content”. Besides, magnesium is observed as the third macronutrient in the studied breads, at levels of 22–32 mg/100 g (6–9%) of the recommended dietary allowance) [41], and three times higher than calcium. Iron, manganese and copper, as micronutrients, were found only at low quantities, < 3 mg/100 g, Table 3. All the mineral levels are within the ranges commonly observed across the globe for breads.
The fortification of bread with bee products did not produce marked changes on the mineral composition of bread. Even though we observed some increment trend on K, Ca, Mg and Fe with the increased addition of BP and BB, those variations are not statistically significant in most cases. This may be due to the large deviation observed between replicates, what could hide the potential impact in mineral enrichment that would be expected when adding the bee products.
Physical characterization
Specific volume
Specific volume is one of the parameters that affect bread quality. A high ratio of volume per weight, which represents aeration of the bread loaf, is required, and, from an economic outlook, strongly influences consumer’s choice [46, 47].
Figure 2, shows the comparison between control and enriched breads. Although the observed changes are small, the loaf volume seems to be reduced for BP bread samples, while for BB bread it remains close to the control. It is known that the interaction between gluten and fibre may limit the gas retention during the dough fermentation process [48], and so, the rising in fibre content in BP breads, Table 2, could explain these differences.
Colour analysis
Colour formation in bread is called browning and is attributed to non-enzymatic browning reactions, which include caramelization and Maillard reactions, during the bread-making process [48, 49]. Complex reactions like caramelization come into existence by heating the carbohydrates, while the Maillard reactions occur between the carbonyl group of reducing carbohydrates and the amino group of free amino acids [49]. Visually, as the ratio of bee pollen and bee bread increased, the colour of the breads became darker. In breads enriched with BP dominated a relatively yellowish colour, while BB fortified breads became brownish. The colour was homogeneous when observing the bread slices, which was the result of mixing the substitute BP and BB with the flour in dry, Fig. 3.
The CIELAB colour system was applied to characterize the fortified breads colour, independent of the observer. The CIELAB system is an approximately uniform three-dimensional colour scale, extensively used to evaluate food colours. In this scale, the coordinate L* measures de degree of lightness, and a* e b* are the horizontal axes defining green/red (-60/60) and blue/yellow (-60/60) chromaticity, respectively [50].
Observing the colour results, Table 4, a decrease in the L* value was noticed in the crusts of BP enriched breads, while a* and b* values increased. The same pattern was detected in the BB enriched breads. For the crumb colour of BP enriched breads, a decrease of the L* and a* values were observed from the control bread to BP5%, while the b* value increased. For BB enriched breads, the crumb colour analysis showed a decrease in the L* values, while both a* and b* values increased. The obtained results are in accordance with previous studies which were focused on functional bread enriched with bee pollen [16]. Yellow-brownish colour of bee pollen and bee bread is essentially related to the accumulation of carotenoids [51]. In terms of consumer’s preferences, the changing in the bread colour can be a desirable attribute of the enriched bread.
Texture profile analysis
The results for the bread crumb and crust texture profile analysis (TPA) are given in Table 5. The TPA parameters, such as hardness, adhesiveness, resilience, cohesiveness, springiness and chewiness of the fortified bread samples were evaluated and compared with control bread. Hardness as the first conspicuous textural parameter is highly related to moisture and fat content [23, 52] and so it is coherent to see, in all cases, higher values for crust compared with crumb, Table 5. The incremental addition of bee products seems to cause an increase in the hardness of the enriched breads, but while the values for BB and BP fortified breads are similar for crumb, the hardness of the crust seems to rise more significantly with the addition of bee pollen. The exception is for the lower additions of BB (1 and 3%), which produced softer crust than the control bread, but become harder at higher percentages of BB. Previous studies refer that the addition of protein can enhance the linkages with the swollen starch granules of bread [53] and so, the observed increase in the protein content of the enriched breads, Table 2, could justify the bread firming. The chewiness of crumb and crust followed the same trend as hardness, with increment as the amount of bee pollen and bee bread becomes higher. Chewiness represents the time and effort of milling a food until ready to swallow [52], and it seems that this effort is lower for bread fortified with BB compared with BP.
