Abstract
Trees progress through various growth stages, each marked by specific responses and adaptation strategies to environmental conditions. Despite the importance of age-related growth responses on overall forest health and management policies, limited knowledge exists regarding age-related effects on dendroclimatic relationships in key subtropical tree species. In this study, we employed a dendrochronological method to examine the impact of rapid warming on growth dynamics and climatic sensitivity of young (40–60 years) and old (100–180 years) Pinus massoniana forests across six sites in central-southern China. The normalized log basal area increment of trees in both age groups increased significantly following rapid warming in 1984. Trees in young forests further showed a distinct growth decline during a prolonged severe drought (2004–2013), whereas those in old forests maintained growth increases. Tree growth was more strongly influenced by temperature than by moisture, particularly in old forests. Spring temperatures strongly and positively impacted the growth of old trees but had a weaker effect on young ones. Old forests had a significantly lower resistance to extreme drought but faster recovery compared to young forests. The “divergence problem” was more pronounced in younger forests due to their heightened sensitivity to warming-induced drought and heat stress. With ongoing warming, young forests also may initially experience a growth decline due to their heightened sensitivity to winter drought. Our findings underscore the importance of considering age-dependent changes in forest/tree growth response to warming in subtropical forest management, particularly in the context of achieving “Carbon Peak & Carbon Neutrality” goals in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, climate warming has emerged as a crucial factor influencing the maintenance or alteration of forest morphology (Bauman et al. 2022; Martinez-Sancho et al. 2022). Previous studies have demonstrated that rapid warming significantly impacts tree vigor, species distribution, and the structure, function, and productivity of forests (McDowell et al. 2015; Rozenberg et al. 2020; Cherubini et al. 2021). Understanding how forests or trees respond to recent climate warming is key to predicting future forest dynamics and formulating effective forest management strategies (Carrasco et al. 2022; Zhang et al. 2023; Hu et al. 2024).
Annual rings are reliable indicators of growth dynamics and natural environments (Cherubini et al. 2021; Gao et al. 2022). Tree rings have been widely used to explore tree growth-climate relationships and the ways in which forests respond to climate change (Begovic et al. 2022; Martinez-Sancho et al. 2022; Zhang et al. 2023). Existing dendrochronological studies indicate that the impacts of climate warming on tree growth remain uncertain, even controversial (Camarero et al. 2015; Bauman et al. 2022). For example, while numerous studies report a generally positive effect of global warming on tree growth or forest productivity due to shifts in plant phenology and increased atmospheric CO2 concentrations (Begovic et al. 2022; Huang et al. 2023), some studies show that rapid warming may also increase respiration rates and drought stress, negatively affecting tree growth or leading to large-scale mortality (McDowell et al. 2015; Bauman et al. 2022). Some studies also indicate that climate warming does not necessarily stimulate tree growth (Clark et al. 2010; Van der Sleen et al. 2015).
Ontogeny is one of the most significant factors influencing tree growth-climate relationships (Au et al. 2022; Begovic et al. 2022). Trees undergo physiological changes as they age and increase in size, developing different strategies to cope with changing environmental conditions (Carrer and Urbinati 2004; Martínez-Vilalta et al. 2007). The climatic sensitivity of forests/trees may undergo diverse changes with increasing age, including enhanced or weakened sensitivity, or no changes (Carrer and Urbinati 2004; Foster et al. 2014; Jiao et al. 2020; Peng et al. 2023). In general, the climatic sensitivity of radial growth increases with age until the tree reaches its maximum height, with some environmental specificity (Carrer and Urbinati 2004; Martínez-Vilalta et al. 2007). For instance, young trees exhibit greater sensitivity than older trees during dry summers due to their developing root systems and low water absorption capacity (Bond 2000; Carrer and Urbinati 2004). However, in temperature-limited environments, old trees may show higher climatic sensitivity than younger ones as a result of their increased use of resources to defend against stress and maintain survival (Carrer and Urbinati 2004). Alternatively, shorter growing seasons result in delayed basipetal movement of growth hormones (Rossi et al. 2008). Therefore, understanding the age-related growth responses of forests/trees to climate warming is crucial for predicting changes in forest productivity, ecosystem services, and carbon sequestration capacity (Camarero et al. 2015; Huang et al. 2021).
Pinus massoniana Lamb. is widely distributed in subtropical regions of China and is commonly used in afforestation in southern China. It plays an important role in the regional carbon balance, in wood production, and in ecosystem services (Ni et al. 2023; Zhang et al. 2023). The species has been widely used for tree ring-based paleoclimate reconstructions for the past two decades due to its sensitivity to climate (Chen et al. 2012; Duan et al. 2012). Given its regional importance, there is a need to understand how its growth reacts to climate change (Liang et al. 2019; Jing et al. 2022b). However, age-dependent growth responses of P. massoniana to climate change remain unexplored. In this study, a dendrochronological investigation of P. massoniana on six sites in the Zhangjiajie region of Hunan Province was undertaken to: (1) determine the impact of rapid warming on the growth of trees in young and old age-class sites, (2) identify any differences in their growth-climate relationships and drought resilience, and (3) examine the variation of their climate-growth relationships over time.
Materials and methods
Study area and climate
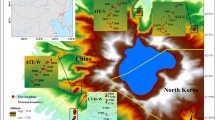
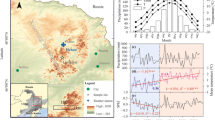
This study was conducted in the Zhangjiajie region (29.38°–29.74° N, 109.77°–111.29° E) of Hunan Province in central-south China (Fig. 1). The area has a humid subtropical continental monsoon climate with hot rainy summers and cold dry winters. According to climate data (1976–2019) from the Sangzhi meteorological station (29.4° N, 110.17° E, 600 m a.s.l.), annual mean temperatures range from 15.7 to 17.2 ℃. The coldest and warmest months are January (4.8 ℃) and July (27.0 ℃), respectively (Fig. 2). Annual total precipitation ranges from 825 to 2,229 mm, with 68% occurring from April to August. The peak monthly mean temperature (July) and precipitation (June) are not synchronized (Fig. 2). The year 1984 marks an abrupt negative peak in temperature for the period 1976–2019. Since 1984, there has been a significant increase in annual mean temperatures at a rate of 0.2 ℃ per decade (R2 = 0.279, p < 0.05). The region experienced a period of prolonged drought stress, as indicated by a high vapor pressure deficit and low relative humidity during 2004–2013, defined as a “drought period” (Fig. 2). The forest vegetation in this area mainly consists of subtropical evergreen conifers and broadleaved species. It is dominated by P. massoniana, admixed Cunninghamia lanceolata (Lamb.) Hook., Liquidambar formasana Hance, Lithocarpus glabra (Thunb.) Nakai, and Cinnamomum camphora (Linn.) Presl. The predominant soils are Ferralsols and Cambisols (Zhao et al. 2009).
