Abstract
Pterocarpus santalinus L. f. (Fabaceae; red sanders) is prized for its wood whose colour and fragrance is due to the presence of santalins that have pharmaceutical and industrial uses. Red sanders is listed as an endangered plant species on the IUCN red data list as a result of the exploitation of its wood and essential oil. This review emphasizes the pollination biology, seed germination, vegetative propagation and micropropagation of P. santalinus. Excessive use of P. santalinus has also caused the emergence of various adulterants, so accurate identification is essential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Historical, cultural, medicinal, and economic importance of red sanders as a basis for conservation
Pterocarpus santalinus L. f. (Fabaceae) is most commonly known as red sandalwood in English, but it also has other common names in several languages (Table S1). Pterocarpus is derived from the Greek words pteron (wing) and karpos (fruit), referring to the winged pod, while santalinus originates from the Latin sandal and inus (meaning similar to), i.e., a plant with characteristics similar to Indian sandalwood, Santalum album L. (Botanical Survey of India 2012). Like African or Nepalese sandalwood (Teixeira da Silva et al. 2016a) and Indian sandalwood (Teixeira da Silva et al. 2016b), P. santalinus is also prized for its hard, dark-purple, bitter heartwood (Navada and Vittal 2014). In India, the natural range of P. santalinus used to be a very restricted area of 15,540 km2 in the southeast (Sarma 1993). Currently, P. santalinus is found exclusively in a well-defined forest tract of Andhra Pradesh in Southern India (Raju and Nagaraju 1999; Prakash et al. 2006; Balaraju et al. 2011), but is also found in the Chinese provinces of Yunnan, Guangdong and Guangxi, and on Hainan Island, where it is referred to as zitan (Kaner et al. 2013).
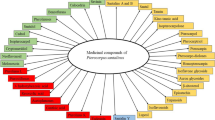
The colour and fragrance of P. santalinus heartwood are derived from santalins while the pleasent aroma is caused by the presence of terpenoids (Kumar et al. 1974). A dye prepared from the heartwood of P. santalinus is used as a stain in light microscopy (Banerjee and Mukherjee 1981; Sen Gupta and Mukherjee 1981), as a coloring agent in pharmaceutical preparations, in food, leather and textile industries (Ankalaiah et al. 2017), and as a textile dye (Gulrajani et al. 2002). The medicinal properties of P. santalinus have been extensively reviewed elsewhere (Navada and Vittal 2014; Azamthulla et al. 2015) and will not be covered in this review. However, multiple uses (Table S2), ethnomedicinal uses (Table S3), and phytochemistry (Table S4) have been provided as supplementary tables to offer a more rounded appreciation of this tree in the context of this review.
The texture and colour differentiate good quality from poor quality trees, with “wavy grain wood texture with intense red color” in the former and “straight grain wood texture with light red color” in the latter (Prakash et al. 2006), and it is the superior quality of P. santalinus that makes it popular in the furniture industry (Prakash et al. 2006; Arunkumar et al. 2011; Arunkumar and Joshi 2014; Azamthulla et al. 2015). In Japan, P. santalinus is used to make carvings and musical instruments, shamisen and koto (Kukrety et al. 2013b; Arunkumar and Joshi 2014; Azamthulla et al. 2015; Ramabrahmam and Sujatha 2016), as well as name seals or hankos. In Buddhism, P. santalinus is considered to be a symbol of holiness, and is thus used for carved statues, as a constituent of incense (Wu et al. 2011), and for cremation (Ramakrishna 1962). In China, P. santalinus wood has a long history of use in furniture and other valuable wood products (Berliner 1996; Kaner et al. 2013).
The export of P. santalinus from India to Europe started in the 17th century, mainly for fabric dyeing (Vedavathy 2004). The Herbal Folklore Research Centre in Tirupati, India, estimated that from 500 planted trees ha−1, at least 500 kg of heartwood per tree can be obtained after 25 years, thus 25 t ha−1 of wood plantation (Vedavathy 2004). At 2004 prices of Rs. 75 kg−1, such a plantation would yield a return of Rs. 177.5 lakhs ha−1 (US$375,000 ha−1) (Vedavathy 2004). Current market prices are, however, unknown to the authors, although prices are likely to be high since natural P. santalinus stands have been in decline as a result of this overexploitation for commercial purposes, earning it an endangered status since 1997 (IUCN 2018).
