Abstract
A critical evaluation of all available phase diagram and thermodynamic data has been performed for Ag-As, Ag-S, As-S and Ag-As-S systems, including a detailed review of the gaseous species involved in the As-S system. Thermodynamic assessments over the whole composition range for these four systems are presented using the CALPHAD method. To predict thermodynamic properties and phase equilibria, the Modified Quasichemical Model for short range ordering was used for the liquid phases, and the Compound Energy Formalism was used for the solid solutions. For the As-S binary system, natural logarithm terms for the temperature dependence of the excess Gibbs energy of the liquid solution have been used. This led to a significant improvement compared with the previous assessment of this system. The optimization of Ag-As-S systems is in good agreement with the existing experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the last decades, the demand for various metals has increased rapidly leading to a growth in their production. The high demand is accompanied by a gradual decline in high-grade ores treated in smelters and an evident rise in complex ores containing high arsenic contents.[1,2,3] This is concerning since arsenic is a toxic impurity that contaminates the surrounding environment and increases the risk of developing health problems.[4] To help the industry develop solutions to overcome the arsenic emission problem, the thermodynamic interaction between arsenic and other elements contained in ores entering the smelting process must be understood. Many transition metal sulfides are found in complex ores, particularly \({\mathrm{Ag}}_{2}\mathrm{S}\). which is most often associated with lead ores and can also be found in small amounts in copper ores.[5] \({\mathrm{Ag}}_{2}\mathrm{S}\) is important for many operations like the manufacturing of photovoltaic cells and it can also be utilized as an electrode material for various applications.[6,7] To study complex systems contained in sulfide ores, it is necessary to develop thermodynamic models of compounds and mixtures for Ag-As-S systems and their associated databases. In this work, the available experimental data of Ag-As, Ag–S, As-S and Ag-As-S systems are critically evaluated, and a set of model parameters is optimized for each system to predict thermodynamic properties and phase equilibria. The Modified Quasichemical Model in the Pair Approximation suggested by Pelton et al.[8,9] was used for the liquid phases, and the Compound Energy Formalism (CEF) suggested by Sundman and Ågren[10] was used for solid solutions. All calculations and optimizations in this work were performed with the FactSage thermochemical software.[11,12] The obtained results are compared with previous evaluations of Ag-As and Ag–S systems by Shi et al.[13] and As-S system by Prostakova et al.[14].
2 Literature Review
2.1 Experimental Determination of Ag-As Binary Phase Diagram
The Ag-As binary system has a single intermediate hcp phase. The experimental studies measuring phase diagram data of this binary system are presented in Table 1. Details of these studies are also discussed and reviewed in this section. Two other studies, by Descamps in 1878[15] and by Friedrich and Leroux in 1906[16] were considered obsolete by Hansen and Anderko[17] and were not considered in his compiled phase diagram. These two studies were also not examined in this work.
2.1.1 Ag-As System
Solid solubility: According to the phase diagram proposed by Heike and Leroux,[18] the maximum solubility of arsenic in silver is constant at 7 at.% As at all temperatures below 868 K. Measurements made by Owen and Rowlands[20] and Owen and Morris[21] show that there is a variation in the maximum solubility of As in Ag as a function of temperature, between approximately 4.5 at.% As at 573 K to 8.8 at.% As at 868 K. The results of Eade and Hume-Rothery[22] confirm the variation in maximum solubility as a function of temperature. However, the proposed maximum solubility is about 0.3 at.% As lower than the values of Owen and Rowlands[20] at 573 K and about 1 at.% As lower at higher temperatures. For the solid solubility of Ag in As, it has been concluded by Heike and Leroux[18] and Broderick and Ehret[19] that the solubility of silver in arsenic is negligeable. Eutectic: According to Heike and Leroux,[18] the eutectic reaction takes place at a temperature of 813 K and a composition of 25.3 at.% As to form solid As and an hcp phase (ξ). Liquidus: Liquidus curves on the silver-rich side have been determined by Heike and Leroux[18] and Eade and Hume-Rothery[22] and they are in good agreement. The liquidus curve on the arsenic-rich side has also been measured by Heike and Leroux[18] under pressure.
2.1.2 Hcp Phase (\(\xi )\)
The Ag-As system contains a single hcp phase (\(\upxi )\). The phase is stable from 647 K (ξ ⇌ Ag + As) to 868 K (Liquid + Ag ⇌ ξ) according to Heike and Leroux,[18] with a likely composition of 10.5 at.% As. However, Broderick and Ehret[19] reported a eutectoid temperature greater than 663 K, which does not agree with the temperature measured by Heike and Leroux.[18] The eutectoid temperature according to Eade and Hume-Rothery[22] takes place between 716 and 723 K and the peritectic temperature between 852 and 858 K, in contrast with the results of Heike and Leroux.[18] At 813 K, King and Massalski[23] measured the compositions of 10.5 and 12.5 at.% As as the limits of the ξ phase. Hall[24] could not accurately measure the limits of the ξ phase but proposed that it corresponds to a formula of \({\mathrm{Ag}}_{9}\mathrm{As}\). Hall[24] was also able to find that the phase is not stable below 729 K, contrary to the temperature reported by Heike & Leroux.[18] No thermodynamic information has been reported for this phase.
2.2 Experimental Determination of Thermodynamic Properties of Ag-As Binary System
For temperatures between 791.8 K and 932.1 K, Lüdecke et al.[25] used the pseudo-isopiestic method to measure the vapor pressure above Ag-As alloys for compositions up to 8 at.% As. The data were then used to determine the activity of As in solid Ag-As alloys, with respect to solid As as the standard state. It was concluded that \(\mathrm{As}\) and \({\mathrm{As}}_{3}\) molecules are not present in the vapor. Additionally, it was observed that the vapor above the very low-concentrated alloys consists mainly of \({\mathrm{As}}_{2}\) molecules, and as the alloy composition in As increases, \({\mathrm{As}}_{4}\) molecules become increasingly dominant in the vapor.
2.3 Experimental Determination of Ag–S Binary Phase Diagram
The Ag–S binary system forms two fields of liquid immiscibility, as well as an intermediate compound, \({\mathrm{Ag}}_{2}\mathrm{S},\) stable at ambient pressure. Jeannot et al.[26] also reported the existence of another compound, \({\mathrm{Ag}}_{4}\mathrm{S}\), at temperatures below 700 K. Kracek[27] is the only one to have measured the \({\mathrm{Ag}}_{2}\mathrm{S}\)-\(\mathrm{S}\) subsystem, and his work is accurate according to Hansen and Anderko.[17] The experimental studies measuring phase diagram data are summarized in Table 2 and discussed in detail in this section (Fig. 1).