The sense of the food sticking to the surface of mastication is measured through the adhesiveness parameter, and breads usually have low adhesiveness [23]. In accordance, the results on our study were also low, below 0.2 gsec, and with no statistical variance with the addition of bee products. Springiness is a desired property for bakery products like muffins and bread and may refer high quality [54], which represents the condition of the food returning to its original shape after the implementation of a deforming force applied on it [23]. The results on Table 5 show that, comparing to the control, the enrichment of bread reduce the elasticity value for the crumb, however, it seems independent of the percentage of fortification and whether is BB or BP.
The other two texture parameters under analysis, resilience and cohesiveness, were quite uniform for all breads, with no statistical difference, which reflects a relative compressive capacity and recovery [55] for the different bread formulations, Table 5.
Sensory evaluation
The sensory scores obtained with the consumer panel for colour, taste, smell, texture, mouthfeel, and overall acceptance are given in Fig. 4. The enrichment of bread with bee pollen seems to improve the evaluation scores on taste, texture and aroma, while the changes in colour are less appreciated by the consumers. This visual perception on colour, particularly for BP5%, seems to contribute significantly to the decision on the overall acceptance, leading the panellists to set the following preference pattern: BP3%> BP1%> Control > BP5%, although very close to each other.
The best evaluation scores were observed in the breads enriched with bee bread, with an improvement comparing with the control, for all the parameters under evaluation. The reason why the breads enriched with BB were more liked may be linked to the sweetness resulting from the honey present in the composition of the bee bread. It is also interesting to notice that, in general, the scores rise with the percentage of BB added particularly for texture and mouthfeel, with a raise of almost 2 points between control and BB5%. For the bread fortified with BB, the negative impact on colour was not observed, and so, the panellists set the following preference pattern: BB5%> BB3%> BB1 > Control.
Microbial stability
Total viable counts
The total viable count (TVC) estimates the population of live microbial loads for food quality assurance, and according to WHO Standard (1994), the acceptable range of TVC is 5.3 log10 CFU/g [56]. From Fig. 5, it is possible to observe that, for the doughs, either in control or fortified doughs, the values are close to each other and within the range of 7.7–7.9 log10 CFU/g. However, the microbial counts decrease significantly after cooking, to values within the mentioned acceptable range, and maintains a similar level after three days. The type of bee product does not seem to cause different trends on the TVC values, however, for the higher amounts of BP or BB (5%), the fortification seems to incite an inhibition on microbial growth. Usually, bee pollen and bee bread are considered as contestable for foods due to its microbial activity, but the cooking process seems to improve the microbial quality of both bee products as food ingredients.
A) and B): Total viable counts (TVC) for control and enriched breads with bee pollen and bee bread, respectively; C) and D): Yeast counts for control and enriched breads with bee pollen and bee bread, respectively; E) and F): Spores of sulphite-reducing clostridia (SRC) counts for control and enriched bread with bee pollen and bee bread, respectively. Different letters (a-c) indicates significant differences (p < 0.05)
Yeasts and moulds
Yeast and moulds were only observed on the doughs, with values around 7.5 ± 0.3 log10 CFU/g. While for the fortified BB dough there was no significant differences between the three formulations, for BP enrichment the doughs for 3 and 5% revealed a small decrease. In all cases, there were no yeasts detected after cooking. The disappearance of the yeast is justified by the high heating process and may be reflected in the bread shelf life. Moulds were not detected in any case, which may be due to the high temperatures of the baking process but also due to the time length of the study (up to 3 days of storage). Other authors referred that mould formation in bread became evident after 7 days [57].
Spores of sulphite-reducing clostridia (SRC)
Clostridium perfringens are anaerobic, spore-forming pathogenic bacteria which produce enterotoxins that can cause diarrhoea in humans. C. perfringens spores can resist the baking process, germinate in the bread and, depending on the quantity, a significant part may inclusively survive the stomach acid conditions [58]. SRC are also indicators of other spore-forming anaerobic and pathogenic bacteria like Clostridium botulinum. In our results, Fig. 5, raw dough contained low amounts of sulphite-reducing Clostridia, however, they were not detected in the cooked samples, making the products safe for consumption.
Conclusions
The contribution of bread to the daily diet is so significate that several fortification approaches have been attempted by the bakery industry to improve the health benefits of this food product. This study explores the incorporation of bee pollen and bee bread in traditional bread recipes, while evaluating its impact in the nutritional value, physical properties, consumer’s acceptability and microbial stability.