This review provides an overview of the reproductive biology, seed germination and micropropagation of P. santalinus as tools for its conservation and large-scale propagation.
Basic flowering biology, and sexual and vegetative reproduction
Pollination and seedset
The P. santalinus tree flowers in the dry season (Rao et al. 2001; Rao and Raju 2002). The flowers are papilionaceous, bisexual, large and and yellow (Rao and Raju 2002). Flowering is discontinuous, blooming at intervals of 2–5 d (Rao et al. 2001). Flowers open at night and the primary pollinators are Apis dorsata, A. cerana indica and A. florea (Rao and Raju 2002). P. santalinus, which shows facultative xenogamy, tends to eliminate growing fruits from self-pollinated flowers, i.e., there is large-scale abortion of flower buds, flowers and fruit (Rao et al. 2001), and has very low fruit set (− 6%), 52% of which set seed (Rao and Raju 2002).
Seed germination
Traditional seed propagation of P. santalinus yields low germination percentages due to a hard testa, poor viability, and sensitivity to temperature (Kumar and Gopal 1975; Dayanand and Lohidas 1988; Anuradha and Pullaiah 1998; Naidu 2001a, b; Naidu and Rajendrudu 2001). Dried, soaked and scarified P. santalinus pods resulted in 49% germination (Kumarasinghe et al. 2003) although seed germination in natural stands or under artificial propagation is generally low (− 30%) (Kumar and Gopal 1975; Dayanand and Lohidas 1988; Kalimuthu and Lakshmanan 1995; Naidu 2001a, b;Naidu and Rajendrudu 2001). Alternate wetting and drying every 48 h enhanced germination, reaching 73% (Vijayalakshmi and Renganayaki 2017). Seed germination, seedling height, and root collar diameter were all significantly stimulated by fire (Kukrety et al. 2013b). Presoaking P. santalinus pods with 500 mg/L gibberellic acid for 24 h resulted in 66.7% seed germination, as well as improved plant growth and seedling survival relative to other treatments with tap water, luke warm water, gibberellic acid, H2SO4 or HCl (Patel et al. 2018).
Vegetative propagation
Vegetative propagation of P. santalinus by semi-hardwood cuttings, cleft grafting, or air layering is not able to produce stock numbers required for effective preservation or for commercial purposes (Kedharnath et al. 1976; Kesava Reddy and Srivasuki 1990). Relative to seed germination, there are almost no studies on ex vitro vegetative propagation for red sanders. However, to provide elite germplasm with desired traits, such as the wavy grain or phytochemicals such as santalins, vegetative propagation under controlled conditions is desirable, and in vitro propagation allows for the production of true-to-type plants via micropropagation such as axillary shoot multiplication or shoot tip culture at a large scale, to continuously produce plantlets with uniform characteristics. In tree biotechnology, such as for Indian sandalwood (Teixeira da Silva et al. 2016b), in vitro propagation also allows for the improvement of desired characteristics such as pathogen resistance or improved wood quality by genetic engineering. The next section assesses the progress of micropropagation of P. santalinus.
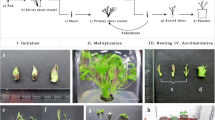
Micropropagation
Explants
The explant source (i.e., mother plant) and the procedure to surface disinfect explants are important aspects underlying the success of a tissue culture protocol (Leifert et al. 1994; Teixeira da Silva et al. 2016c). Information about the explants used for the in vitro propagation of P. santalinus, as well as surface disinfection protocols, are summarised in Table 1. P. santalinus seedlings derived from in vitro seed germination have been a popular source of explants, while in vitro germinated seedlings, shoot tips, cotyledons, hypocotyls, mesocotyl and nodes have also served as popular sources of explants for culture initiation since they do not require surface disinfection (Lakshmi Sita et al. 1992; Anuradha and Pullaiah 1999a,b; Chaturani et al. 2005; Rajeswari and Paliwal 2008; Balaraju et al. 2011; Vipranarayana et al. 2012; Warakagoda and Subasinghe 2013). In terms of ex vitro sources of explants, Prakash et al. (2006) used young terminal shoot cuttings collected from mature trees in winter as the explant; Ashrafee et al. (2014) used leaf segments from 1 to 2 year old plants while Sarita et al. (1988) used nodes and terminal cuttings.