2.4 \(Ag\) - \({Ag}_{2}S\) Subsystem
Solid solubility: The experiments by Friedrich and Leroux[28] show that the solid solubility of sulfur in silver is very small, probably less than 0.17 at.% S. The solid solubility has been measured subsequently by Barbouth and Oudar,[29] Raub and Röschel[30] and Fueki et al.[31]. As shown in Fig. 2, the data obtained by Raub and Röschel[30] are in very good agreement with those of Fueki et al.[31]. However, the measurements performed by Barbouth and Oudar[29] show a lower solubility. Monotectic: The \(\mathrm{Ag}\)-\({\mathrm{Ag}}_{2}\mathrm{S}\) subsystem forms a miscibility gap and the meaured monotectic temperatures are in excellent agreement: 1177 K by Bisset,[32] 1178 K by Friedrich and Leroux[28] and Jaeger and van Klooster,[33] and 1179 \(\pm\) 1 K by Kracek.[27] The boundary of the miscibility gap was determined by Rosenqvist[34] and Fukatu and Kozula.[35] The boundary proposed by Fukatu and Kozula[35] is slightly wider on the silver rich side. The critical temperature is 1398 \(\pm\) 25 K according to Rosenqvist.[34] Eutectic: The eutectic reaction that forms \(\mathrm{Ag}\) and \({\mathrm{Ag}}_{2}\mathrm{S}\) lies at approximately 32 at.% S at a temperature of 1080 K by Friedrich and Leroux,[28] 1079 K by Jaeger and van Klooster,[33] 1077 K by Bissett[32] and 1077 \(\pm\) 2 K by Kracek.[27] Liquidus: The liquidus curves measured by by Friedrich and Leroux,[28] Jaeger and van Klooster,[33] Bisset,[32] Kracek,[27] Rosenqvist[34] and Fukatu and Kozula[35] are in very good agreement. Contrary to what was stated by Jeannot et al.[26], no indication of \({\mathrm{Ag}}_{4}\mathrm{S}\) was found.[32]
2.4.1 \({Ag}_{2}S\)-\(S\) Subsystem
Solid solubility: No information has been reported regarding the solid solubility of silver in sulfur. It is most likely to be negligeable according to.[42] Monotectic: The \({\mathrm{Ag}}_{2}\mathrm{S}\)-\(\mathrm{S}\) subsystem forms a miscibility gap and the monotectic temperature is 1013 K according to Kracek[27] and 1017 K according to Rau.[37] There has been no experimental determination of the phase boundary of the miscibility gap. Eutectic: The eutectic reaction that forms \(\mathrm{S}\) and \({\mathrm{Ag}}_{2}\mathrm{S}\) occurs at a temperature of 392 K according to Kracek.[27]
2.4.2 \({Ag}_{2}S\) Compound
The natural form of \({\mathrm{Ag}}_{2}\mathrm{S }\) is acanthite with a monoclinic structure. A reversible polymorphic transformation takes place to form argentite which has a body centered cubic structure (bcc).[43] In mineralogy, argentite refers to a pseudomorph of acanthite after argentite.[44] A third polymorphic transformation takes place to form a face-centered cubic phase (fcc).[45] The transformation temperatures reported in the literature for are shown in Table 3. The transformation temperatures of \({\mathrm{Ag}}_{2}\mathrm{S}\) vary with composition, especially at higher temperatures, which indicates its non-stoichiometry. According to the experiments performed by Kracek,[27] the \({\mathrm{Ag}}_{2}\mathrm{S}\) compound undergoes the first polymorphic transformation at a temperature of \(450.8 \pm 0.7\) K with excess sulfur and \(449.3 \pm 0.5\) K with excess silver. The second polymorphic transformation varies largely with composition: \(895 \pm 3\) K with excess sulfur and \(859\pm 3\) K with excess silver.[27] \({\mathrm{Ag}}_{2}\mathrm{S }\) melts congruently and the reported melting temperatures are also presented in Table 3. According to Hansen and Anderko,[17] the lower values of the melting point reported can be explained by the insufficient protection against oxidation or a small excess of silver or sulfur. A narrow \({\mathrm{Ag}}_{2}\mathrm{S}\) homogeneity range is suggested, and the different results are in very good agreement. Wagner[36] determined the solubility of Ag in \({\mathrm{Ag}}_{2}\mathrm{S}\) by meausring the electomotive force (emf) of the cell \(\mathrm{Ag}/\mathrm{AgI}/{\mathrm{Ag}}_{2}\mathrm{S}\)/Pt for temperatures from 433 to 573 K. Using the \({\mathrm{H}}_{2}\mathrm{S}/{\mathrm{H}}_{2}\) and the dew point methods, Rau[37] constructed a defect model from \({\mathrm{S}}_{2}\) fugacity measurements and calculated the complete homogeneity range of \({\mathrm{Ag}}_{2}\mathrm{S}\). Bonnecaze et al.[38, 39] also determined the homogeneity range from 296 to 449 K and 466 K to 823 K, and the non stoichiometry was controled by coulometry using the cell \(\mathrm{Ag}/{\mathrm{RbAg}}_{4}{\mathrm{I}}_{5}/{\mathrm{Ag}}_{2+\mathrm{e}}\mathrm{S}/\mathrm{Pt}.\) Ditman and Kulikova[40] determined the excess sulfur that \({\mathrm{Ag}}_{2}\mathrm{S}\) can solubilize by dew point method at temperatures of \(503\pm 10\) K and 743 \(\pm\) 25 K. Finally, Reye[41] determined by an electrochemical investigation the solubility limit of \({\mathrm{Ag}}_{2}\mathrm{S}\) at a temperature of 723 K. The various results agree in that the low temperature form has nearly an ideal stoichiometric composition (Fig. 3).
The heat of formation of \({\mathrm{Ag}}_{2}\mathrm{S}\) determined using a variety of techniques are listed in Table 4 and are in good agreement. Certain values were obtained by evaluation of the third law of galvanic cell data and equilibrium data for the following reactions:
Several studies have been conducted to measure the heat capacity of \({\mathrm{Ag}}_{2}\mathrm{S}\) phases, and some results do not agree. Over the range 323 K to 673 K, Perrott and Fletcher[50] measured the heat capacity of the stoichiometric \({\mathrm{Ag}}_{2}\mathrm{S}\) using adiabatic calorimetry. They also measured the heat capacity of non-stoichiometric \({\mathrm{Ag}}_{2}\mathrm{S}\) containing 1 mol % excess silver or 1 mol % excess sulfur. The absolute accuracy of their measurements was estimated as \(\pm\) 10%. As can be seen from Fig. 4, the data measured for the stoichiometric compound differ greatly from the other measurements. The main difference comes from an additional transition reported at 623 K. This was explained by the partial disordering of the stoichiometric compound, analogous to that observed for AgI. Gr \(\o\) nvold and Westrum[53] rejected this explanation and suggested that this non reproducible transition might be associated with the presence of another phase in their sample. The heat capacity of \({\mathrm{Ag}}_{2}\mathrm{S}\) was also measured using calorimetry by Bellati and Lussana[46] for temperatures from 320 to 490 K, by Gultyaev and Petrov[63] from 80 to 300 K, by Walsh et al.[64] from 13 to 296 K, and by Jost and Kubaschewski[65] from 200 to 300 K. Okazaki and Takano[59] also measured the heat capacity using adiabatic scanning calorimetry from 350 to 1200 K. Thompson and Flengas[52] also determined the heat capacity from 448 to 997 K using drop calorimetry. Gr \(\o\) nvold and Westrum[53] performed the measurements from 5 to 1000 K using adiabatic-shield calorimetry with a sample with stoichiometric composition and a sample with \({\mathrm{Ag}}_{2}{\mathrm{S}}_{1.0526}\) composition. The results of both samples agree and only the values obtained for the stoichiometric composition are shown in Fig. 4. Gusev and Sadovniko[57] measured the heat capacity for a coarse-crystalline sample and for nanoparticles. The difference in particle size resulted in different heat capacity values. A good agreement was obtained between the results of Thompson and Flengas 1971,[52] Grønvold and Westrum,[53] and Gusev and Sadovnikov,[57] showing a slight decrease over the range of 460 K to 620 K. The experimental data of Grønvold and Westrum[53] are the most reliable according to Sadonikov and Gusev.[66] The standard entropy obtained from low temperature heat capacity measurements and second law evaluation of galvanic cell measurements are presented in Table 4.
According to the mass spectroscopic studies of Glazov and Korenchuk[76] and Dutchak et al.,[77] \({\text{Ag}}_{2} {\text{S}}\) thermally decomposes according to the following reaction:
Vapor pressure measurements were also performed using the Knudsen effusion method. Piacente et al.[78] also measured the vapor pressure using torsion and Knudsen effusion methods and found no significant difference using cells of different materials (graphite, pyrophyllite and quartz). Smith and Smeltzer[79] carried sulfidation tests of iron using \({\text{Ag}}_{2} {\text{S}}\) and reported a \({\text{P}}_{{{\text{S}}_{2} }}\) value for a temperature of 1023 K. The vapor pressure data are presented in Fig. 2.
2.5 Experimental determination of thermodynamic properties of Ag–S binary system
Rosenqvist[34] determined the partial pressure of \({\mathrm{S}}_{2}\) by measuring the ratio \({\mathrm{H}}_{2}\mathrm{S}/{\mathrm{H}}_{2}\) of the gas in equilibrium with Ag–S alloys. The measurements were performed for alloys with compositions up to 33.3 at.% S, between 773 and 1573 K, according to the following equilibrium:
The composition of the gas mixture was determined from its density obtained by magnetic balancing. Fukatsu and Kozuka[35] determined the emf for the following cell: \(\mathrm{Pt},\mathrm{ Air}|{\mathrm{ZrO}}_{2}(+\mathrm{CaO})\)|Ag–S, \({\mathrm{SO}}_{2},\mathrm{ Ir},\mathrm{Kanthal}\), and from the measured oxygen partial pressures, the partial pressure of \({\mathrm{S}}_{2}\) was calculated between 867 and 1538 K, for compositions up to 32 at.% S. Yazawa, et al.[80] also measured the partial pressure of sulfur over Ag–S liquid alloys up to 33 at.% S. The measurements were performed between 1273 and 1473 K by bubbling nitrogen through the melt, and the sulphur content was determined by chemical analysis. The different measurements of the partial pressure of \({\mathrm{S}}_{2}\) are in good agreement.