Distribution of sampling sites and weather station. All maps used in this figure were prepared using the 2023 edition of the standard map of China [censor code: GS(2023)2767]. The map was released by the Ministry of Natural Resources of China and downloaded from National Platform for Common Geospatial Information Services (http://bzdt.ch.mnr.gov.cn/)
a, b Climatic diagrams for the study area and variations in (c) precipitation, d mean temperature, e vapor pressure deficit (VPD), and f relative humidity 1976–2019. The straight lines in c–f represent linear fitted trends before and after the abrupt increase in mean temperatures in 1984. The R-squared and slope (k) of each model are listed. The yellow-shaded area highlights a long-term drought during 2004–2013. T, mean temperature; Tmin, minimum temperature; Tmax, maximum temperature; P, precipitation. *p < 0.05
Fieldwork and dendrochronological methods
Two age groups were established, young (40–60 years, YOUNG) and old (100–180 years, OLD) classes of P. massoniana forests, considering that the oldest tree was less than 200 years old. Three replicate sites were designated for each age group to mitigate the impact of site conditions on results. In July 2021, six forest stands with elevations ranging from 270 to 831 m were selected as sampling sites (Table 1). At each site, at least 20 healthy P. massoniana trees were sampled in stands with minimal disturbances. Two increment cores per tree were taken at breast height (1.3 m) using a 5.15 mm inner diameter increment borer (Haglöf, Sweden). In some cases, only one core was sampled to reduce the damage to particularly small trees. All cores were numbered, stored in straws, and transported to the laboratory.
In the laboratory, all samples were air-dried and glued onto wooden mounts. Core surfaces were sanded with progressively finer grades of sandpaper until tree-ring boundaries were clearly visible under the microscope. Each tree’s annual rings were visually cross-dated to identify possible absent or false rings and assigned an accurate calendar year using the skeleton-plot method (Stokes and Smiley 1968). Ring widths were measured to an accuracy of 0.01 mm using the LINTAB™ 6 tree-ring width measuring system (Frank Rinntech, Germany). The accuracy of measurements and cross-dating was statistically tested with the COFECHA program (Holmes 1983). Samples younger than 15 years or with poor cross-dating quality were excluded from the study. A total of 287 cores from 164 trees were used for further analysis (Table 1).
Raw ring width sequences were detrended by fitting a negative exponential curve or linear regression function (ModNegExp) with the “dplR” package (Bunn 2008) in R software (R Core Team 2021). All tree-ring indices from each site were then combined into a single standard chronology using Tukey’s biweight robust mean to minimize the effects of abnormal values (Fig. 3) (Cook 1985). The arithmetic mean method was used to calculate the regional tree-ring chronologies of P. massoniana for young (YOUNG) and old (OLD) age classes. Statistical characteristics of the ring width chronologies for each site are shown in Table 1.
Basal area increment measurements
Basal area increment (BAI) provides a more accurate representation of growth than tree-ring width because it eliminates variations in growth due to increasing stem circumference and size adjustments in growth (Zhu et al. 2018a; Jing et al. 2022b). The ring width measurements were converted to BAI using the formula:
where, \(R_{n}^{2}\) and \(R_{n - 1}^{2}\) are the squared stem radial increments corresponding to rings formed in years n and n − 1, respectively. After implementing the pith offset (Rozas 2003), raw ring widths were converted to BAI values using the BIAPlt software (available at http://www.ldeo.columbia.edu/treering-laboratory/resources/software). For a more intuitive comparison of P. massoniana BAI growth at different sites, the BAI sequence was log-transformed and normalized using the Z-core method (Jing et al. 2022b).
Climate data
Instrumental climate data from the nearby Sangzhi Meteorological Station were obtained from the China Meteorological Data Service Centre (http://data.cma.cn). Monthly climate data from 1976 to 2019 were used in this study because the shortest chronology started in 1976. Climate variables included total precipitation (P), relative humidity (Rh), and mean (T), minimum (Tmin), and maximum (Tmax) temperatures.
Vapor pressure deficit (VPD), the amount of vapor stored in the air until the saturation point at a given temperature is reached, is a proven indicator of drought (Martinez-Sancho et al. 2022). Monthly VPD data were calculated from the monthly mean temperature and relative humidity using the formula:
where Ta is air temperature, and Rh is relative humidity.
Seasonal climate data were defined as previous winter (pWin, previous December–current February), current spring (SPR, March–May), current summer (SUM, June–August), and current autumn (AUT, September–November).
Extreme drought events and indices of resistance and recovery
Based on previous studies (Jing et al. 2022b) and our correlation analysis, we found that autumn drought plays an important role in tree growth. Extreme drought years were characterized based on the averaged autumn VPD values (Martinez-Sancho et al. 2022). First, the first order differences of VPD in autumn from 1976 to 2019 were calculated. Years in the first order differences series with values higher than the mean plus 1.5 times the standard deviation were defined as extreme drought years (Fang and Zhang 2019; Zhu et al. 2023). The years 1988, 1990, 2001, and 2019 were identified as extreme drought years.
The ability of trees to continue growing under drought stress is represented by the resistance (Rt), which is the reduction of radial growth during drought compared to before the drought. The capacity of trees to restart growth following drought is represented by the recovery (Rc), the post-drought radial growth compared to that during the drought (Lloret et al. 2011; Fang and Zhang 2019; Zhu et al. 2023). Tree resilience to drought was estimated using the two components (Rt and Rc), calculated for each tree following the formulas provided by Lloret et al. (2011):
where Dr indicates the ring width indices or raw ring widths in the drought year, and PreDr and PostDr indicate the mean ring width indices or raw ring widths during the three years before and after the drought. The year 2019 was not considered in calculating drought resilience as data for the three subsequent years were not available.
Statistical analysis
A segmented test was conducted to examine long-term trends in temperature using the R package ‘segmented’ (Muggeo 2008). The year 1984 was identified as an abrupt point of temperature increase between 1976 and 2019. A linear regression model was used to investigate temporal trends in climate variation and radial growth before and after 1984. Pearson correlation coefficients (R) between main climatic variables and ring width chronologies were calculated to reveal the impact of climate change on tree growth. In this study, growth-climate relationships were calculated for a 12-month period from the previous December to the current November, and for a 4-season period from the previous winter to the current autumn. Moving correlation analysis between seasonal climate and tree growth was also applied to the period 1976–2019 with a 20 year moving window to detect temporal changes in growth-climate relationships. Correlation and moving correlation analyses were performed using the R package ‘treeclim’ (Zang and Biondi 2015).
Results
Growth characteristics and drought resilience of P. massoniana
The LXY chronology showed a positive correlation with the LPH (R = 0.36, p < 0.05) and LPL (R = 0.4, p < 0.01) chronologies. Additionally, the LPL (R = 0.55, p < 0.01) was positively correlated with the LPH chronology (R = 0.4, p < 0.01). The LJS and LJN chronologies also demonstrated a positive correlation (R = 0.51, p < 0.01) (Table 2). The normalized log BAI sequences revealed that tree growth on old and young age-class sites significantly increased after the onset of rapid warming in 1984. Trees in old age-class sites still maintained a similar trend of growth enhancement during the long-term severe drought period 2004–2013, while trees in young age-class sites showed a slight decline in the dry period (Fig. 4). The OLD group trees have a significantly lower resistance (p < 0.0001) to drought but higher recovery (p < 0.05) than the YOUNG group trees (Fig. 5).