Basal medium
The most commonly used and preferred basal medium for in vitro studies on P. santalinus is Murashige and Skoog (1962) (MS) medium (Table 2). Lakshmi Sita et al. (1992) used Gamborg’s B5 medium (Gamborg et al. 1968) with 2% sucrose and 0.8% agar to multiply axillary shoots from shoot tips derived from seedlings germinated in vitro. Chaturani et al. (2006) used Anderson medium (Anderson 1980) and Vitis medium (Chee and Pool 1987) to germinate seed. Anuradha and Pullaiah (1999a) employed half-strength B5 medium supplemented with 0.05% activated charcoal to germinate seeds in vitro then used B5 medium with 8.88 µM 6-benzyladenine (BA) for shoot tip culture.
In vitro propagation from predetermined meristems
Three predetermined meristems were employed in P. santalinus tissue culture: shoot tips, cotyledonary nodes and nodes from mature trees (Table 2). Shoot tips from in vitro germinated seedlings were used by Lakshmi Sita et al. (1992), Anuradha and Pullaiah (1999a), and Balaraju et al. (2011) for shoot tip culture, with either BA as the most effective cytokinin (Lakshmi Sita et al. 1992; Anuradha and Pullaiah 1999a) or a combination of BA and thidiazuron (Balaraju et al. 2011). Cotyldenory nodes were sucessfully applied for the in vitro propagation of P. santalinus (Anuradha and Pullaiah 1999a; Arockiasamy et al. 2000; Rajeswari and Paliwal 2008; Warakagoda and Subasinghe 2013). BA, alone or combined with other cytokinins or auxins, has frequently been utilized for the micropropagation of P. santalinus (Table 2). Prakash et al. (2006) cultured nodes of mature trees directly on filter paper bridges employing liquid MS medium containing 1.16–9.30 µM kinetin or 1.11–8.88 µM BA, as well as an antioxidant, observing that 4.44 µM BA was optimum for bud break and shoot multiplication.
In vitro propagation (callogenesis, regeneration and somatic embryogenesis)
Leaf, cotyledon, root, internode and nodal segments (presumably with axillary buds) from in vitro P. santalinus seedlings formed callus, but shoot regeneration was not reported (Chaturani et al. 2005). Callus was induced from leaves and internodes of P. santalinus by Ashrafee et al. (2014) solely to assess antibiotic activity against Aeromonas and Pseudomonas but regeneration was not assessed. Details of effective plant growth regulator concentrations and combinations, medium composition, and explant type, as well as their effects on morphogenesis are presented in Table 2.
Rooting and acclimatization
Sucessful roooting of in vitro-raised plants followed by effective acclimatization and successful transfer of in vitro-propagated plants to field conditions is the final objective of any micropopagation protocol and care is needed to avoid hyperhydricity in in vitro-raised plants, which tend to display poor rooting efficiency (Ruffoni and Savona 2013; Teixeira da Silva et al. 2017b). Rooting and acclimatization protocols for in vitro-raised shoots of P. santalinus are summarized in Table 2. Only a few studies have quantified the survival of micropropagated plants (Lakshmi Sita et al. 1992; Prakash et al. 2006; Rajeswari and Paliwal 2008; Balaraju et al. 2011; Warakagoda and Subasinghe 2013). Among the 12 reports on P. santalinus tissue culture, in vitro rooting employed full-strength, half-strength and quarter-strength MS medium (Table 2). According to Arockiasamy et al. (2000), quarter-strength MS medium supplemented with 5.71 µM IAA was effective for 76.2% rooting of shoots derived from cotyledenory nodes, but acclimatization and survival of plantlets were not reported. Vipranarayana et al. (2012) applied a pulse treatment of 7.34 µM IBA in half-strength MS but details about survival were not reported. Rajeswari and Paliwal (2008) achieved 85% plantlet survival ex vitro after a pulse treatment of 5 µM IAA and 1 µM IBA for 25 d. In contrast, Warakagoda and Subasinghe (2013) showed limited success (46% of plantlets rooted) ex vitro with 49,000 µM IBA, possibly because of the excessively high concentration of this auxin.