Bee pollen and bee bread are known for their richness in protein, lipid and mineral content, so it was predictable that its incorporation as flour substitute would input some changes on the nutritional composition of the fortified breads. Indeed, the progressively increased incorporation of bee products also increased the protein of the enriched breads up to 20%, while the amount of lipids raised from 0.1 to 0.7%. The impact on the fibre content of bread was not equal, with a different trend between the fortified breads with bee pollen (reinforce fibre content) and bee bread (weakened fibre content), linked with the fibre level on the raw material. For minerals, although there was an incremental trend, this behaviour did not cause statistically significant changes. It is relevant to highlight that the recipes used can be classified as “low salt content” bread, with salt values below 130 mg/100 g.
The enrichment with bee products seems also to induce some changes in the physical characteristics of the bread, particularly in colour. The bread crust and crumb lose some lightness with the increasing amount of BP or BB, together with a yellowing or browning shift, respectively. The texture profile of bread is also affected, with the fortified bread becoming firming, due to the increase in the protein content of the enriched breads, at the same time that some losing in elasticity is observed.
From the consumer’s point of view, the nutritional and physical changes of the enriched breads with bee products received a very good acceptability from the sensory panel, with the best scores given for the bread enriched with 5% of BB. These outputs, together with the microbial stability observed on the bread after the cooking process, are good indicators for the future introduction of these new products on the market as functional daily foods.
References
K. Banwo, A.O. Olojede, A.T. Adesulu-Dahunsi, D.K. Verma, M. Thakur, S. Tripathy, S. Singh, A.R. Patel, A.K. Gupta, C.N. Aguilar, G.L. Utama, Functional importance of bioactive compounds of foods with potential health benefits: a review on recent trends. Food Biosci. 43, 101320 (2021). https://doi.org/10.1016/j.fbio.2021.101320
A. Annunziata, R. Vecchio, Functional foods development in the european market: a consumer perspective. J. Funct. Foods. 3, 223–228 (2011). https://doi.org/10.1016/j.jff.2011.03.011
B. Plasek, A. Temesi, The credibility of the effects of functional food products and consumers’ willingness to purchase/willingness to pay- review. Appetite. 143, 104398 (2019). https://doi.org/10.1016/j.appet.2019.104398
T. Wandersleben, E. Morales, C. Burgos-Díaz, T. Barahona, E. Labra, M. Rubilar, H. Salvo-Garrido, Enhancement of functional and nutritional properties of bread using a mix of natural ingredients from novel varieties of flaxseed and lupine, LWT 91 (2018) 48–54
P. Conte, A. Del Caro, P.P. Urgeghe, G.L. Petretto, L. Montanari, A. Piga, C. Fadda, Nutritional and aroma improvement of gluten-free bread: is bee pollen effective? LWT. 118, 108711 (2020). https://doi.org/10.1016/j.lwt.2019.108711
A.S. Desai, T. Beibeia, M.A. Brennan, X. Guo, X.-A. Zeng, C.S. Brennan, Protein, amino acid, fatty acid composition, and in vitro digestibility of bread fortified with Oncorhynchus tschawytscha powder, Nutrients 10 (2018) 1923
V.D. Capriles, J.A.G. Arêas, Novel approaches in gluten-free breadmaking: interface between food science, nutrition, and health. Compr. Reviews Food Sci. Food Saf. 13, 871–890 (2014)
R. Klopsch, S. Baldermann, F.S. Hanschen, A. Voss, S. Rohn, M. Schreiner, S. Neugart, Brassica-enriched wheat bread: unraveling the impact of ontogeny and breadmaking on bioactive secondary plant metabolites of pak choi and kale. Food Chem. 295, 412–422 (2019)