Temporal variation of normalized log basal area increment (BAI) for Pinus massoniana in the study area. Growth patterns for young and old age classes are fitted by linear regressions from 1984 to 2020 (red) and 2004 to 2013 (blue). 1984 marks the abrupt negative peak of the mean annual temperature. Yellow-shaded areas (2004–2013) indicate drought years. The R-squared and slope (k) of each model are listed. The data for YOUNG and OLD groups were obtained by averaging the raw BAI values corresponding to the same age-class sites and then logarithmically normalizing them. **p < 0.01
Boxplots of mean resistance (Rt) and recovery (Rc) indices of trees in young and old age classes to extreme droughts in 1988, 1990, and 2001; resistance and recovery indices are calculated using raw ring widths and tree ring indices. The data for the YOUNG and OLD groups are a mixture of samples from the young and old age-class sites. *p < 0.05, ****p < 0.0001
Relationship between tree growth and climate
Tree growth in old and young age classes had a strong positive correlation with temperatures (T, Tmax, and Tmin) in January to May and the current spring, especially for trees in old age-class sites (Fig. 6). However, trees in old and young age classes responded differently to Tmin in September, with a negative correlation (p < 0.05) with growth in the young LJN and LJS sites but a weak correlation with old age-class sites (Fig. 6).
Correlations between tree ring width indices and monthly (from the previous December to the current November) and seasonal climate variables. YOUNG and OLD mean regional tree-ring chronologies of Pinus massoniana at young and old age-class sites. P, precipitation; Rh, relative humidity; VPD, vapor pressure deficit; T, mean temperature; Tmax, maximum temperature; and Tmin, minimum temperature; pWin, previous winter; SPR, current spring; SUM, current summer; AUT, current autumn. *p < 0.05
Drought in autumn had a strong effect on the radial growth of trees in old age-class sites, but no effect on the growth of trees in young age-class sites (Fig. 6). The Rh and VPD in November had a significant (p < 0.05) positive and negative correlation with the radial growth of trees in old age-class sites, respectively (Fig. 6). Precipitation had slight impacts on radial growth on all sites. The consistency of tree growth-climate relationships on old age-class sites was stronger than on young age-class sites.
Temporal stability of tree growth-climate relationships
A moving correlation analysis revealed a clear shift in growth-climate relationships around 1984, a year of rapid warming (Fig. 7). The relationships between temperatures (T and Tmin) during the current spring and tree growth on the OLD age group maintained a stable, and positive correlation. However, the relationship shifted from a significant (p < 0.05) positive correlation to a weak one in the YOUNG age group (Fig. 7). The negative impacts of Tmin in the current autumn on tree growth in the OLD and YOUNG age groups strengthened in recent decades, with the OLD age group trees shifting from positive (p < 0.05) to negative correlations, and the YOUNG age group trees transitioning from weak correlations to negative (p < 0.05) ones.
Moving correlation analysis between tree-ring chronologies and main seasonal climate factors during 1976–2019. Pearson correlation coefficients were computed for 20-year moving windows. YOUNG and OLD mean regional tree-ring chronologies of Pinus massoniana at young and old age-class sites. P, precipitation; Rh, relative humidity; VPD, vapor pressure deficit; T, mean temperature; Tmax, maximum temperature; and Tmin, minimum temperature; pWin, previous winter; SPR, current spring; SUM, current summer; AUT, current autumn. *p < 0.05
The correlation between previous winter precipitation and tree growth in the OLD age group gradually shifted from weak positive to negative in recent decades. In contrast, it turned from weakly negative to significantly (p < 0.05) positive for trees in the YOUNG age group (Fig. 7). The negative impacts of relative humidity in the current autumn for trees in both YOUNG and OLD age groups strengthened with time; specifically, their correlations shifted from positive to negative or from significantly positive to weakly positive. The correlation between autumn VPD and tree growth in the YOUNG and OLD age groups was gradually opposite to the relationships between autumn Rh and tree growth (Fig. 7).
Discussion
Growth enhancement of young and old P. massoniana forests
The normalized log BAI of P. massoniana significantly increased after 1984 across all sites. This growth enhancement has been reported by Li et al. (2020), and similar patterns have been observed with other subtropical tree species (Carrasco et al. 2022). These studies are in agreement with the general belief that subtropical forest ecosystems are likely to benefit or at least withstand rising temperatures (Boisvenue and Running 2006; Reyes-Fox et al. 2014). In recent decades, increasing atmospheric CO2 has been an important factor contributing to enhanced growth (Camarero et al. 2015; Zhu et al. 2021). As early as 1984, Lamarche et al. (1984) attributed the improved growth of high elevation Pinus aristate Engelm and Pinus longaeva Bailey in the western United States during the late twentieth century to higher atmospheric CO2. Elevated CO2 levels may increase photosynthetic rates and light energy utilization efficiency (Reyes-Fox et al. 2014) and improve intrinsic water-use efficiency (Martinez-Sancho et al. 2022). Additionally, temperature has been identified as a key factor driving cambium reactivation in conifers in the Northern Hemisphere (Gao et al. 2022; Huang et al. 2023). Rapid warming promotes an earlier start and later finish of cambium activities, extending the growing period, which could be another important factor contributing to the growth enhancement of P. massoniana (Duan et al. 2012). Gao et al. (2022) also found that the prolonged growing season from climate warming significantly promoted growth in humid areas but not arid ones. Other possible reasons for growth enhancement of P. massoniana require further investigation. For example, the El Niño-Southern Oscillation has been shown to significantly increase the growth of P. massoniana in subtropical China by delaying the arrival of summer monsoons (Li et al. 2020). Moreover, no detrending process was applied to BAI data in this study. The substantial growth improvement of trees in young age-class sites may be partly attributed to the age-related trend in BAI growth. According to the typically sigmoidal pattern of BAI growth in individual trees, this rapid growth increase in young trees could occur because of an increasing canopy as the trees age (Johnson and Abrams 2009).
Furthermore, the radial growth of trees in young age-class sites showed a clear decline during the long-term drought of 2004–2013, while trees in old age-class sites basically enhanced growth. It suggests that trees in old age-class sites still benefit from warming, even under severe drought. This is understandable because trees in young age class exhibit increasing sensitivities to winter droughts as climate warming intensifies, while trees in old age class do not (Fig. 7) (Carrer and Urbinati 2004; Au et al. 2022). Winter drought may weaken tree hydraulic-carbohydrate systems, resulting in slower growth in the following growing season (Earles et al. 2018). A plausible explanation is that older trees have a higher water absorption capacity and can access water from deeper soil layers because of their more developed and extensive root systems (Puhe 2003). To some extent, these root systems buffer the adverse effects of winter drought (Au et al. 2022; Zhu et al. 2023). Additionally, competition is a major factor affecting tree growth. Competition for resources between neighboring trees adversely affects growth and may even lead to decline or death, especially for saplings with limited competitiveness (Gómez-Aparicio et al. 2011; Liang et al. 2019).