Variability in quality and quality control
There is a problem with the adulteration and falsification of plant material in the P. santalinus market. The heartwood of Adenanthera pavonina Willd. (Mimosaceae), known as ‘Ranjana’ and ‘Raktakambal’ in West Bengal and ‘Bari Gumchi’ in the northern parts of India, is often sold as a fake substitute for P. santalinus, while artificially colored wood shavings and the sawdust of some other trees are also sold on the market as cheap substitutes (Botanical Survey of India 2012). In China, the manufacture of furniture utilizes Dalbergia louvelii R. Vig. (violet rosewood) as a substitute for P. santalinus since both plants have a very similar appearance and anatomical characteristics, and cheaper D. louvelii is often illegally used to impersonate the valuable P. santalinus (Zhang et al. 2014). Zhang et al. (2014) used conventional infrared spectroscopy (FT-IR), second derivative infrared (SD-IR) spectroscopy and two-dimensional correlation infrared (2DCOS-IR) spectroscopy to differentiate furniture made of P. santalinus wood from furniture made from D. louvelii. They observed that P. santalinus wood had a higher holocellulose content than D. louvelii wood while D. louvelii had more NaOH- and benzyl-alcohol-based extracts than P. santalinus.
The size and age of trees affects the heartwood content and wood density of P. santalinus (Suresh et al. 2017). Woody anatomy such as grain waviness can be used to delimit and identify P. santalinus (Rawat and Uniyal 1996; Gasson and MacLachlan 2010). The Botanical Survey of India (2012) used various anatomical methods such as maceration, scanning electron microscopy, exo- and endomorphic features, and fluorescence analysis to correctly identify P. santalinus wood samples.
Molecular markers are regularly utilized to measure the degree of genetic variation within natural or breeding populations, and have been extensively used in Indian sandalwood research (Teixeira da Silva et al. 2017a). In P. santalinus, RAPD (random amplified polymorphic DNA)-based marker analysis was used to detect variations in micropropagated plants raised from shoot tips, verifying that in fact no variation existed (Balaraju et al. 2011). RAPD was also used by Usha et al. (2013) to detect variation among nursery-grown plants. Variation in genetic distance among natural accessions, detected by RAPD markers, reflected a high level of DNA polymorphism due to outcrossing (Padmalatha and Prasad 2007; Usha et al. 2013). Jhansi Rani and Usha (2013) developed a sequence characterized amplified region (SCAR) marker to differentiate wavy from straight-grained plants at the seedling stage. Jyothi et al. (2014) reported differences in the quantity of genomic DNA in samples collected from different locations in Andhra Pradesh, India.
Therefore, quality control, as assessed by anatomical or chemical methods, is essential to verify the originality of P. santalinus wood while molecular methods serve to confirm genetic stability.
Conclusions and future perspectives
This review highlights key advances in the tissue culture-based biotechnology of economically important Pterocarpus santalinus. To date, effective protocols for seed surface disinfection and in vitro germination exist. There are also effective protocols for direct shoot regeneration from a range of explants or through callus induction. In most cases, explants are derived from seeds or seedlings which are not suitable for clonal propagation (Table 1). Therefore, a clonal method should be developed from vegetative tissues of elite germplasm. Rooting and survival of micropropagated plants remain a major limitation to the success of P. santalinus tissue culture and should be optimized in the future, for example by using CO2 enrichment and vessels that allow for maximized aeration without impacting relative humidity levels within the culture vessel (Teixeira da Silva et al. 2005). The ability to stably produce units that allow for germplasm conservation would then stimulate the need for cryoconservation (Teixeira da Silva and Engelmann 2017; Bi et al. 2017), including through the application of synthetic seeds (Sharma et al. 2013). Analytic hierarchy, which is a multicriteria decision-making tool, is valuable for incorporating the perceptions of stakeholders when planning the conservation and restoration of a P. santalinus population (Kukrety et al. 2013a, c). The micropropagation and biotechnology of another commercially important tree in this genus, P. marsupium (Indian kino tree), have recently been reviewed (Teixeira da Silva et al. 2018).