M.D. Wilcox, P. Cherry, P.I. Chater, X. Yang, M. Zulali, E.J. Okello, C.J. Seal, J.P. Pearson, The effect of seaweed enriched bread on carbohydrate digestion and the release of glucose from food. J. Funct. Foods. 87, 104747 (2021). https://doi.org/10.1016/j.jff.2021.104747
M. Haber, M. Mishyna, J.J.I. Martinez, O. Benjamin, The influence of grasshopper (Schistocerca gregaria) powder enrichment on bread nutritional and sensorial properties, Lwt-Food Sci Technol 115 (2019) 108395. ARTN 108395 https://doi.org/10.1016/j.lwt.2019.108395
M. Kieliszek, K. Piwowarek, A.M. Kot, S. Błażejak, A. Chlebowska-Śmigiel, I. Wolska, Pollen and bee bread as new health-oriented products: a review. Trends in Food Science Technology. 71, 170–180 (2018)
Q.-Q. Li, K. Wang, M.C. Marcucci, A.C.H.F. Sawaya, L. Hu, X.-F. Xue, L.-M. Wu, F.-L. Hu, Nutrient-rich bee pollen: a treasure trove of active natural metabolites. J. Funct. Foods. 49, 472–484 (2018)
K. Komosinska-Vassev, P. Olczyk, J. Kaźmierczak, L. Mencner, K. Olczyk, Bee pollen: chemical composition and therapeutic application. Evidence-Based Complement. Altern. Med. 2015, 297425 (2015)
A. Vásquez, T.C. Olofsson, The lactic acid bacteria involved in the production of bee pollen and bee bread. J. Apic. Res. 48, 189–195 (2009)
A.C. Urcan, L.A. Marghitas, D.S. Dezmirean, O. Bobis, V. Bonta, C.I. Muresan, R. Margaoan, Chemical Composition and Biological Activities of Beebread – Review, Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Animal Science and Biotechnologies 74 (2017) 6. https://doi.org/10.15835/buasvmcn-asb:12646
P. Conte, A. Del Caro, F. Balestra, A. Piga, C. Fadda, Bee pollen as a functional ingredient in gluten-free bread: a physical-chemical, technological and sensory approach. LWT. 90, 1–7 (2018)
J.S. Novais, M.L. Absy, F.d.A.R.d. Santos, Pollen types collected by Tetragonisca angustula (Hymenoptera: Apidae) in dry vegetation in northeastern Brazil, Eur. J. Entomol. 111 (2014)
W. Von Der Ohe, L. Oddo, M. Piana, M. Morlot, P. Martin, Harmonized methods of melissopalynology. Apidologie. 35, S18–S25 (2004)
W. Horwitz, G. Latimer, Association of Official Analytical Chemists International, Official Methods of Analysis of AOAC International 18th ed (Gaithersburg, AOAC International, 2006)
A.L. Rabie, J.D. Wells, L.K. Dent, The nitrogen content of pollen protein. J. Apic. Res. 22, 119–123 (1983)
A. Tomás, S.I. Falcão, P. Russo-Almeida, M. Vilas-Boas, Potentialities of beebread as a food supplement and source of nutraceuticals: Botanical origin, nutritional composition and antioxidant activity. J. Apic. Res. 56, 219–230 (2017)
C. Fuenmayor, B.C. Zuluaga, D.C. Díaz M, M. Quicazán de C, M. Cosio, S. Mannino, Evaluation of the physicochemical and functional properties of colombian bee pollen. Revista MVZ Córdoba. 19, 4003–4014 (2014)
M. Carocho, P. Morales, M. Ciudad-Mulero, V. Fernandez-Ruiz, E. Ferreira, S. Heleno, P. Rodrigues, L. Barros, I.C. Ferreira, Comparison of different bread types: Chemical and physical parameters. Food Chem. 310, 125954 (2020)
E. Regulation, No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Eur. Comm. Off J. Eur. Union. 20, 168–213 (2011)
C.R. Encina-Zelada, V. Cadavez, J.A. Teixeira, U. Gonzales-Barron, Optimization of quality properties of gluten-free bread by a mixture design of xanthan, guar, and hydroxypropyl methyl cellulose gums. Foods. 8, 156 (2019)
C. De Carli, V. Aylanc, K.M. Mouffok, A. Santamaria-Echart, F. Barreiro, A. Tomás, C. Pereira, P. Rodrigues, M. Vilas-Boas, S.I. Falcão, Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 213, 486–497 (2022)
I.S. Organization, ISO 6887-1, : (2017) Microbiology of food and animal feeding stuffs — Preparation of test samples, initial suspension and decimal dilutions for microbiological examination — Part 1: General rules for the preparation of the initial suspension and decimal dilutions Part 1: General rules for the preparation of the initial suspension and decimal dilutions, 2022