2.6 Experimental Determination of As-S Binary Phase Diagram
The As-S binary system contains three stoichiometric intermediate compounds stable at ambient pressure: \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\), \({{\mathrm{As}}_{4}\mathrm{S}}_{4}\) et \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\). Phase diagram data for this binary system are challenging to measure. For compositions lower than 45 at.% As, the measurements are hard to record due to the glass formation tendency of the alloys. And for compositions higher than 45 at.% As, the thermodynamically stable \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) tends to not crystallize from synthetic melts, creating a metastable system. The experimental studies measuring phase diagram data are summarized in Table 5 and discussed in detail in this section.
2.6.1 \(S\)-\({{As}_{2}S}_{3}\) Subsystem
\(\mathrm{As}\)-\(\mathrm{S}\) alloys with compositions lower than 45 at.% As are highly viscous according to the experiments of Jonker.[81] Since the liquid–solid equilibrium is reached very slowly, it was not possible to measure the liquidus curve of the \(\mathrm{S}\)-\({{\mathrm{As}}_{2}\mathrm{S}}_{3}\) subsystem using thermal analysis. Therefore, Jonker[81] estimated the liquidus curve by comparing the viscosities of several alloys with that of \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) at its melting temperature. Thus, at temperatures between 473 and 673 K, the viscosities of pure sulfur and alloys at the compositions of 5, 10, 20 and 28.6 at.% As were evaluated quantitatively. It was possible to estimate two points of the liquidus curve. Jonker[81] also analytically determined the vapor curve in equilibrium with boiling liquid of known compositions and identified an azeotropic point at the composition of \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\). It was concluded that the vapor coexisting with the liquid is rich in sulfur. Blachnik et al.[87] also failed to measure the liquidus curve due to the high alloy viscosities in this subsystem.
2.6.2 \({{As}_{2}S}_{3}\)-\({As}_{4}{S}_{4}\) Subsystem
According to measurements by Jonker,[81] a eutectic reaction takes place at a temperature of 565 K to form \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) and \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) at a composition around 43 at.% As. It was also concluded that the vapor coexisting with the liquid is rich in arsenic. However, according to Fedorova and Epova,[88] \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) is incongruent and decomposes at temperatures above 569 ± 2 K. A monotectic reaction occurs at a temperature of 576 ± 2 K. According to the suggested phase diagram of Blachnik et al.,[87] \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) and \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) form by a eutectic reaction at a temperature of 573 K for a composition around of 43 at.% As.
2.6.3 \({As}_{4}{S}_{4}\) - \(As\) Subsystem
According to the work of Jonker,[81] an invariant equilibrium between the gaseous phase, the liquid phase and solid arsenic exists at an estimated temperature of 807 K at 52.2 at.% As. Since the experiments were performed at atmospheric pressure, no liquid phase was formed for alloys very rich in arsenic due to the sublimation. For this subsystem, the vapor is richer in arsenic relative to the liquid in equilibrium. Clark[82] estimated that at a temperature of 873 K, the saturated liquid contains 60 ± 0.2 at.% As. He also confirms that the vapor phase is richer in arsenic compared to the liquid in equilibrium. According to the results obtained by Barton,[83] a monotectic reaction takes place at a temperature of 1070 ± 5 K. The miscibility gap of two liquids forms between the compositions of 83 ± 3 and 97 ± 3 at.% As. According to Ceolin et al.[85], the monotectic reaction involves a vapor phase in addition to solid arsenic and the two liquids. The reaction takes place at a temperature of 1056 ± 1 K, with an upper critical temperature of 1210 K. The metallographic examination confirms the presence of the miscibility gap. The monotectic reaction takes place at a temperature of 1048 ± 3 K according to the experiments of Fedorova and Epova.[86] Also, the formation of \({\text{As}}_{4} {\text{S}}_{3}\) was reported for the first time by Fedorova and Epova.[86] The compound is incongruent and stable only at temperatures below 404 ± 3 K. The eutectic reaction between \({\text{As}}_{4} {\text{S}}_{4}\) and As takes place at a temperature of 452 ± 4 K and at a composition around 56 at.% As. The limits of the miscibility gap could not be determined by Blachnik et al.[87] due to technical difficulties. The liquidus curves of Blachnik et al.[87] and Ceolin et al.[85] agree, unlike the curve measured by Barton[83] found at lower arsenic compositions. The phase diagram measured by Blachnik et al.[87] does not show the formation of the thermodynamically stable \({\text{As}}_{4} {\text{S}}_{3}\). The metastable eutectic temperature forming \({\text{As}}_{4} {\text{S}}_{4}\) and As is 515 K, while the eutectic temperature forming \({\text{As}}_{4} {\text{S}}_{3}\) and \({\text{As}}_{4} {\text{S}}_{4}\) in the suggested stable phase diagram is 473 ± 4 K.
2.6.4 \(As_{2} S_{3}\) Compound
The natural crystalline form of \({\text{As}}_{2} {\text{S}}_{3}\) is orpiment. At high pressures, the crystalline form undergoes two polymorphic transformations according to the experiments of Timofeeva et al.[84], at 463 K and 483 K. At atmospheric pressure \({\text{As}}_{2} {\text{S}}_{3}\) occurs only in one form.[89] The synthesized \({\text{As}}_{2} {\text{S}}_{3}\) is usually amorphous and almost impossible to crystallize. Also, \({\text{As}}_{2} {\text{S}}_{3}\) melt is a strong glass-forming liquid at ambient pressure, with a viscosity of 8000 \({\text{Pa}}.{\text{s}}\) near the melting temperature.[90] Pure \({\text{As}}_{2} {\text{S}}_{3}\) crystals cannot be prepared from \({\text{As}}_{2} {\text{S}}_{3}\) melts. However, the amorphization of \({\text{As}}_{2} {\text{S}}_{3}\) crystals is relatively easy. The optically induced transition of crystalline \({\text{As}}_{2} {\text{S}}_{3}\) to the amorphous state studied by Frumar et al.[91] results in a glass transition temperature \({\text{T}}_{{\text{g}}}\) of 453 K. Slightly higher \({\text{T}}_{{\text{g}}}\) values have been reported for synthetic samples.[89, 92,93,94] The temperatures and heats of fusion for crystalline \({\text{As}}_{2} {\text{S}}_{3}\) are presented in Table 6. Espeau et al.[89] measured and compared the melting temperatures obtained from synthetic and natural samples. According to the DSC results, with a heating rate of 5 K.\({\text{ min}}^{ - 1}\), the average melting temperature obtained for the synthetic samples is 601 K, while the average for the natural samples of two different sources is 596 K and 591 K. Even though elemental analyzes showed that the synthetic sample and the natural samples have the same composition, Espeau et al.[89] suggests that the difference may be due to the higher purity of the synthetic sample. The average heat of fusion \(\left( {\Delta H_{{{\text{fusion}}}} } \right)\) for the synthetic samples is 26.7 kJ/mol, and for natural samples from the different sources are 29.8 kJ/mol and 27.4 kJ/mol.