However, our results do not agree with previous studies in China’s subtropical forests that reported a significant decline in radial growth of P. massoniana in recent decades due to warming-related drought (Li et al. 2019; Jing et al. 2022b). Elevated temperatures may enhance respiration and drought stress, increasing the consumption of limited water resources and resulting in a decline in growth (Zhu et al. 2018a; Jiao et al. 2020). In contrast to these studies, our study area experienced lower annual temperatures that did not exceed the optimal threshold for limiting P. massoniana growth. Furthermore, the contrasting findings may also be attributed to differences in intensity of solar radiation (photosynthetic capacity) (Muller-Landau et al. 2021) and soil characteristics (water and nutrient holding capacity) between different regions (Li et al. 2019; Jing et al. 2022b). There are still some intriguing possibilities to explore, such as a comparative study between healthy and declining pine forests to verify whether the evidence of growth decline on sensitive sites exposed to extreme drought and thermal stress is conclusive. More research is needed to thoroughly understand the processes and implications of warming on P. massoniana growth in southern China.
Growth-climate relationships of P. massoniana in young and old forests
The radial growth of P. massoniana was more strongly associated with temperature than precipitation. This is consistent with studies on P. massoniana in other subtropical areas of China (Chen et al. 2012; Duan et al. 2012) and other temperate tree species in northeast China (Zhu et al. 2020). Precipitation is typically not a limiting factor for tree growth in humid environments, especially in mountainous regions (Duan et al. 2012; Jevšenak et al. 2021). Our study area, characterized by a humid subtropical climate with 1412 mm of rain annually, satisfies the water needs for tree growth. This is why existing paleoclimate reconstructions based on tree ring widths of P. massoniana in subtropical areas of China are primarily focused on temperature rather than precipitation or drought indices (Chen et al. 2012; Duan et al. 2012).
Spring temperatures, especially in March, had a significant positive effect on P. massoniana growth, particularly for trees in old age-class sites (Fig. 6). This result agrees with the observation that warm spring conditions boost P. massoniana growth (Duan et al. 2012; Fu et al. 2017). A rise in spring temperatures is typically associated with an early onset of cambial activity (Duan et al. 2012) and high photosynthesis (Reyes-Fox et al. 2014). Warm, early spring temperatures promote the activity of microorganisms, enhancing litter decomposition and nitrogen mineralization and thereby releasing more nutrients to support growth (Fu et al. 2017). High early-spring temperatures also protect trees from frost damage (Duan et al. 2012). Trees in old age-class sites were more sensitive to spring temperatures than those in young age-class sites because they have well-developed organs and are more able to take advantage of favorable early spring conditions. For example, the extensive foliage and deep root systems of older trees allow more efficient access to water and nutrients and to synthesize photosynthates (Bond 2000; Puhe 2003). In addition, older trees, having experienced numerous spring seasons, might have developed specific resilience to typical spring temperature changes. This adaptation could demonstrate a positive relationship with spring temperatures. For example, older trees exhibit more stable and predictable phenological patterns, including the timing of bud burst and flowering, which are often triggered by temperature cues (Rollinson and Kaye 2012). This stability leads to tree growth being more synchronized (strong positive correlation) with spring temperatures.
Furthermore, September VPD exhibited a strong negative correlation, and Rh exhibited a strong positive correlation with P. massoniana trees in old age-class sites, but no correlation with trees in young age-class sites. This suggests that older trees were more sensitive to drought towards the end of the growing season. A plausible explanation is that younger trees store carbon during periods of high stress and allocate it more towards respiration than growth, potentially increasing resistance to external mortality factors (McDowell et al. 2011) and avoiding drought-induced carbon starvation (Huang et al. 2021). However, older trees may prioritize allocating resources to maintaining existing structures and metabolic functions over active growth (Malhi 2012). Towards the end of the growing season, the transition to dormancy and the need to store resources for winter may alter resource allocation, potentially making older trees more sensitive to water stress (Schultz et al. 2013). Additionally, drought stress toward the end of the growing season may impact various phenological processes influenced by water availability such as leaf senescence and nutrient reabsorption, making older trees more vulnerable (Estiarte and Peñuelas 2015). Moreover, differences in the growth-climate relationships of P. massoniana among sites within the same age group might be attributed to heterogeneous microenvironments caused by topography and elevation, including factors such as solar radiation, air humidity, and soil properties (Gómez-Aparicio et al. 2011; Zhu et al. 2018b; Rozenberg et al. 2020). These factors could immediately or eventually affect radial growth, leading to diverse tree growth responses to climate among sites (Zhu et al. 2018a, 2021; Jing et al. 2022a). For example, trees at the high-elevation LPH site exhibited a significant positive correlation with temperature from January to March, whereas trees at the low-elevation LPL site showed a weak correlation. This is in agreement with that high-elevation trees are usually more sensitive to temperature than those at lower elevations (Rozenberg et al. 2020; Jing et al. 2022a). Additionally, trees on the shady slope at the LJN site had positive correlations with temperature and Rh in winter. In contrast, trees on the sunny slope at the LJS site were weakly correlated with these factors. This difference may be related to variations in winter microclimates between sunny and shady slopes, including factors like sunshine hours, temperature, evapotranspiration, and snow cover (Branson and Shown 1990; Tang and Fang 2006).
Drought resilience of young and old P. massoniana forest trees
There were significant differences in drought resistance and recovery between young and old trees. It is important to clarify that older trees are generally considered more resistant to drought than younger ones due to their more extensive root systems and greater accumulation of water and nutrient reserves (Puhe 2003; Zhu et al. 2023). However, we found that trees in the YOUNG group exhibited higher resistance to but lower recovery from drought compared to trees in the OLD group (Fig. 5). This is in agreement with research on ponderosa pine (Pinus ponderosa Douglas ex C. Lawson) in remote forests of the Rocky Mountains (Lloret et al. 2011) and certain angiosperm and gymnosperm species across North and South America, Eurasia, Africa, and Oceania (Au et al. 2022).
The lower resistance in older trees may indicate a reduction in vitality, consistent with the extensively documented age-related reduction in tree growth (Lutz et al. 2018) and the frequently disproportionate mortality of older trees (Foster et al. 2014; McDowell et al. 2015). This could also reflect growth and survival tradeoffs, whereby stress-induced growth reductions in older trees reflect a survival strategy (Dobbertin 2005; Körner 2017; Piovesan and Biondi 2021). Another explanation involves non-structural carbohydrates: during periods of drought, older trees may have a bias to converting non-structural carbon to starch for recovery growth, while younger trees may convert it to soluble sugars to defend against the negative effects of drought (Dietze et al. 2014; Martínez-Vilalta et al. 2016).
Moreover, older trees, being more resilient, may have experienced cumulative stress over the years such as damage from pests, diseases, or previous drought events. This accumulated stress could make them more susceptible to additional stressors, including drought, especially toward the end of the growing season (Niinemets 2010). As noted previously, older trees allocate resources differently than younger ones (Malhi 2012), potentially making older trees more vulnerable to drought (Schultz et al. 2013). However, older trees usually have a larger storage capacity, accumulating considerable nutrients, carbohydrates, and water. These resources enable the canopy and the root system to continue functioning or to re-establish after the stress period, resulting in better recovery from extreme drought (Linares et al. 2010; Begovic et al. 2022).