I.S. Organization, ISO 4833-1: 2013. Microbiology of the food chain — Horizontal method for the enumeration of microorganisms — Part 1: Colony count at 30°C by the pour plate technique, 2019
I.S. Organization, ISO 7218:2007 Microbiology of food and Animal Feeding stuffs — General Requirements and Guidance for Microbiological Examinations (General requirements and guidance for microbiological examinationsInternational Organization for Standardization, Geneva, 2013)
I.S. Organization, ISO 21527-1, : 2008. Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of yeasts and moulds Part 2: Colony count technique in products with water activity less than or equal to 0, 95, International Standard Organization Geneva, 2008
I.S. Organization, ISO 15213:2003 - Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of sulfite-reducing bacteria growing under anaerobic conditions, 2015
M.G. Campos, S. Bogdanov, L.B. de Almeida-Muradian, T. Szczesna, Y. Mancebo, C. Frigerio, F. Ferreira, Pollen composition and standardisation of analytical methods. J. Apic. Res. 47, 154–161 (2008)
M. Salehifar, M. Shahedi, Effects of Oat Flour on Dough Rheology, texture and Organoleptic Properties of Taftoon Bread, J. Agric. Sci. Technol. 9 (2007)
P. Gélinas, Active dry yeast: Lessons from patents and science. Compr. Reviews Food Sci. Food Saf. 18, 1227–1255 (2019)
E.W. Jr Herbert, H. Shimanuki, Chemical composition and nutritive value of bee-collected and bee-stored pollen. Apidologie. 9, 33–40 (1978)
L.M. Estevinho, S. Rodrigues, A.P. Pereira, X. Feás, Portuguese bee pollen: palynological study, nutritional and microbiological evaluation. Int. J. Food Sci. Technol. 47, 429–435 (2012)
M. Thakur, V. Nanda, Composition and functionality of bee pollen: a review. Trends in Food Science Technology. 98, 82–106 (2020)
A.Å. Kostić, M.B. Pešić, M.D. Mosić, B.P. Dojčinović, M.M. Natić, J.Ä. Trifković, Mineral content of bee pollen from Serbia, Arhiv za higijenu rada i toksikologiju 66 (2015) 251–258
V. Liolios, C. Tananaki, A. Papaioannou, D. Kanelis, M.-A. Rodopoulou, N. Argena, Mineral content in monofloral bee pollen: investigation of the effect of the botanical and geographical origin. J. Food Meas. Charact. 13, 1674–1682 (2019)
S.M. Mohammad, N.-K. Mahmud-Ab-Rashid, N. Zawawi, Botanical origin and nutritional values of bee bread of stingless bee (Heterotrigona itama) from Malaysia, Journal of Food Quality 2020 (2020)
A. Torrinha, M. Oliveira, S. Marinho, P. Paiga, C. Delerue-Matos, S. Morais, Mineral content of various portuguese breads: characterization, Dietary Intake, and Discriminant Analysis. Molecules. 24 (2019). https://doi.org/10.3390/molecules24152787
P. Strazzullo, L. D’Elia, N.-B. Kandala, F.P. Cappuccio, Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies, Bmj 339 (2009)
F.P. Cappuccio, M. Beer, P. Strazzullo, European Salt Action, Population dietary salt reduction and the risk of cardiovascular disease. A scientific statement from the european Salt Action Network. Nutr. Metab. Cardiovasc. Dis. 29, 107–114 (2018). https://doi.org/10.1016/j.numecd.2018.11.010
D. República., N. Law, ◦ 75/2009 from 12 of August. First series, 2009, Nº155. - Diário da República I Série, 2009, pp. 5225–5226
G.T. Ferrari, C. Proserpio, L.K. Stragliotto, J.M. Boff, E. Pagliarini, V.R. de Oliveira, Salt reduction in bakery products: a critical review on the worldwide scenario, its impacts and different strategies, Trends in Food Science Technology 129 (2022)
A.-S. Hager, A. Wolter, M. Czerny, J. Bez, E. Zannini, E.K. Arendt, M. Czerny, Investigation of product quality, sensory profile and ultrastructure of breads made from a range of commercial gluten-free flours compared to their wheat counterparts. Eur. Food Res. Technol. 235, 333–344 (2012)
P. Chareonthaikij, T. Uan-On, W. Prinyawiwatkul, Effects of pineapple pomace fibre on physicochemical properties of composite flour and dough, and consumer acceptance of fibre‐enriched wheat bread. Int. J. Food Sci. Technol. 51, 1120–1129 (2016)