Since the synthesized \({\text{As}}_{2} {\text{S}}_{3}\) is usually glassy and difficult to crystallize, several studies have used natural orpiment to determine its thermodynamic properties. Černošek et al.[98] successfully synthesized high purity \({\text{As}}_{2} {\text{S}}_{3}\) crystals for the first time in 1999 and questioned the purity of the natural samples used in the previous studies. As can be seen from Table 7, there is a large discrepancy between the suggested heat of formation values by the different sources, ranging from − 83 kJ/mol to − 169 kJ/mol. Britzke et al.[99] obtained the first enthalpy of formation value of − 145 kJ/mol by combustion calorimetry in oxygen. From the data of Britzke et al.[99], Wagman et al.[100] recalculated a value of − 169 kJ/mol, and Mills[101] a value of 159 ± 42 kJ/mol. Neverov et al.[102] calculated two enthalpy of formation values from the reactions of arsenic sulfide with an alkaline solution of NaOH saturated with bromine as well as with an alkaline solution of NaOH saturated with chlorine. The values obtained are − 138 ± 12 kJ/mol and − 126 ± 10 kJ/mol respectively. Barton[83] does not agree with such negative values of \(\Delta H_{formation}^{\circ }\) of orpiment and estimated a value of − 96.23 kJ/mol from his equilibrium study on As-Fe-S alloys. From the combustion energy of the glassy \({\text{As}}_{2} {\text{S}}_{3}\) measured in fluorine by Johnson et al.[103], a value of \(\Delta H_{formation}^{\circ }\) of the crystalline compound of − 91.6 ± 4, 8 kJ/mol was derived, agreeing with the value of Barton.[83] This is also close to the value of − 88.7 ± 3.8 kJ/mol recalculated by O'Hare.[104] Using high temperature calorimetry, a value of − 83 ± 3.8 kJ/mol was obtained by Bryndzia and Kleppa.[105] Babanly et al.[106] calculated a value of − 94.09 ± 2.11 kJ/mol from emf measurements, with a liquid electrolyte d \({\text{KBr}}\) in glycol with 0.01% mass of \({\text{Br}}_{3} {\text{As}}\) and solid arsenic as reference electrode. Nordstrom and Archer[107] evaluated and fitted the different enthalpy of formation values reported in the literature with a weighted least-squares regression and suggested a value of 85.8 kJ/mol. Enthalpy of formation values for amorphous \({\text{As}}_{2} {\text{S}}_{3}\) are also shown in Table 7. The heat capacity of crystalline \({\text{As}}_{2} {\text{S}}_{3}\) was first measured at low temperatures by Romanovsky and Tarasov[108] using adiabatic calorimetry between 65 and 300 K. The heat capacity was also measured at higher temperatures by Blachnik et al.[87] using DSC between 300 and 650 K. For vitreous \({\text{As}}_{2} {\text{S}}_{3} ,\) heat capacities were also measured at low temperatures by Hattori et al.,[109] Kageyama et al.,[110] Tarasov and Zhdanov,[111] Haggerty et al.[112] and Schnaus et al.[113] and are in reasonable agreement. Only the heat capacity data for the crystallin \({\text{As}}_{2} {\text{S}}_{3}\) are presented in Fig. 4. The standard entropy calculated from these data are presented in Table 7.
The compound \({\text{As}}_{2} {\text{S}}_{3}\) sublimates congruently, and the experiments determining the composition of its vapor are not in agreement. The electron diffraction study results suggested by Lu and Donohue[119] show that \({\text{As}}_{4} {\text{S}}_{6}\) molecules are probably the only species present in the vapor state. The interpretation of the results of this study is very doubtful according to Rogstad.[120] Based on their mass spectrometric analyses, Janai and Rudman[121] also suggest that the compound evaporates as \({\text{As}}_{4} {\text{S}}_{6}\) based on the appearance of all its possible fragments in the spectra. The composition of the vapor in equilibrium with \({\text{As}}_{2} {\text{S}}_{3}\) was also studied by Ban and Knox[122] using mass spectrometry with laser-induced vaporization. It was concluded that vaporization of \({\text{As}}_{2} {\text{S}}_{3}\) forms \({\text{S}}_{2}\) and \({\text{As}}_{3} {\text{S}}_{{\text{n}}}\) species (n = 1, 2, 3, 4), with abundance of \({\text{As}}_{3} {\text{S}}\) and \({\text{As}}_{3} {\text{S}}_{4}\) species. According to the mass spectrometry by Faure et al.[123], the vaporization of \({\text{As}}_{2} {\text{S}}_{3}\) forms gaseous AsS and \({\text{S}}_{2}\) The mass spectrum was obtained by scanning the ion accelerating voltage at a constant magnetic field, instead of the usual practice of varying the magnetic field at a constant ion accelerating voltage. This method used to obtain the spectrum does not allow the identification of species with higher masses according to Brittain et al.[124]. Moreover, according to Chakraborti and Lynch,[125] the presence of AsS species in their results can be associated with the destruction of larger molecules due to the high ionization potentials used. The high temperature mass spectrometry by Pashinkin et al.[126] reveals the following gaseous specie: \({\text{As}}_{2} {\text{S}}_{2} ,\) \({\text{As}}_{3} {\text{S}}_{3}\) and \({\text{As}}_{4} {\text{S}}_{4}\). Since the measurements were made with ionizing electron energies of the order of 50 eV, the results also reflect the presence of products associated with the destruction of larger molecules according to Brittain et al.[124] and Chakraborti and Lynch.[125] High temperature mass spectrometry performed by Brittain et al.[124] was obtained with an ionizing potential of 15 eV. The results show that the vaporization of the compound \({\text{As}}_{2} {\text{S}}_{3}\) mainly produces the following gaseous species: \({\text{As}}_{4} {\text{S}}_{4}\), \({\text{As}}_{4} {\text{S}}_{3}\), \({\text{S}}_{2}\) and a minor amount of \({\text{As}}_{4} {\text{S}}_{5}\). It was also concluded that the vapor does not contain \({\text{AsS}},\) \({\text{As}}_{2} {\text{S}}_{2}\) and \({\text{As}}_{3} {\text{S}}_{3}\). Thus, the process of vaporization is mainly done according to the following reactions:
The ion intensity data indicate that reaction 5 predominates over reaction 6. Also, the following reaction contributes slightly to the process:
According to the results obtained by Brunetti et al.[127], reactions 5 and 6 take place independently of temperature. Several experiments determined the vapor pressures of \({\text{As}}_{2} {\text{S}}_{3}\). Figure 5 shows data from each experiment. Using the Knudsen-Langmuir technique, Hsiao and Schlechten[128] determined the vapor pressures for temperatures between 453 and 603 K. The vapor pressure values were labeled by Hsiao and Schlechten[128] as “apparent” because of the uncertainty as to how much is due to evaporation and how much to dissociation. Chakraborti and Lynch[125] rejected the values measured by Hsiao and Schlechten.[128] Isakova and Nesterov[129] determined the vapor pressures for temperatures between 623 and 723 K using an indirect statistical method based on the amount of condensate formed during cooling in a closed container. When processing experimental data, it was assumed that only high dosage \({\text{As}}_{2} {\text{S}}_{3}\) molecules are in the vapor phase. Since the obtained vapor pressures measured are very close to those obtained for \({\text{As}}_{4} S_{4} ,\) Chakraborti and Lynch[125] suggested the presence of a small amount of \({\text{As}}_{4} {\text{S}}_{4}\) in the solid sample used by Isakova and Nesterov.[129] Isakova[130] determined the vapor pressures using flux and boiling point methods between 623 and 773 K. Ustyugov et al.[131] determined the vapor pressures with the static method between 729 and 966 K using a quartz membrane manometer. Faure et al.[123] determined the vapor pressures with the Knudsen method between 497 and 569 K, and Novoselova and Pashinkin[132] expressed the vapor pressure as a function of temperature. Gospodinov and Pashinki[133] determined the vapor pressures with the Knudsen method between 476 and 536 K, and the Langmuir method between 470 and 546 K. Brunetti et al.[127] determined the total vapor pressures with the torsion-effusion method between 501 and 583 K. The boiling temperature of the compound \({\text{As}}_{2} {\text{S}}_{3}\) is around 980 K according to Jonker[81] and it corresponds to an azeotropic point.
2.6.5 \({As}_{4}{S}_{4}\) Compound
\({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) Is found in nature in a crystalline form called realgar and it can easily change to orpiment with light exposure.[120] The formula of realgar is also given by \(\mathrm{AsS}\) or \({\mathrm{As}}_{2}{\mathrm{S}}_{2}\) in some references. Since the mineral consists of \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) rings,[134] the formula considered in this work is \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\). Realgar undergoes a polymorphic transformation to form pararealgar. Since both forms are monoclinic,[135] the transformation enthalpy is very small. The experiments performed by Rolland[136] show that the transformation temperature for the stoichiometric compound occurs at 525 K and it varies between 512 and 536 K in the presence of elemental As and \({\mathrm{As}}_{2}{\mathrm{S}}_{3},\) respectively. The compound has a very narrow homogeneity range,[136] and it is still not experimentally investigated. The measured transformation temperatures are shown in Table 8. Hall[24] defined three distinguishable optical properties of \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\), and more recently, a third form was reported by Bindi et al.,[137] analogous to the new mineral bonazziite found in Khaidarkan deposit in Kyrgystan. The \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) compound is reported as congruent, except for the measurements of Fedorova et al.[138] that show an incongruent compound. The measured melting temperatures are shown in Table 8.