The tradeoff between resistance and recovery can be related to the mathematical relations between Rt and Rc. A significantly decreased Dr in the presence of similar PreDr and PostDr levels will invariably produce low Rt and high Rc values. Galiano et al. (2011) found that if both resistance and recovery following periods of drought partly depend on the amount of accumulated reserves, a tradeoff between resistance and recovery might happen, leading to high resistance and low recovery, or vice versa. The observed enhanced tree growth in old age-class sites could be attributed to high recovery. Nevertheless, it is important to note that the response of trees to drought stress is complex and can vary significantly based on species, environmental conditions, and individual tree characteristics. More research at a larger regional scale is needed to test the findings of age-related differences in the responses of P. massoniana to extreme drought and the mechanisms behind them.
Temporal variability in dendroclimatic relationships of P. massoniana
The positive correlations between winter-spring temperatures and tree growth on young age-class sites gradually weakened after the onset of rapid warming in 1984. This reduced growth sensitivity to temperature is known as the “divergence problem” (D’Arrigo et al. 2008). Wang et al. (2014) found that five subtropical tree species, including P. massoniana, Pinus taiwanensis Hayata, Schima superba Gardn et Champ., C. lanceolata (Lamb.) Hook., and Castanopsis eyrei (Champ. ex Benth.) Tutcher, in southeastern China have a weaker response to temperatures in recent decades than in the early decades of the twentieth century.
A high winter-spring temperature can increase drought stress by accelerating air/soil water evaporation and plant transpiration (Atkin et al. 2007). In addition, photosynthetic enzyme activity may decrease as temperatures increase beyond a particular threshold (Lindner et al. 2010), resulting in a narrow growth ring. Increased water use efficiency may also be a key reason for the diminished effect of temperature on trees (McMahon et al. 2010; Van der Sleen et al. 2015). The results of a study by Cai et al. (2020) show similar responses by P. massoniana at low elevations, whereas at high elevations, it showed a positive correlation with temperature. Trees typically benefit from warming in cold, high elevation environments (Zhu et al. 2018a; Rozenberg et al. 2020). The strong correlation between temperature and tree growth at high elevations suggests that warming may eventually encourage the expansion of the tree line and provide a more favorable environment. However, the “divergence problem” was not found in older P. massoniana trees (OLD group), possibly as a result of their weaker sensitivity to warming-related drought (Carrer and Urbinati 2004; Au et al. 2022).
In general, climate warming significantly increased the carbon fixation of P. massoniana, especially by older forests, which indicates that they benefit more from climate warming (Begovic et al. 2022) or suffer less damage in a warming climate than younger forests (Wang et al. 2019). Furthermore, the negative effects of warming are mainly caused by drought. Because older trees can recover quickly from drought, they are less affected by warming. These factors could also help explain why older forests benefit more from warming. However, drought stress in our study area will increase if warming continues, reducing photosynthesis and increasing respiration (Lindner et al. 2010). The growth of young trees will likely decrease due to warming-related drought stress, resulting in a long-term decline in the carbon sink of young P. massoniana forests in the subtropical region. Old P. massoniana forests are likely to continue removing atmospheric carbon at increasing rates for many decades beyond 100 years (Lutz et al. 2018).
Pinus massoniana is one of the most important afforestation tree species in southern China. Therefore, from a climate mitigation perspective, the preservation of older forests/trees should be prioritized because of their greater capacity for carbon sequestration and storage (Körner 2017). It is also crucial to choose different tree species to develop an optimal species portfolio to compensate for the different degrees of carbon capture of different age classes due to continued warming. Some moisture conditions also should be changed, such as irrigation or watering, when carrying out afforestation, which could offset the negative impacts of global warming. Moreover, when dealing with extreme droughts, more focus should be given to post-drought protection for young P. massoniana forests/trees, while preventative measures should be prioritized for older forests/trees to maintain higher productivity. These findings could help the Chinese government implement “Ecosystem Conservation and Restoration Projects” and achieve the “Carbon Peak & Carbon Neutrality” goals, the most important national policies now being implemented by the Chinese government.
Conclusion
In this study, a dendrochronological approach assessed the impact of rapid warming on the growth of P. massoniana in old and young forests. Trees in both young and old age-class sites exhibited significant growth enhancement (BAI) after 1984. The radial growth of P. massoniana was generally more strongly linked with temperature than with moisture, indicating that temperature was the key factor influencing growth in the study area. The growth of older trees was more sensitive to climate factors, such as spring temperatures (positive) and autumn vapor pressure deficits (negative). Different climate-growth responses of P. massoniana on nearby sites with similar ages might be caused by heterogeneous microenvironments. Trees in older forests recover faster from extreme droughts, while those in younger forests are more resistant to drought stress.
The “divergence problem” of P. massoniana was found in young forest sites but not in old forest sites due to differences in drought sensitivity. Trees in old age-class sites had a positive correlation with spring temperatures after warming, in line with their continuing growth enhancement even under long-term severe drought. The results suggest that trees in old forests may be able to enhance growth under continued warming in coming decades, but those in younger forests may experience more severe growth decline or increased mortality. Our findings may provide new perspectives on paleoclimate reconstructions as well as on the estimation of the effects of climate warming on forest dynamics and biomass accumulation in subtropical forests. They may be helpful in formulating sustainable forest management strategies for subtropical forests of different ages in southern China.