References
Anderson WC (1980) Tissue culture propagation of red and black raspberries, Rubus idaeus and R. occidentalus. Acta Hort 112(112):13–20
Ankalaiah C, Mastan T, Reddy MS (2017) A study on the density, population structure and regeneration of red sanders Pterocarpus santalinus (Fabales: Fabaceae) in a protected natural habitat—Sri Lankamalleswara Wildlife Sanctuary, Andhra Pradesh, India. J Threat Taxa 9(9):10669–10674
Anuradha M, Pullaiah T (1998) Investigations on germination of Pterocarpus santalinus (red sanders) with special reference to in-vitro seed culture. Indian For 124:309–314
Anuradha M, Pullaiah T (1999a) Propagation studies of red sanders (Pterocarpus santalinus L.f.) in vitro—an endangered taxon of Andhra Pradesh, India. Taiwania 44(3):311–324
Anuradha M, Pullaiah T (1999b) In vitro seed culture and induction of enhanced axillary branching in Pterocarpus santalinus and a method for rapid multiplication. Phytomorphology 49:157–163
Arockiasamy S, Ignacimuthu S, Melchias G (2000) Influence of growth regulators and explant type on in vitro shoot propagation and rooting of red sandal wood (Pterocarpus santalinus L.). Indian J Exp Biol 38(12):1270–1273
Arunkumar AN, Joshi G (2014) Pterocarpus santalinus (red sanders) an endemic, endangered tree of India: current status, improvement and the future. J Trop For Environ 4:1–10
Arunkumara KKIU, Walpola BC, Subasinghe S, Yoon M-H (2011) Pterocarpus santalinus Linn. f. (Rath handun): a review of its botany, uses, phytochemistry and pharmacology. J Korean Soc Appl Biol Chem 54:495–500
Ashrafee TS, Rahman MM, Chakraborty A, Prodhan SH (2014) Antibacterial potentiality of red sandalwood callus against pathogenic isolates of Aeromonas and Pseudomonas. Univers J Plant Sci 2(4):86–91
Azamthulla M, Balasubramanian R, Kavimani S (2015) A review on Pterocarpus santalinus Linn. World J Pharm Res 4:282–292
Balaraju K, Agastian P, Ignacimuthu S, Park K (2011) A rapid in vitro propagation of red sanders (Pterocarpus santalinus L.) using shoot tip explants. Acta Physiol Plant 33(6):2501–2510
Banerjee A, Mukherjee AK (1981) Chemical aspects of santalin as a histological stain. Stain Technol 56(2):83–85
Berliner N (1996) Beyond the screen: Chinese furniture of the 16th and 17th centuries. Mus Fine Arts, Boston
Bi W-L, Pan C, Hao X-Y, Cui Z-H, Kher MM, Marković Z, Wang Q-C, Teixeira da Silva JA (2017) Cryopreservation of grapevine (Vitis spp.)—a review. In Vitro Cellular Dev Biol Plant 53(5):449–460
Botanical Survey of India (2012) Identification protocol for red sanders. In: Pharmacognosy of negative listed plants. Ministry of Environment and Forests, India, pp 171–181. http://wccb.gov.in/WriteReadData/userfiles/file/PROTOCOL%20FOR%20Red%20sanders%20identification.pdf
Chaturani GDG, Jayatilleke MP, Subasinghe S (2005) In vitro callus formation of red sandalwood (Pterocarpus santalinus L.) as affected by explant type and different levels of 2,4-D and BAP. In: Proceedings of 10th annual forestry & environmental symposium. University of Sri Jayewardenpura, Gangodawila, Sri Lanka, p 37
Chaturani GDG, Subasinghe S, Jayatilleke MP (2006) In-vitro establishment, germination and growth performance of red sandalwood (Pterocarpus santalinus L.). Trop Agric Res Ext 9:116–130
Chee R, Pool RM (1987) Improved inorganic media constituents for in-vitro shoot multiplication of Vitis. Sci Hortic 32:85–89
Dayanand T, Lohidas T (1988) Effect of different treatments on pod germination of red sanders (Pterocarpus santalinus Linn. f). Indian J For 11:87–88