The heat of formation values of \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) reported in the literature are presented in Table 9. Britzke et al.[99] obtained a first value of − 242 kJ/mol by combustion calorimetry in oxygen. From the data of Britzke et al.[99], Wagman et al.[100] recalculated a value of − 285.4 kJ/mol, and Mills[101] a value of − 284.5 \(\pm\) 42 kJ/mol. Neverov et al.[102] calculated a \({\Delta H}_{formation}^{\circ }\) value of − 236 \(\pm\) 12 kJ/mol from the reactions of arsenic sulfide with an alkaline solution of NaOH saturated with bromine. These values are erroneous according to Barton,[83] who estimated a value of − 145.6 ± 6 kJ/mol based on the equilibrium studies in As-Fe-S alloys. Data from vapor phase analyzes seem to indicate a value closer to that of Barton[83] according to Mah.[145] A value of − 138.1 ± 6.7 kJ/mol was obtained from fluorine bomb calorimetry measurements by Johnson et al.[103], which is close to the value suggested by Barton. The \({\Delta H}_{formation}^{\circ }\) value obtained by mass spectroscopy by Steblevskii et al.[146] is − 127.6 ± 5.2 kJ/mol. This value agrees with that calculated by O'Hare[104] of − 127.5 ± 5.3 kJ/mol. Using high temperature calorimetry, the \({\Delta H}_{formation}^{\circ }\) obtained by Bryndzia et al.[105] is − 108.8 ± 1.6 kJ/mol, which is less negative than other reported values. This may be due to an incomplete chemical reaction in the synthesis calorimeter according to O'Hare.[104] In the most recent study performed by Babanly et al.[106], the \({\Delta \mathrm{H}}_{\mathrm{formation}}^{\circ }\) calculated from the emf measurements is − 158.4 ± 4.4 kJ/mol. The heat capacity of synthetic AsS was determined for low temperatures by Weller and Kelley.[147] Heat capacities of synthetic \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) was also measured by DSC at higher temperatures by Blachnik et al.[87] and Chattopadhyay et al.[143], but the obtained results do not fully agree. According to Chattopadhyay et al.[143] this is due to the partial evaporation of the sample during the measurements by Blachnik et al.[87]. Also, according to Emelina et al.,[148] Chattopadhyay et al.[143] obtained the most reliable data. The standard entropy derived from the heat capacity data is presented in Table 9.
Several experiments studied the composition of the vapor in equilibrium with \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\). By determining the vapor density at several temperatures, Szarvasy and Messinger[149] concluded that the vapor contains mainly \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) molecules at temperatures below 823 K, and at higher temperatures, the molecules dissociate into \({\mathrm{As}}_{2}{\mathrm{S}}_{2}\). At temperatures above 1473 K, Szarvasy and Messinger[149] suggested that another dissociation might occur. Castro et al.[150] confirm that the gaseous \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) dissociates in two stages at high temperatures to \({\mathrm{As}}_{2}{\mathrm{S}}_{2}\) and \(\mathrm{AsS}.\) According to the results of the mass spectrometry performed by Munir et al.[151], the vaporization of synthetic and natural realgar produces a vapor that mainly contains \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) species at high temperatures. The following species were also found in minor quantities: \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\), \({\mathrm{As}}_{4}\) and \({\mathrm{As}}_{2}\), and their presence decreases with increasing temperature. Therefore, it was concluded that the process of vaporization takes place according to the following reaction:
This agrees with the results of Brittain et al.[124] who used mass spectrometry and mass-torsion effusion. It was concluded that at least 90% of the vapor produced following the sublimation of realgar comprises the gaseous \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) species. Also, it was determined that the vapor pressure of the two forms, \(\mathrm{\alpha }\)-\({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) and \(\upbeta\)-\({\mathrm{As}}_{4}{\mathrm{S}}_{4}\), are similar. Various experiments determined the vapor pressures of \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\). The measured data are shown in Fig. 5. Using spoon gauge, Strathdee and Pidgeon[152] performed vapor pressure measurements for temperatures between 573 and 773 K. Ustyugov et al.[131] also determined the vapor pressures for temperatures between 663 and 838 K using the same technique. The data obtained by Ustyugov et al.[131] have a systematic error caused by the non-uniformity of the thermal field according to Novoselova and Pashinkin.[132] This is due the large sizes of the membrane chambers. Munir et al.[151] performed vapor pressure measurements using torsion-effusion for temperatures between 452 and 534 K and using torsion-Langmuir between 411 and 465 K. Godovikov et al.[144] also performed vapor pressure measurements using a quartz membrane manometer for temperatures between 614 and 814 K. Their pressures are lower than those measured by Strathdee and Pidgeon[152] and Ustyugov et al.[131] Finally, measurements were performed by Pashinkin et al.[153] between 470 and 580 K using the Knudsen method, and the results are in good agreement with those by Munir et al.[151].
2.6.6 \({As}_{4}{S}_{3}\) Compound
The natural form of \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) is dimorphite. There is few thermodynamic information in the literature for this compound and the data available are inconsistent across the sources. \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) undergoes a polymorphic transformation and the measured transformation temperatures are shown in Table 10. The orthorombic structure of the synthetic forms has been determined by Whitfield,[154, 155] and has been confirmed for the natural forms by Gavezzoti et al.[156]. Since both forms are orthorombic, the transformation enthalpy is very small.[87] Another modification from crystalline to plastic-crystalline has been found by Blachnik et al.[87] and Chattopadhyay et al.[157]. It is probably of tetragonal symmetry similar to the one found in \({\mathrm{P}}_{4}{\mathrm{S}}_{3}\) and it possibly only occurs under non-equilibrium conditions according to Blachnik et al.[87]. The compound is reported as congruent and the measured melting temperatures are shown in Table 11. Fedorova and Epova[86] measured a much lower melting point of 404 \(\pm\) 3 K which is incongruent with no polymorphic transformation.
Based on emf measurements, Babanly et al.[106] calculated a \({\Delta \mathrm{H}}_{\mathrm{formation}}^{\circ }\) value of − 125.5 kJ/mol for \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\). Using the second law of thermodynamics, Pashinkin et al.[158] calculated a value of − 111.1 kJ/mol. A lower value of − 82.4 kJ/mol from Isabaev et al.[159] was cited by Prostakova et al.[14] without reporting the measurement method. The heat capacity of \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) was first measured by Blachnik et al.[87] using DSC for temperatures between 130 and 600 K. These measurements are uncertain because the sample has undergone partial evaporation according to Chattopadhyay et al.[143] Measurements were also performed using DSC by Chattopadhyay et al.[143] between 100 and 400 K. Pashinkin et al.[158] approximated the heat capacity of \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) by structural analogy to \({\mathrm{P}}_{4}{\mathrm{S}}_{3}\). As shown in Fig. 4, the heat capacity values from each source agree only in a limited temperature range. The standard thermodynamic properties of \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) are presented in Table 11.
The vaporization behavior of solid \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) is still not well established in the literature. As reported by Pashinkin et al.[158], the process of vaporization of \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) may occur according to the following reaction:
Godovikov et al.[138] measured the saturation vapor pressure of \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) at temperatures above its melting temperature, between 600 and 780 K. Using the Knudsen method, Pashinkin et al.[158] measured the vapor pressure of the metastable \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) plastic phase between 400 and 430 K and the stable plastic phase between 434 and 481 K. Pashinkin et al.[158] also measured the vapor pressure of \({{\mathrm{As}}_{4}{\mathrm{S}}_{3}}_{(oP28)}+{{\mathrm{As}}_{4}{\mathrm{S}}_{3}}_{(o**)}\) for temperatures between 402 and 429 K, and of \({{\mathrm{As}}_{4}{\mathrm{S}}_{3}}_{(oP28)}\) between 394 and 418 K using the Langmuir method. All measured data are presented in Fig. 5.