References
Atkin OK, Scheurwater I, Pons TL (2007) Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol 174(2):367–380. https://doi.org/10.1111/j.1469-8137.2007.02011.x
Au TF, Maxwell JT, Robeson SM, Li JB, Siani SMO, Novick KA, Dannenberg MP, Phillips RP, Li T, Chen ZJ, Lenoir J (2022) Younger trees in the upper canopy are more sensitive but also more resilient to drought. Nat Clim Change 12(12):1168–1174. https://doi.org/10.1038/s41558-022-01528-w
Bauman D, Fortunel C, Delhaye G, Malhi Y, Cernusak LA, Bentley LP, Rifai SW, Aguirre-Gutierrez J, Menor IO, Phillips OL, McNellis BE, Bradford M, Laurance SGW, Hutchinson MF, Dempsey R, Santos-Andrade PE, Ninantay-Rivera HR, Chambi Paucar JR, McMahon SM (2022) Tropical tree mortality has increased with rising atmospheric water stress. Nature 608(7923):528–533. https://doi.org/10.1038/s41586-022-04737-7
Begovic K, Schurman JS, Svitok M, Pavlin J, Langbehn T, Svobodova K, Mikolas M, Janda P, Synek M, Marchand W, Vitkova L, Kozak D, Vostarek O, Cada V, Bace R, Svoboda M (2022) Large old trees increase growth under shifting climatic constraints: aligning tree longevity and individual growth dynamics in primary mountain spruce forests. Glob Change Biol 29(1):143–164. https://doi.org/10.1111/gcb.16461
Boisvenue C, Running SW (2006) Impacts of climate change on natural forest productivity-evidence since the middle of the 20th century. Glob Change Biol 12(5):862–882. https://doi.org/10.1111/j.1365-2486.2006.01134.x
Bond BJ (2000) Age-related changes in photosynthesis of woody plants. Trends Plant Sci 5(8):349–353. https://doi.org/10.1016/s1360-1385(00)01691-5
FA Branson, LM Shown (1990) Contrasts of vegetation, soils, microclimates, and geomorphic processes between north-and south-facing slopes on Green Mountain near Denver, Colorado. Department of the Interior, US Geological Survey
Bunn AG (2008) A dendrochronology program library in R (dplR). Dendrochronologia 26(2):115–124. https://doi.org/10.1016/j.dendro.2008.01.002
Cai QF, Liu Y, Qian HJ, Liu RS (2020) Inverse effects of recent warming on trees growing at the low and high altitudes of the Dabie mountains, subtropical China. Dendrochronologia 59:125649. https://doi.org/10.1016/j.dendro.2019.125649
Camarero JJ, Gazol A, Galvan JD, SanguesaBarreda G, Gutierrez E (2015) Disparate effects of global-change drivers on mountain conifer forests: warming-induced growth enhancement in young trees versus CO2 fertilization in old trees from wet sites. Glob Change Biol 21(2):738–749. https://doi.org/10.1111/gcb.12787
Carrasco G, Almeida AC, Falvey M, Olmedo GF, Taylor P, Santibanez F, Coops NC (2022) Effects of climate change on forest plantation productivity in Chile. Global Change Biol 28(24):7391–7409. https://doi.org/10.1111/gcb.16418
Carrer M, Urbinati C (2004) Age-dependent tree-ring growth responses to climate in Larix decidua and Pinus cembra. Ecology 85(3):730–740. https://doi.org/10.1890/02-0478
Chen F, Yuan YJ, Wei WS, Sl Yu, Zhang TW (2012) Reconstructed temperature for Yong’an, Fujian, Southeast China: linkages to the Pacific ocean climate variability. Glob Planet Change 86–87:11–19. https://doi.org/10.1016/j.gloplacha.2012.01.005
Cherubini P, Battipaglia G, Innes JL (2021) Tree vitality and forest health: Can tree-ring stable isotopes be used as indicators? Curr for Rep 7(2):69–80. https://doi.org/10.1007/s40725-021-00137-8
Clark DB, Clark DA, Oberbauer SF (2010) Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Glob Change Biol 16(2):747–759. https://doi.org/10.1111/j.1365-2486.2009.02004.x
Cook ER (1985) A time series analysis approach to tree-ring standardization. University of Arizona, Tucson
D’Arrigo R, Wilson R, Liepert B, Cherubini P (2008) On the ‘divergence problem’ in northern forests: a review of the tree-ring evidence and possible causes. Glob Planet Change 60(3):289–305. https://doi.org/10.1016/j.gloplacha.2007.03.004
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65(1):667–687. https://doi.org/10.1146/annurev-arplant-050213-040054
Dobbertin M (2005) Tree growth as indicator of tree vitality and of tree reaction to environmental stress: a review. Eur J Forest Res 124(4):319–333. https://doi.org/10.1007/s10342-005-0085-3
Duan J, Zhang QB, Lv L, Zhang C (2012) Regional-scale winter-spring temperature variability and chilling damage dynamics over the past two centuries in southeastern China. Clim Dynam 39(3–4):919–928. https://doi.org/10.1007/s00382-011-1232-9
Earles JM, Stevens JT, Sperling O, Orozco J, North MP, Zwieniecki MA (2018) Extreme mid-winter drought weakens tree hydraulic-carbohydrate systems and slows growth. New Phytol 219(1):89–97. https://doi.org/10.1111/nph.15136
Estiarte M, Peñuelas J (2015) Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: effects on nutrient proficiency. Glob Change Biol 21(3):1005–1017. https://doi.org/10.1111/gcb.12804
Fang O, Zhang QB (2019) Tree resilience to drought increases in the Tibetan Plateau. Glob Change Biol 25(1):245–253. https://doi.org/10.1111/gcb.14470
Foster JR, D’Amato AW, Bradford JB (2014) Looking for age-related growth decline in natural forests: unexpected biomass patterns from tree rings and simulated mortality. Oecologia 175(1):363–374. https://doi.org/10.1007/s00442-014-2881-2
Fu LY, Lei XD, Hu ZD, Zeng WS, Tang SZ, Marshall P, Cao L, Song XY, Yu L, Liang JJ (2017) Integrating regional climate change into allometric equations for estimating tree aboveground biomass of Masson pine in China. Ann Forest Sci 74(2):42. https://doi.org/10.1007/s13595-017-0636-z
Galiano L, Martínez-Vilalta J, Lloret F (2011) Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol 190(3):750–759. https://doi.org/10.1111/j.1469-8137.2010.03628.x
Gao S, Liang EY, Liu RS, Babs F, Camarero JJ, Fu YSH, Piao SL, Rossi S, Shen MG, Wang T, Peñuelas J (2022) An earlier start of the thermal growing season enhances tree growth in cold humid areas but not in dry areas. Nat Ecol Evol 6(4):397–404. https://doi.org/10.1038/s41559-022-01668-4
Gómez-Aparicio L, García-Valdés R, RuÍz-Benito P, Zavala MA (2011) Disentangling the relative importance of climate, size and competition on tree growth in Iberian forests: implications for forest management under global change. Glob Change Biol 17(7):2400–2414. https://doi.org/10.1111/j.1365-2486.2011.02421.x
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull 43:51–67
Hu Z, Zhu L, Liu S, Lei P, Zhang R, Cherubini P (2024) Xylem adjustment and growth response of early- and late-successional tree species to rapid warming. Eur J for Res (early Access). https://doi.org/10.1007/s10342-023-01655-9