Gamborg OLL, Miller RAA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50(1):151–158
Gasson P, MacLachlan IR (2010) PCA of CITES listed Pterocarpus santalinus (Leguminosae) wood. IAWA J 31(2):121–138
Gulrajani ML, Bhaumik S, Oppermann W, Hardtmann G (2002) Kinetic and thermodynamic studies on red sandalwood. Indian J Fibre Text Res 27(1):91–94
IUCN (2018) CAMP workshops on medicinal plants, India (January 1997) 1998. Pterocarpus santalinus. In: The IUCN red list of threatened species. Version 2016.2. http://www.iucnredlist.org/details/32104/0. Accessed 21 May 2018
Jhansi Rani S, Usha S (2013) Development of RAPD and specific SCAR markers for the identification of Pterocarpus santalinus L. J Cell Tissue Res 13:3809–3816
Jyothi CP, Chandrashekar R, Lakshmi B (2014) Isolation of Pterocarpus santalinus L. genomic DNA, for quality check and quantification with reference to Telangana region, Andhra Pradesh, India. Indian J Sci 8:21–24
Kalimuthu K, Lakshmanan KK (1995) Effect of different treatments on pod germination of Pterocarpus species. Indian J For 18:104–106
Kaner J, Jiufang L, Yongji X, Ioraş F (2013) A re-evaluation of woods used in Chinese historic furniture (part two). Bull Transilv Univ Brasov Ser II For Wood Ind Agric Food Eng 6:31–40
Kedharnath S, Rawat MS, Uniyal DP, Kantham DL (1976) Studies on field grafting and the growth of grafts in red sanders. Indian For 102:761–765
Kesava Reddy K, Srivasuki KP (1990) Vegetative propagation of red sanders (Pterocarpus santalinus Linn.). Indian For 116:536–540
Kukrety S, Dwivedi P, Jose S, Alavalapati JRR (2013a) Stakeholders’ perceptions on developing sustainable Red Sanders (Pterocarpus santalinus L.) wood trade in Andhra Pradesh, India. For Policy Econ 26(C):43–53
Kukrety S, Gezan S, Jose S, Alavalapati JRR (2013b) Facilitating establishment of advance regeneration of Pterocarpus santalinus L.—an endangered tree species from India. Restor Ecol 21(3):372–379
Kukrety S, Jose S, Alavalapati JRR (2013c) Exploring stakeholders’ perceptions with analytic hierarchy process—a case study of red sanders (Pterocarpus santalinus L.) restoration in India. Restor Ecol 21(6):777–784
Kumar A, Gopal M (1975) A note on temperature sensitivity for germination of Pterocarpus santalinus seed. Seed Sci Technol 3:605–613
Kumar N, Ravindranath B, Seshadri TR (1974) Terpenoids of Pterocarpus santalinus heartwood. Phytochemistry 13:633–636
Kumarasinghe HKMS, Subasinghe S, Arunakumara KKIU (2003) Studies on seed propagation of rathhadun (Pterocarpus santalinus Linn.) as affected by method of soaking and scarification. In: Proceedings of 9th annual forestry and enviorenmcnt symposium, p 57
Lakshmi Sita G, Sreenatha KS, Sujata S (1992) Plantlet production from shoot tip cultures of red sandalwood (Pterocarpus santalinus L.). Curr Sci 62:532–535
Leifert C, Morris CE, Waites WM (1994) Ecology of microbial saprophytes and pathogens in tissue culture and field-grown plants: reasons for contamination problems in vitro. Crit Rev Plant Sci 13:139–183
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int Plant Propag Soc Proc 30:421–427
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naidu CV (2001a) Improvement of seed germination in red sanders (Pterocarpus santalinus Linn. F.) by plant growth regulators. Indian J Plant Physiol 6:205–207
Naidu CV (2001b) Seed pretreatment methods to improve germination red sanders (Pterocarpus santalinus) Linn. F. Indian J For 24:342–343