2.7 Thermodynamic Properties of the Gas in As-S Binary System
In addition to the complexity of the vaporization mechanism of As-S system, there is still a gap in the thermodynamic information available for the gaseous species. In fact, the thermodynamic properties for \({{\mathrm{As}}_{4}{\mathrm{S}}_{5}}_{(\mathrm{g})}\) and \({{\mathrm{As}}_{2}{\mathrm{S}}_{3}}_{(\mathrm{g})}\) are still unavailable in the literature. Mah[145] calculated the standard enthalpy of formation for the predominant species \({{\mathrm{As}}_{4}{\mathrm{S}}_{4}}_{(\mathrm{g})}\) and \({\mathrm{AsS}}_{(\mathrm{g})}\) but the values have significant uncertainties. A standard enthalpy of formation of 0.189 \(\pm\) 0.038 kJ/mol for \({\mathrm{AsS}}_{(\mathrm{g})}\) was obtained based on the dissociation energy of 0.385 ± 0.038 kJ /mol from Sullivan.[160] For gaseous \({{\mathrm{As}}_{4}{\mathrm{S}}_{4}}_{(\mathrm{g})}\), a standard enthalpy of formation of − 2.092 \(\pm\) 10.5 kJ /mol was obtained based on the sublimation studies of Munir et al.[151] and Gospodinov and Pashinkin.[133]
2.8 Experimental Determination of Ag-As-S Ternary Phase Diagram
Several minerals have compositions within the Ag-As-S ternary system, including proustite and xanthoconite \(({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3})\) which are very abundant, smithite and trechmannite (\({\mathrm{AgAsS}}_{2}\)) as well as a rare sulfosalt bearing called billingsleyite (\({\mathrm{Ag}}_{7}{\mathrm{AsS}}_{6}\)). Just like the system As-S, the Ag-As-S system tends to form as a glass under certain conditions. Measurements of phase diagram data of this system are presented in Table 12.
2.8.1 \({{\varvec{A}}{\varvec{g}}}_{2}{\varvec{S}}\)-\({{\varvec{A}}{\varvec{s}}}_{2}{{\varvec{S}}}_{3}\) System
In the ternary \(\mathrm{Ag}\)-\(\mathrm{As}\)-\(\mathrm{S}\) system, the quasi-binary \({\mathrm{Ag}}_{2}\mathrm{S}\)-\({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) is the most important experimentally and mineralogically.[165] Jaeger and van Klooster,[33] Elli and Giudici[161] and Kovaleva et al.[163] evaluated the \({\mathrm{Ag}}_{2}\mathrm{S}\)-\({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) phase diagram and reported two congruently melting compounds along this system: \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) and \({\mathrm{AgAsS}}_{2}.\) The measurements could not be performed for the entire composition range due to glass formation at high \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) compositions. Solid solubility: No solid solubility has been reported in the \({\mathrm{Ag}}_{2}\mathrm{S}\)-\({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) system. Eutectic: According to Jaeger and van Klooster,[33] the eutectic reaction that forms \({\mathrm{Ag}}_{2}\mathrm{S}\) and \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) takes place at a temperature of 742 K and a composition of 29 at.% \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\). The eutectic reaction that forms \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) and \({\mathrm{AgAsS}}_{2}\) takes place at a temperature of 672 K and a composition around 58 at.% \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\). This agrees with the eutectic measurements of Elli and Giudici[161] and Kovaleva et al.[163] Liquidus: The liquidus of \({\mathrm{Ag}}_{2}\mathrm{S}\)-\({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) measured by Kovaleva et al.[163] is slightly higher than the one measured by Jaeger and van Klooster.[33] The liquidus measurements of \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\)-\({\mathrm{AgAsS}}_{2}\) determined by Jaeger and van Klooster[33] and Kovaleva et al.[163] are in good agreement and are higher than the liquidus measured by Wehmeier et al.[162] Gaudin and McGlashan[170] proposed a tentative \({\mathrm{Ag}}_{2}\mathrm{S}\)-\({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) phase diagram under non equilibrium conditions based on selective iridescent filming and reported an additional incongruent phase “A”. Sommerlad[171] reported the synthesis of \({\mathrm{Ag}}_{5}{\mathrm{AsS}}_{4}\), and Hall[24] also reported an additional intermediate phase along this quasi-binary system. The phase has possibly a composition of \({\mathrm{Ag}}_{5}{\mathrm{AsS}}_{4}\) by analogy with stephanite (\({\mathrm{Ag}}_{5}{\mathrm{SbS}}_{4}\)).[24] Roland[165] was not able to detect the formation of this phase.
2.8.2 \({{\varvec{A}}{\varvec{g}}}_{3}{\varvec{A}}{\varvec{s}}{{\varvec{S}}}_{3}\) Compound
\({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) Corresponds the mineral xanthoconite that undergoes a polymorphic transformation to form proustite as seen from Table 13. For this compound, Bryndzia and Kleppa[75] determined a standard heat of formation of \(111.3 \pm 3.4\) kJ/mol using high-temperature calorimetry. This value is slightly lower than the one calculated by Gasanova et al.[172] from emf measurements of the following cell: \((-)\mathrm{Pt}\left|\mathrm{Ag }\left(\mathrm{reference electrode}\right)\right|{{\mathrm{Ag}}_{4}{\mathrm{RbI}}_{5}}_{\left(\mathrm{s}\right)}|({\mathrm{Ag}}_{2}\mathrm{S}\)-\({\mathrm{As}}_{2}{\mathrm{S}}_{3}\)-\(\mathrm{S})|\mathrm{Pt}(+)\). The measurements were made at temperatures from 300 to 380 K and the calculated enthalpy is \(121.4 \pm 2.8\) kJ/mol. Using adiabatic calorimetry, Gorbunov et al.[173] and Gurevich et al.[174] measured the heat capacity of the compound in the 10 K to 350 K range and they are in very good agreement. The standard thermodynamic properties of \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) are presented in Table 14. Godovikov et al.[175] and Fedorova et al.[176] suggested that \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) vaporizes incongruently according to the following reaction:
Godovikov et al.[175] also calculated the vapor pressure as a function of temperature based on quartz manometer measurements for 846 K to 1013 K. Using the Knudsen method, Fedorova et al.[176] measured the vapor pressure for temperatures between 673 and 723 K. Godovikov et al.[138] also measured the vapor pressure for temperatures between 769 and 901 K using the isoteniscope method.
2.8.3 \({\varvec{A}}{\varvec{g}}{\varvec{A}}{\varvec{s}}{{\varvec{S}}}_{2}\) Compound
\({\mathrm{AgAsS}}_{2}\) Is present in nature as the mineral trechmannite and it undergoes a polymorphic transformation to form smithite. The standard heat of formation of \({\mathrm{AgAsS}}_{2}\) by Bryndzia and Kleppa[75] and Gasanova et al.[172] are in very good agreement. Using adiabatic calorimetry, Gurevich et al.[174] measured the heat capacity of the compound in the 10 K to 350 K range. The measured standard thermodynamic properties and transformation temperatures are presented in Tables 14 and 15, respectively. Godovikov et al.[175] and Fedorova et al.[176] suggested that \({\mathrm{AgAsS}}_{2}\) vaporizes incongruently according to the following reaction:
Using the Knudsen method, Fedorova et al.[176] measured the vapor pressure for temperatures between 575 and 623 K. Godovikov et al.[138] calculated the vapor pressure as a function of temperature based on quartz manometer measurements from 709 to 980 K. Using the same technique, Fedorova et al.[183] also calculated the vapor pressure for temperatures from 724 to 1013 K. The measurements of Godovikov et al.[138] and Fedorova et al.[183] are in good agreement.
2.8.4 \({{\varvec{A}}{\varvec{g}}}_{7}{\varvec{A}}{\varvec{s}}{{\varvec{S}}}_{6}\) , \({{\varvec{A}}{\varvec{g}}}_{2}{\varvec{A}}{\varvec{s}}{{\varvec{S}}}_{2}\) and \({\varvec{A}}{\varvec{g}}{\varvec{A}}{\varvec{s}}{\varvec{S}}\) Compounds
The only standard entropy and heat of formation values available in the literature for \({\mathrm{Ag}}_{7}{\mathrm{AsS}}_{6}\) are from Gasanova et al.[172]. The values are presented in Table 14. The compound undergoes a polymorphic transformation around 523 K, but no enthalpy of transformation is available. There are still no studies done to investigate the properties and the melting temperatures of \({\mathrm{Ag}}_{2}{\mathrm{AsS}}_{2}\) and \(\mathrm{AgAsS}\). Values of heat of formation of these compounds predicted by density functional theory (DFT) are given in Table 16 as a reference.