Huang JB, Hammerbacher A, Gershenzon J, Van Dam NM, Sala A, McDowell NG, Chowdhury S, Gleixner G, Trumbore S, Hartmann H (2021) Storage of carbon reserves in spruce trees is prioritized over growth in the face of carbon limitation. P Natl Acad Sci USA 118(33):e2023297118. https://doi.org/10.1073/pnas.2023297118
Huang JG, Zhang YL, Wang MH, Yu XH, Deslauriers A, Fonti P, Liang E, Mäkinen H, Oberhuber W, Rathgeber CBK, Tognetti R, Treml V, Yang B, Zhai LH, Zhang JL, Antonucci S, Bergeron Y, Camarero JJ, Campelo F, Čufar K, Cuny HE, De Luis M, Fajstavr M, Giovannelli A, Gričar J, Gruber A, Gryc V, Güney A, Jyske T, Kašpar J, King G, Krause C, Lemay A, Liu F, Lombardi F, del Castillo EM, Morin H, Nabais C, Nöjd P, Peters RL, Prislan P, Saracino A, Shishov VV, Swidrak I, Vavrčík H, Vieira J, Zeng Q, Liu Y, Rossi S (2023) A critical thermal transition driving spring phenology of Northern Hemisphere conifers. Glob Change Biol 29(6):1606–1617. https://doi.org/10.1111/gcb.16543
Jevšenak J, Tychkov I, Gričar J, Levanič T, Tumajer J, Prislan P, Arnič D, Popkova M, Shishov VV (2021) Growth-limiting factors and climate response variability in Norway spruce (Picea abies L.) along an elevation and precipitation gradients in Slovenia. Int J Biometeorol 65(2):311–324. https://doi.org/10.1007/s00484-020-02033-5
Jiao L, Chen K, Wang SJ, Liu XP (2020) Stability evaluation of radial growth of Picea schrenkiana in different age groups in response to climate change in the eastern Tianshan Mountains. J Mt Sci 17(7):1735–1748. https://doi.org/10.1007/s11629-019-5703-5
Jing M, Zhu L, Cherubini P, Yuan D, Li Z, Wang X, Liu S (2022a) Responses of radial growth of Pinus massoniana and Castanopsis eyrei to climate change at different elevations in south China. Ecol Indic 145:109602. https://doi.org/10.1016/j.ecolind.2022.109602
Jing M, Zhu L, Liu S, Cao Y, Zhu Y, Yan W (2022b) Warming-induced drought leads to tree growth decline in subtropics: evidence from tree rings in central China. Front Plant Sci 13:964400. https://doi.org/10.3389/fpls.2022.964400
Johnson SE, Abrams MD (2009) Age class, longevity and growth rate relationships: protracted growth increases in old trees in the eastern United States. Tree Physiol 29(11):1317–1328. https://doi.org/10.1093/treephys/tpp068
Körner C (2017) A matter of tree longevity. Science 355(6321):130–131. https://doi.org/10.1126/science.aal2449
Lamarche VC, Graybill DA, Fritts HC, Rose MR (1984) Increasing atmospheric carbon dioxide: tree ring evidence for growth enhancement in natural vegetation. Science 225(4666):1019–1021. https://doi.org/10.1126/science.225.4666.1019
Li Y, Dong Z, Chen D, Zhao S, Zhou F, Cao X, Fang K (2019) Growth decline of Pinus massoniana in response to warming induced drought and increasing intrinsic water use efficiency in humid subtropical China. Dendrochronologia 57:125609. https://doi.org/10.1016/j.dendro.2019.125609
Li JY, Huang JG, Tardif JC, Liang HX, Jiang SW, Zhu HX, Zhou P (2020) Spatially heterogeneous responses of tree radial growth to recent El Niño southern-oscillation variability across East Asia subtropical forests. Agric for Meteorol 287:107939. https://doi.org/10.1016/j.agrformet.2020.107939
Liang HX, Huang JG, Ma QQ, Li JY, Wang Z, Guo XL, Zhu HX, Jiang SW, Zhou P, Yu BY, Luo DW (2019) Contributions of competition and climate on radial growth of Pinus massoniana in subtropics of China. Agric for Meteorol 274:7–17. https://doi.org/10.1016/j.agrformet.2019.04.014
Linares JC, Camarero JJ, Carreira JA (2010) Competition modulates the adaptation capacity of forests to climatic stress: insights from recent growth decline and death in relict stands of the Mediterranean fir Abies pinsapo. J Ecol 98(3):592–603. https://doi.org/10.1111/j.1365-2745.2010.01645.x
Lindner M, Maroschek M, Netherer S, Kremer A, Barbati A, Garcia-Gonzalo J, Seidl R, Delzon S, Corona P, Kolström M, Lexer MJ, Marchetti M (2010) Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol Manag 259(4):698–709. https://doi.org/10.1016/j.foreco.2009.09.023
Lloret F, Keeling EG, Sala A (2011) Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120(12):1909–1920. https://doi.org/10.1111/j.1600-0706.2011.19372.x
Lutz JA, Furniss TJ, Johnson DJ, Davies SJ, Allen D, Alonso A, Anderson-Teixeira KJ, Andrade A, Baltzer J, Becker KML, Blomdahl EM, Bourg NA, Bunyavejchewin S, Burslem DFRP, Cansler CA, Cao K, Cao M, Cárdenas D, Chang LW, Chao KJ, Chao WC, Chiang JM, Chu C, Chuyong GB, Clay K, Condit R, Cordell S, Dattaraja HS, Duque A, Ewango CEN, Fischer GA, Fletcher C, Freund JA, Giardina C, Germain SJ, Gilbert GS, Hao Z, Hart T, Hau BCH, He F, Hector A, Howe RW, Hsieh CF, Hu YH, Hubbell SP, Inman-Narahari FM, Itoh A, Janík D, Kassim AR, Kenfack D, Korte L, Král K, Larson AJ, Li Y, Lin Y, Liu S, Lum S, Ma K, Makana JR, Malhi Y, McMahon SM, McShea WJ, Memiaghe HR, Mi X, Morecroft M, Musili PM, Myers JA, Novotny V, de Oliveira A, Ong P, Orwig DA, Ostertag R, Parker GG, Patankar R, Phillips RP, Reynolds G, Sack L, Song G-ZM, Su SH, Sukumar R, Sun IF, Suresh HS, Swanson ME, Tan S, Thomas DW, Thompson J, Uriarte M, Valencia R, Vicentini A, Vrška T, Wang X, Weiblen GD, Wolf A, Wu SH, Xu H, Yamakura T, Yap S, Zimmerman JK (2018) Global importance of large-diameter trees. Glob Ecol Biogeogr 27(7):849–864. https://doi.org/10.1111/geb.12747
Malhi Y (2012) The productivity, metabolism and carbon cycle of tropical forest vegetation. J Ecol 100(1):65–75. https://doi.org/10.1111/j.1365-2745.2011.01916.x
Martinez-Sancho E, Treydte K, Lehmann MM, Rigling A, Fonti P (2022) Drought impacts on tree carbon sequestration and water use-evidence from intra-annual tree-ring characteristics. New Phytol 236(1):58–70. https://doi.org/10.1111/nph.18224
Martínez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F (2016) Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecol Monogr 86(4):495–516. https://doi.org/10.1002/ecm.1231
Martínez-Villta J, Vanderklein D, Mencuccini M (2007) Tree height and age-related decline in growth in Scots pine (Pinus sylvestris L.). Oecologia 150(4):529–544. https://doi.org/10.1007/s00442-006-0552-7
McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M (2011) The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol 26(10):523–532. https://doi.org/10.1016/j.tree.2011.06.003
McDowell NG, Nathan G, Allen CD (2015) Darcy’s law predicts widespread forest mortality under climate warming. Nat Clim Change 5(7):669–672. https://doi.org/10.1038/nclimate2641
McMahon SM, Parker GG, Miller DR (2010) Evidence for a recent increase in forest growth. Proc Natl Acad Sci U S A 107(8):3611–3615. https://doi.org/10.1073/pnas.0912376107