M. Gómez, F. Ronda, C.A. Blanco, P.A. Caballero, A. Apesteguía, Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol. 216, 51–56 (2003)
T. Croguennec, Non-enzymatic browning, John Wiley & Sons, Handbook of food science technology 1: Food alteration food quality, 2016
J. Carreño, A. Martínez, L. Almela, J.A. Fernández-López, Proposal of an index for the objective evaluation of the colour of red table grapes. Food Res. Int. 28, 373–377 (1995). https://doi.org/10.1016/0963-9969(95)00008-A
I. Barene, I. Daberte, S. Siksna, Investigation of bee bread and development of its dosage forms. Medicinos Teorija ir Praktika. 21, 16–22 (2014). https://doi.org/10.15591/mtp.2015.003
L.S. Young, 22 - Applications of texture analysis to dough and bread, in: S.P. Cauvain (Ed.) Breadmaking (Second Edition), Woodhead Publishing2012, pp. 562–579
S. Bhise, A. Kaur, Baking quality, sensory properties and shelf life of bread with polyols. J. Food Sci. Technol. 51, 2054–2061 (2014)
M.E. Matos, T. Sanz, C.M. Rosell, Establishing the function of proteins on the rheological and quality properties of rice based gluten free muffins. Food Hydrocoll. 35, 150–158 (2014)
U. Gonzales-Barron, R. Dijkshoorn, M. Maloncy, T. Finimundy, R.C. Calhelha, C. Pereira, D. Stojković, M. Soković, I.C. Ferreira, L. Barros, Nutritive and bioactive properties of mesquite (Prosopis pallida) flour and its technological performance in breadmaking. Foods. 9, 597 (2020)
A. Saddozai, K. Samina, Microbiological status of bakery products available in Islamabad. Pakistan J. Agricultural Res. 22, 93–96 (2009)
J. Ju, Y. Xie, H. Yu, Y. Guo, Y. Cheng, Y. Chen, L. Ji, W. Yao, Synergistic properties of citral and eugenol for the inactivation of foodborne molds in vitro and on bread. LWT. 122, 109063 (2020). https://doi.org/10.1016/j.lwt.2020.109063
A.M. Katsurayama, P.M. Planas, S.T.A. Dantas, B.F. Rossi, E.C.R. Bonsaglia, G.L.R. da Silva, V.L.M. Rall, Microbiological quality of Doner kebab sold in retail in Sao Paulo-Brazil/Qualidade Microbiológica do Churrasco grego (Doner Kebab) vendido no varejo em São Paulo–Brazil. Brazilian J. Dev. 6, 11639–11648 (2020)
Acknowledgements
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support by national funds FCT/MCTES to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2021). Thanks to the European Regional Development Fund (ERDF) through the Regional Operational Program North 2020, within the scope of Project PDR2020-1.0.1-FEADER-031734: “DivInA-Diversification and Innovation on Beekeeping Production” and project GreenHealth, Norte-01-0145-FEDER-000042. Thanks to national funding by FCT- Foundation for Science and Technology, through the institutional scientific employment program-contract with Soraia I. Falcão. Thanks to Joana Serra from Nopabril LDA, from Bragança, Portugal, for the support in the bread making process.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Seymanur Ertosun: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, Visualization. Soraia I. Falcão: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing. Volkan Aylanc: Conceptualization, Methodology. Andreia Tomás: Methodology. Paulo Russo-Almeida: Methodology. Paula Rodrigues: Conceptualization, Methodology, Validation. Miguel Vilas-Boas: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition
Corresponding authors
Ethics declarations
Conflict of interest
The authors report there are no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ertosun, S., Falcão, S.I., Aylanc, V. et al. The impact of bee product incorporation on the processing properties, nutritional value, sensory acceptance, and microbial stability of bread. Food Measure 18, 451–463 (2024). https://doi.org/10.1007/s11694-023-02172-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02172-4