2.9 Previous Thermodynamic Assessment of Ag-As-S Systems
The binary Ag-As system was previously assessed by Shi et al.[13]. The liquid phase was described by the substitutional solution model \({\left(\mathrm{Ag},\mathrm{ As}\right)}_{1}\), using the PARROT program of the Thermo-Calc software.[187] Shi et al.[13] also performed a thermodynamic assessment of the binary Ag–S system using the same program of the Thermo-Calc software. The liquid was modeled by the associate model \({\left(\mathrm{Ag},{\mathrm{Ag}}_{2}\mathrm{S},\mathrm{ S}\right)}_{1}\) and \({\mathrm{Ag}}_{2}\mathrm{S}\) was considered as a stoichiometric compound. The sub-solidus phase equilibria in the As-S system were calculated by Emelina et al.[148] by constructing convex envelopes of primary thermodynamic surfaces using PhDi software.[188] The As-S system was also assessed by Prostakova et al.[14] with FactSage thermochemical software using the Modified Quasichemical Model (MQM) for the liquid phase similarly to the present work. However, different choices were made that distinguish the two optimizations. First, \({\mathrm{As}}_{2}{\mathrm{S}}_{5}\) is obtained only at high pressures and Prostakova et al.[14] included it in their optimization. This compound is not considered in the present assessment. Also, different coordination numbers are set in this work. This choice is made because Liu et al.[189] investigated the structural stability of various \(\mathrm{As}\)-\(\mathrm{S}\) clusters based on DFT calculations and demonstrated that \({\left({\mathrm{As}}_{2}{\mathrm{S}}_{3}\right)}_{\mathrm{n}}\) has the highest stability. The stability is due to the 4p-orbital in As atom binding with 3p-orbital of S atom, which decreases the energy level of the highest occupied molecular orbital. Additionally, Tsuchihashi et al.[190] studied the glasses in the \(\mathrm{As}\)-\(\mathrm{S}\) system, and the results show that the hardness, \({\mathrm{T}}_{\mathrm{g}}\) and the thermal expansion are maximum at 40 at.% As, which corresponds to the composition of \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\). Hattori et al.[109] also reported that thermal conductivity of As-S glasses shows a maximum near the composition of \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\). Since Tsuchihashi et al.[190] confirms that the SRO arrangement of glassy \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) is similar to the crystalline structure, the maximum SRO is set in this work to occur at a composition of 40 at.% As. This selected SRO composition was also confirmed to be the most appropriate by studying the measurements made for the ternary \(\mathrm{Ag}\)-\(\mathrm{As}\)-\(\mathrm{S}\) system as well as the favored oxidation state of arsenic. Thus, the values of the coordination numbers set in this work are \({\mathrm{Z}}_{\mathrm{AsS}}^{\mathrm{As}}\)=3 and \({\mathrm{Z}}_{\mathrm{AsS}}^{\mathrm{S}}\)=2, contrary to the values of \({\mathrm{Z}}_{\mathrm{AsS}}^{\mathrm{As}}\)=\({\mathrm{Z}}_{\mathrm{AsS}}^{\mathrm{S}}\)=3 used in the work of Prostakova et al.[14]. Finally, the most significant distinction between the two optimizations comes from the parameters of the excess Gibbs energy of the liquid solution. Linear terms to describe the liquid as used by Prostakova et al.[14] result in that the liquid is internally unstable at high temperatures and form inverted miscibility gaps, even if the real As-S liquid is not prone to separation. To correct the excess Gibbs energy of the liquid at high temperatures, CTln(T) terms were added in this work to avoid the formation of inverted liquid miscibility gaps in this system. This led to an improvement compared with the previous assessment of this system.
3 Thermodynamic Modeling
All calculations and optimizations in this work were performed with the FactSage thermochemical software.[11, 12] The properties of pure solid and liquid Ag, As and S are from SGTE.[191] The properties of the gaseous species \(\mathrm{S}\), \({\mathrm{S}}_{2}\), \({\mathrm{S}}_{3}\), \({\mathrm{S}}_{4}\), \({\mathrm{S}}_{5}\), \({\mathrm{S}}_{6}\), \({\mathrm{S}}_{7}\) and \({\mathrm{S}}_{8}\) are from JANAF Thermochemical Tables.[192] The properties of the gaseous species \(\mathrm{As}\), \({\mathrm{As}}_{2}\), \({\mathrm{As}}_{3}\) and \({\mathrm{As}}_{4}\) are from Degterov et al.[193] and other of their unpublished optimizations and evaluations. The properties of the gaseous species \(\mathrm{AsS}\) and \({\mathrm{As}}_{4}{\mathrm{S}}_{4}\) are from Barin[194] based on the calculations of Mah.[145] The gaseous AgS is from Mills,[101] \(\mathrm{Ag}\) is from Barin et al.[195] and \({\mathrm{Ag}}_{2}\) is from Wagman et al.[196]
3.1 Thermodynamic Model for the Liquid Phase in the Binary Systems
The Modified Quasichemical Model in the Pair Approximation suggested by Pelton et al.[8, 9] was used to model the liquid phases to consider the short-range ordering (SRO). For a liquid binary A-B system, the following pair exchange reaction is considered between the A and B atoms on neighbouring lattice sites:
\(\left(\mathrm{A}-\mathrm{A}\right)\), \(\left(\mathrm{B}-\mathrm{B}\right)\) and \(\left(\mathrm{A}-\mathrm{B}\right)\) are the first nearest neighbor (FNN) atom pairs, and \({\Delta \mathrm{g}}_{\mathrm{AB}}\) is the non-configurational Gibbs energy change for the formation of 2 mol of \(\left(\mathrm{A}-\mathrm{B}\right)\) pairs. The pair and the overall mole (site) fractions are defined respectively as:
\({\mathrm{n}}_{\mathrm{A}}\), \({\mathrm{n}}_{\mathrm{B}}\) represent the number of moles of A and B, \({\mathrm{n}}_{\mathrm{AB}}\) represents the number of moles of pairs (A-B). If the coordination number of A is \({\mathrm{Z}}_{\mathrm{A}}\), the “coordination equivalent” fraction of A is defined as:
The “coordination equivalent” fraction of B is defined similarly. The Gibbs energy of the binary liquid is given by the following equation:
\({\mathrm{g}}_{\mathrm{A}}^{\circ }\) and \({\mathrm{g}}_{\mathrm{B}}^{\circ }\) are the molar Gibbs energies of the pure liquid components, and \({\Delta \mathrm{S}}^{\mathrm{configuration}}\) is the configurational entropy of mixing given by randomly distributing the (A-A), (B-B) and (A-B) pairs. The non-configurational Gibbs energy change for the formation of 2 mol of \(\left(\mathrm{A}-\mathrm{B}\right)\) pairs is expended in terms of the pair fractions:
\(\Delta {g}_{AB}^{\circ }\), \({g}_{AB}^{i0}\) and \({g}_{AB}^{0j}\) are the temperature dependant model parameters that are optimized in this work for the Ag-As, Ag–S and As-S systems. The coordination numbers can be varied with composition to reproduce the SRO, as given by Eq 18 and 19. The composition of maximum SRO is determined by the ratio \({Z}_{B}\) / \({Z}_{A}\):
\({\mathrm{Z}}_{\mathrm{AA}}^{\mathrm{A}}\) and \({\mathrm{Z}}_{\mathrm{AB}}^{\mathrm{A}}\) are the coordination numbers when the nearest neighbours of an A atom are A atoms and B atoms respectively. \({\mathrm{Z}}_{\mathrm{BB}}^{\mathrm{B}}\) and \({\mathrm{Z}}_{\mathrm{BA}}^{\mathrm{B}}\) are defined similarly. In this work, \({\mathrm{Z}}_{\mathrm{AgAg}}^{\mathrm{Ag}}\), \({\mathrm{Z}}_{\mathrm{AsAs}}^{\mathrm{As}}\) and \({\mathrm{Z}}_{\mathrm{SS}}^{\mathrm{S}}\) are set to be 6 for consistency with previous work.
3.2 Thermodynamic Model for the Liquid Phase in the Ternary System
Geometric models can be used to estimate the excess Gibbs energy of a ternary system from the parameters of the three binary subsystems.[197] For a given geometric model, the excess Gibbs energy of the ternary system at any composition p is calculated from the binary parameters at points a, b and c. The difference between the models is the placement of these three points which is very important to correctly interpolate the ternary system. After trying several extrapolation methods, the Kohler-Toop type extrapolation technique, with Ag as the asymmetric component, provided the most satisfactory description of the ternary system. It is then the selected technique in this work to optimize the ternary Ag-As-S liquid phase (Table 17).