Muggeo VM (2008) Segmented: an R package to fit regression models with broken-line relationships. R News 8(1):20–25. https://doi.org/10.1159/000323281
Muller-Landau HC, Cushman KC, Arroyo EE, Martinez Cano I, Anderson-Teixeira KJ, Backiel B (2021) Patterns and mechanisms of spatial variation in tropical forest productivity, woody residence time, and biomass. New Phytol 229(6):3065–3087. https://doi.org/10.1111/nph.17084
Ni Y, Xiao W, Liu J, Jian Z, Li M, Xu J, Lei L, Zhu J, Li Q, Zeng L, Cherubini P (2023) Radial growth-climate correlations of Pinus massoniana in natural and planted forest stands along a latitudinal gradient in subtropical central China. Agric for Meteorol 334:109422. https://doi.org/10.1016/j.agrformet.2023.109422
Niinemets Ü (2010) Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. For Ecol Manag 260(10):1623–1639. https://doi.org/10.1016/j.foreco.2010.07.054
Peng Z, Zhang Y, Zhu L, Guo M, Lu Q, Xu K, Shao H, Mo Q, Liu S (2023) Spatial and temporal patterns of the sensitivity of radial growth response by Picea schrenkiana to regional climate change in the Tianshan Mountains. J Forestry Res 34(6):1669–1681. https://doi.org/10.1007/s11676-023-01629-y
Piovesan G, Biondi F (2021) On tree longevity. New Phytol 231(4):1318–1337. https://doi.org/10.1111/nph.17148
Puhe J (2003) Growth and development of the root system of Norway spruce (Picea abies) in forest stands—a review. For Ecol Manag 175(1):253–273. https://doi.org/10.1016/S0378-1127(02)00134-2
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reyes-Fox M, Steltzer H, Trlica MJ, McMaster GS, Andales AA, LeCain DR, Morgan JA (2014) Elevated CO2 further lengthens growing season under warming conditions. Nature 510(7504):259–262. https://doi.org/10.1038/nature13207
Rollinson CR, Kaye MW (2012) Experimental warming alters spring phenology of certain plant functional groups in an early successional forest community. Glob Change Biol 18(3):1108–1116. https://doi.org/10.1111/j.1365-2486.2011.02612.x
Rossi S, Deslauriers A, Anfodillo T, Carrer M (2008) Age-dependent xylogenesis in timberline conifers. New Phytol 177(1):199–208. https://doi.org/10.1111/j.1469-8137.2007.02235.x
Rozas V (2003) Tree age estimates in Fagus sylvatica and Quercus robur: testing previous and improved methods. Plant Ecol 167(2):193–212. https://doi.org/10.1023/A:1023969822044
Rozenberg P, Chauvin T, Escobar-Sandoval M, Huard F, Shishov V, Charpentier JP, Sergent AS, Vargas-Hernandez JJ, Martinez-Meier A, Pâques L (2020) Climate warming differently affects Larix decidua ring formation at each end of a French Alps elevational gradient. Ann Forest Sci 77(2):54. https://doi.org/10.1007/s13595-020-00958-w
Schultz J, Appel H, Ferrieri A, Arnold T (2013) Flexible resource allocation during plant defense responses. Front Plant Sci. https://doi.org/10.3389/fpls.2013.00324
Stokes MA, Smiley TL (1968) An introduction to tree-ring dating. University of Arizona Press, Tucson
Tang Z, Fang J (2006) Temperature variation along the northern and southern slopes of Mt. Taibai China. Agric for Meteorol 139(3):200–207. https://doi.org/10.1016/j.agrformet.2006.07.001
Van der Sleen P, Groenendijk P, Vlam M, Anten NPR, Boom A, Bongers F, Pons TL, Terburg G, Zuidema PA (2015) No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat Geosci 8(1):24–28. https://doi.org/10.1038/ngeo2313
Wang X, Pederson N, Chen Z, Lawton K, Zhu C, Han S (2019) Recent rising temperatures drive younger and southern Korean pine growth decline. Sci Total Environ 649:1105–1116. https://doi.org/10.1016/j.scitotenv.2018.08.393
Wang X, Li Z, Ma K (2014) Decreased sensitivity of tree growth to temperature in Southeast China after the 1976/’77 regime shift in Pacific climate. Sains Malays 43(1):9–19
Zang C, Biondi F (2015) treeclim: an R package for the numerical calibration of proxy-climate relationships. Ecography 38(4):431–436. https://doi.org/10.1111/ecog.01335
Zhang R, Hu Z, Cherubini P, Cooper DJ, Zhu L, Lei P (2023) Tree-ring data reveal trees are suffering from severe drought stress in the humid subtropical forest. For Ecol Manag 546:121330. https://doi.org/10.1016/j.foreco.2023.121330
Zhao T, Yang B, Zheng H (2009) Assessment of the erosion control function of forest ecosystems based on GIS: a case study in Zhangjiajie National Forest Park, China. Int J Sustain Dev World Ecol 16(5):356–361. https://doi.org/10.1080/13504500903205022
Zhu L, Cooper DJ, Yang J, Zhang X, Wang X (2018a) Rapid warming induces the contrasting growth of Yezo spruce (Picea jezoensis var. microsperma) at two elevation gradient sites of northeast China. Dendrochronologia 50:52–63. https://doi.org/10.1016/j.dendro.2018.05.002
Zhu L, Wang X, Pederson N, Chen Z, Cooper DJ, Zhang Y, Li Z (2018b) Spatial variability in growth-climate relationships of Amur cork tree (Phellodendron amurense) and their connections with PDO in Northeast China. J Geophys Res Biogeo 123(5):1625–1636. https://doi.org/10.1029/2017jg004292
Zhu L, Cooper DJ, Yuan D, Li Z, Zhang Y, Liang H, Wang X (2020) Regional scale temperature rather than precipitation determines vessel features in earlywood of Manchurian ash in temperate forests. J Geophys Res Biogeosci. https://doi.org/10.1029/2020JG005955
Zhu L, Liu S, Arzac A, Cooper DJ, Jin Y, Yuan D, Zhu Y, Zhang X, Li Z, Zhang Y, Liang H, Wang X (2021) Different response of earlywood vessel features of Fraxinus mandshurica to rapid warming in warm-dry and cold-wet areas. Agric for Meteorol 307:108523. https://doi.org/10.1016/j.agrformet.2021.108523
Zhu L, Zhang J, Camarero JJ, Cooper DJ, Cherubini P, Yuan D, Wang X (2023) Drivers and spatiotemporal patterns of post-drought growth resilience of four temperate broad-leaved trees. Agric for Meteorol 342:109741. https://doi.org/10.1016/j.agrformet.2023.109741
Acknowledgements
We are grateful to the Forestry Bureau staff for field assistance.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was funded by the National Natural Science Foundation of China (42107476, 31901241), the China Postdoctoral Science Foundation (2020M682600), the China Postdoctoral International Exchange Fellowship Program (PC2021099) and the Natural Science Foundation of Hunan Province (2021JJ41075).
The online version is available at https://link.springer.com/.
Corresponding editor: Tao Xu.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, W., Zhu, L., Zhu, L. et al. Old Pinus massoniana forests benefit more from recent rapid warming in humid subtropical areas of central-southern China. J. For. Res. 35, 88 (2024). https://doi.org/10.1007/s11676-024-01740-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11676-024-01740-8