Naidu CV, Rajendrudu G (2001) Influence of kinetin and nitrogenous salts on seed germination of red sanders (Pterocarpus santalinus Linn. f.). Seed Sci Technol 29:669–672
Navada KK, Vittal RR (2014) Ethnomedicinal value of Pterocarpus santalinus (Linn. f.), a Fabaceae member. Orient Pharm Exp Med 14:313–317
Padmalatha K, Prasad MNV (2007) Morphological and molecular diversity in Pterocarpus santalinus L. f—an endemic and endangered medicinal plant. Med Aromat Plant Sci Biotechnol 1:263–273
Padmalatha K, Prasad MNV (2008) In vitro plant regeneration of Pterocarpus santalinus L.f (red sanders)—an endangered medicinal plant and important timber tree. Tree For Sci Biotechnol 2:1–6
Patel HS, Tandel MB, Prajapati VM, Amlani MH, Prajapati DH (2018) Effect of different pre-sowing treatments on germination of red sanders (Pterocarpus santalinus L. f.) in net house condition. Int J Chem Stud 6:876–879
Prakash E, Sha Valli Khan PS, Sreenivasa Rao TJV, Meru ES (2006) Micropropagation of red sanders (Pterocarpus santalinus L.) using mature nodal explants. J For Res 11:329–335
Rajeswari V, Paliwal K (2008) In vitro plant regeneration of red sanders (Pterocarpus santalinus L.f.) from cotyledonary nodes. Indian J Biotechnol 7:541–546
Raju KK, Nagaraju A (1999) Geobotany of red sanders (Pterocarpus santalinus)—a case study from the southeastern portion of Andhra Pradesh. Environ Geol 37:340–344
Ramabrahmam V, Sujatha M (2016) Red sanders in Rayalaseema region of Andhra Pradesh: importance to commercial & medicinal value. IOSR J Pharm Biol Sci 11:57–60
Ramakrishna A (1962) The red sanders and its future. Indian For 88:202–206
Rao SP, Raju AJS (2002) Pollination ecology of the red sanders Pterocarpus santalinus (Fabaceae), an endemic and endangered tree species. Curr Sci 83:1144–1148
Rao SP, Atluri JB, Reddi CS (2001) Intermittent mass blooming, midnight anthesis and rockbee pollination in Pterocarpus santalinus (Fabaceae). Nord J Bot 21:271–276
Rawat MS, Uniyal DP (1996) Identification of wavy grained red sanders (Pterocarpus santalinus) at nursery stage. Indian For 122:831–833
Ruffoni B, Savona M (2013) Physiological and biochemical analysis of growth abnormalities associated with plant tissue culture. Hortic Environ Biotechnol 54:191–205
Sarita S, Bhatnagar SP, Bhojwani SS (1988) Preliminary investigations on micropropagation of leguminous timber tree Pterocarpus santalinus. Phytomorphology 38:41–45
Sarma CR (1993) Study of outturn and value of red sanders. J Trop For 9:125–131
Sen Gupta PC, Mukherjee AK (1981) Newer applications of the histological stain prepared from Pterocarpus santalinus. Stain Technol 56:79–82
Sharma S, Shahzad A, Teixeira da Silva JA (2013) Synseed technology—a complete synthesis. Biotechnol Adv 31:186–207
Suresh K, Hegde M, Deenathayalan P, Karthick Kumar P, Thangapandi M, Gurudev Singh B, Krishnakumar N (2017) Variation in heartwood formation and wood density in plantation-grown red sanders (Pterocarpus santalinus). In: Pandey K, Ramakantha V, Chauhan S, Arun Kumar A (eds) Wood is good. Springer Singapore, Singapore, pp 139–151
Teixeira da Silva JA (2012) Is BA (6-benzyladenine) BAP (6-benzylaminopurine)? Asian Aust J Plant Sci Biotechnol 6(Special Issue 1):121–124
Teixeira da Silva JA, Engelmann F (2017) Cryopreservation of oil palm (Elaeis guineensis Jacq.). Cryobiology 77:82–88
Teixeira da Silva JA, Giang DTT, Tanaka M (2005) Micropropagation of sweet potato (Ipomoea batatas) in a novel CO2-enriched vessel. J Plant Biotechnol 7(1):67–74
Teixeira da Silva JA, Kher MM, Soner D, Nataraj M (2016a) African sandalwood or Nepalese sandalwood: a brief synthesis. Notulae Scientia Biologicae 8:57–61
Teixeira da Silva JA, Kher MM, Soner D, Page T, Zhang X, Nataraj M, Ma G (2016b) Sandalwood: basic biology, tissue culture, and genetic transformation. Planta 243:847–887
Teixeira da Silva JA, Kulus D, Zhang X, Zeng SJ, Ma GH, Piqueras A (2016c) Disinfection of explants for saffron (Crocus sativus L.) tissue culture. Environ Exp Biol 14(4):183–198
Teixeira da Silva JA, Kher MM, Soner D, Nataraj M, Dobránszki J, Millar MA (2017a) Santalum molecular biology: molecular markers for genetic diversity, phylogenetics and taxonomy, and genetic transformation. Agrofor Syst. https://doi.org/10.1007/s10457-017-0075-8 (in press)
Teixeira da Silva JA, Sharma M, Hossain M, Dobránszki J, Cardoso JC, Zeng SJ (2017b) Acclimatization of in vitro-derived Dendrobium. Hortic Plant J 3(3):110–124
Teixeira da Silva JA, Kher MM, Soner D, Nataraj M (2018) Indian kino tree (Pterocarpus marsupium): propagation, micropropagation, and biotechnology. Environ Exp Biol 16(1):1–8
Thimijan RW, Heins RD (1983) Photometric, radiometric, and quantum light units of measure: a review of procedures for interconversion. HortScience 18:818–822
Usha R, Rani SJ, Prasuna TG (2013) Genetic relationship between quality and non quality wood of Pterocarpus santalinus L. (red sanders), an endemic tree species by using molecular markers. J Chem Pharm Sci 6:189–194
Vedavathy S (2004) Cultivation of endemic red sanders for international trade. Nat Prod Radiance 3:83–84
Vijayalakshmi KP, Renganayaki PR (2017) Preliminary study on germination of pre-treated seed of red sanders under nursery conditions from Tamil Nadu. Agric Update 12:956–959
Vipranarayana S, Prasad TNVKV, Damodharam T (2012) In vitro seed germination and induction of enhanced shoot multiplication in Pterocarpus santalinus Linn. f: an endemic medicinal plant of Seshachalam Hills, Tirumala. Int J Pure Appl Sci Technol 9:118–126
Warakagoda PS, Subasinghe S (2013) In vitro propagation of Pterocarpus santalinus L. (red sandalwood) through tissue culture. J Natl Sci Found Sri Lanka 41:53–63
Wu SF, Chang FR, Wang SY, Hwang TL, Lee CL, Chen SL, Wu CC, Wu YC (2011) Anti-inflammatory and cytotoxic neoflavonoids and benzofurans from Pterocarpus santalinus. J Nat Prod 74:989–996
Zhang F, Xu C-H, Li M-Y, Huang A-M, Sun S (2014) Rapid identification of Pterocarpus santalinus and Dalbergia louvelii by FTIR and 2D correlation IR spectroscopy. J Mol Struct 1069:89–95
Acknowledgements
The authors thank Dr. Emilia Caboni (Agricultural Research Council (CRA), Fruit Tree Research Centre, Rome, Italy), Dr. Randall Niedz (U.S. Department of Agriculture, Agricultural Research Service, U.S. Horticultural Research Laboratory, FL, USA) and Dr. Ivana Gribaudo (Istituto Protezione Sostenibile delle Piante—CNR, Grugliasco, Italy) for ideas, comments and suggested improvements to an early version of the manuscript. The authors also thank Prof. M.N.V. Prasad (Department of Plant Sciences, University of Hyderabad, India) for some suggestions on a later version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The online version is available at http://www.springerlink.com
Corresponding editor: Yu Lei.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Teixeira da Silva, J.A., Kher, M.M., Soner, D. et al. Red sandalwood (Pterocarpus santalinus L. f.): biology, importance, propagation and micropropagation. J. For. Res. 30, 745–754 (2019). https://doi.org/10.1007/s11676-018-0714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0714-6