3.3 Thermodynamic Model for \({{A}{g}}_{2}{S}\) Solid Solutions
The low temperature monoclinic form is a semiconductor and has nearly an ideal stoichiometric composition.[43, 198] The second bcc form has a high conductivity due to the increase of number of vacancies in the silver sublattice and the transfer of silver atoms to adjacent vacancy sites. Silver is distributed among the tetrahedrally and octahedrally coordinated positions.[199] At higher temperatures, the tetrahedral occupancy increases at the expense of the octahedral occupancy, and above 533 K the occupied sites are entirely tetrahedral.[199] The sulfur atoms arrangement stays unchanged.[199, 200] Based on this information and by analogy to the model established by Waldner[201] for the \({\mathrm{Cu}}_{2}\mathrm{S}\) phase, a simplified sub-lattice model is used for \({\mathrm{Ag}}_{2}\mathrm{S}\) solid solutions and is presented in Table 7.
4 Results and Discussion
The optimization of the thermodynamic parameters was conducted using the FactSage thermochemical software. The Ag-As-S systems are assessed based on the literature data presented in the previous sections, and the optimized thermodynamic model parameters are presented in Table 18. The calculated Ag-As binary phase diagram is shown in Fig. 1, along with the experimental data. No information on the composition of maximum short-range ordering (SRO) of this system is reported. Nevertheless, analysis of the measured phase diagram data shows an increase of the slope of the tangent line of the liquidus curves around 20 at.% As. This indicates a strong maximum SRO around this composition. Thus, the coordination numbers \({\mathrm{Z}}_{\mathrm{AgAs}}^{\mathrm{Ag}}\) and \({\mathrm{Z}}_{\mathrm{AgAs}}^{\mathrm{As}}\) are set in this work to 1.5 and \(6\) respectively to reproduce SRO at 20 at.% As. There is also an inflection point on the liquidus of the arsenic rich side which is not uncommon. An inflection is considered traditionally as an indication of a tendency for an instable liquid phase at temperatures below the liquidus, meaning that it is prone to separation. It can also appear due to the properties of fusion of pure arsenic.[203] As seen from Fig. 1, the calculated eutectic and liquidus curves reproduce the measured data very well. However, silver solubilizes slightly less arsenic compared to the measured values below 625 K and above 900 K. The hcp phase (ξ) may not occur as a mineral according to Hall,[24] but it is still modeled in this work. The optimized Gibbs free energy of the phase is shown in Table 18. As explained in the previous section, there is a disagreement regarding the formation and the decomposition temperatures of the hcp phase. Elliot,[204] Baren[205] and Shi et al.[13] selected peritectic and eutectoid temperatures in accordance with the measurements of Eade and Hume-Rothery.[22] The same choice is made in this work since the measurements of Eade and Hume-Rothery[22] are considered more reliable than those of Heike and Leroux.[18] The activity data suggested by Lüdecke et al.[25] are very scattered and could not be reproduced in the work by Shi et al.[13]. After examining the experimental method and the inconsistency with phase diagram data, the activity data suggested by Lüdecke et al.[25] are considered doubtful and is not used in the present optimization.
The calculated Ag–S binary phase diagram is shown in Fig. 2, and the different phases are identified with Pearson symbols. According to the integral molar quantities of the liquid phase determined by Fukatsu and Kozuka,[35] the SRO is maximum at a composition of \({\mathrm{Ag}}_{2}\mathrm{S}\), which is expected. Considering the sharpness of the curves, the coordination numbers \({\mathrm{Z}}_{\mathrm{AgS}}^{\mathrm{Ag}}\) and \({\mathrm{Z}}_{\mathrm{AgS}}^{\mathrm{S}}\) are set in this work to 1.5 and \(3\) respectively to reproduce SRO at 33.33 at.% As. The solubility of sulfur in silver is very small as shown in Fig. 2. The most reliable solubility data obtained by Raub and Roschel[30] and Fueki et al.[31] are in very good agreement with the solubility calculated in this work. As reported in the literature, the low temperature form of \({\mathrm{Ag}}_{2}\mathrm{S}\) is considered stoichiometric, and the high temperature forms are considered non-stoichiometric. The homogeneity range, the non-symmetrical polymorphic temperatures, and the partial pressure of \({\mathrm{S}}_{2}\) over the solid solutions are well reproduced. The optimized Gibbs free energies of the \({\mathrm{Ag}}_{2}\mathrm{S}\) phases are presented in Table 18.
Table 3 shows that the selected heat of fusion of \({\mathrm{Ag}}_{2}\mathrm{S}\) is slightly higher than what was measured experimentally by Kelly[206] and nearly double of what was measured by Thompson and Flengas[52] and Koh and Itagaki.[62] This choice was necessary to adequately reproduce not only the binary Ag–S phase diagram, but also the \({\mathrm{Ag}}_{2}\mathrm{S}\) liquidus in the quasi-binary \({\mathrm{Ag}}_{2}\mathrm{S}\)-\({\mathrm{As}}_{2}{\mathrm{S}}_{3}\). As shown in Fig. 2(d), the sulphur contained in \({\mathrm{Ag}}_{2}\mathrm{S}\) completely vaporizes as observed by Piacente et al.[78] and becomes pure silver before this completely vaporizes too.
The calculated heat capacity of \({\mathrm{Ag}}_{2}\mathrm{S}\) as function of temperature is presented in Fig. 4(a). As was concluded by Sadonikov and Gusev,[66] the experimental data of Grønvold and Westrum[53] are considered the most reliable in the context of this work. Liu et al.[208] developed recently the zentropy theory to understand and predict anomalies of the physical properties of phases and this theory could be used to address the spikes of Fig. 4(a).
The optimization of the binary As-S system presents a great challenge. First, there are many contradictions and uncertainties in the measurements of this system as explained in the literature review. This is mainly due to the complexity of performing experiments of polymer structured samples. Above its melting temperature, sulfur is organized as ring shaped \({\mathrm{S}}_{8}\) molecules, and at higher temperatures, it can form long polymer chains \({\mathrm{S}}_{\mathrm{n}}\) with high molecular weight.[209, 210] The addition of arsenic to sulfur causes a significant increase in polymer chains and a reduction of \({\mathrm{S}}_{8}\) molecules. This leads to glass formation and metastable tendencies for a wide range of compositions in the As-S system.[211, 212] Also, the high vapor pressure in the arsenic rich region and its toxicity add to the difficulty of performing experiments to study this binary system. The calculated As-S binary phase diagram is shown in Fig. 5 and the optimized thermodynamic model parameters are presented in Table 18. Two calculations are superimposed on the figure, one with only the solids and the liquid phase, and the other including the gaseous species. As justified in Sect. 2.8, the coordination numbers set in this work are \({\mathrm{Z}}_{\mathrm{AsS}}^{\mathrm{As}}\)=3 and \({\mathrm{Z}}_{\mathrm{AsS}}^{\mathrm{S}}\)=2. By adding CTlnT terms to the model parameters of the liquid, no artifact miscibility gap is generated up to 6000 K. However, this also leads to the an unusual shape of the actual miscibility gap excisting in the arsenic rich region, hence it is represented by a dotted line. The extrapolation of this gap to the Ag-As-S ternary system is shown in the isothermal section of Fig. 7. As stated in Sect. 2.6, the thermodynamic information available in the literature for the gaseous species in the As-S system is incomplete. Despite this, the liquid was still optimized using the limited information available to reproduce the liquid–vapor equilibrium as best as possible. The azeotrope at the composition of \({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) measured by Jonker[81] is well reproduced. But this work suggests more than one azeotrope, as shown in Fig. 5(a), which was also predicted by Emelina.[148] Also, it is important to acknowledge that the MQM model used to optimize the liquid phase describes a monomeric melt and does not consider the complex structure of liquid As-S which tends to form polymer chains.
There is a large discrepancy in the measured standard enthalpies of formation of the three solid compounds in the As-S system, as presented in Tables 7, 9 and 11. In this work, low enthalpy values were selected for each compound. This was necessary to avoid stabilizing the liquid too much and consequently pushing the gas curve towards very high temperatures. With the few contradicting thermodynamic data available in the literature for \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\), it is difficult to predict the behaviour of this compound very accurately. In this work, only the two orthorhombic crystalline phases were modeled, the plastic phase found by Blachnik et al.[87] and Chattopadhyay et al.[157] was not considered. The transformation and melting temperatures measured by Chattopadhyay et al.[157] are the most reliable in the context of this work and their values were selected in this optimization. However, it was not possible to optimize the system to have a congruent compound as found by Chattopadhyay et al.[157], so \({\mathrm{As}}_{4}{\mathrm{S}}_{3}\) is incongruent in this work as it was measured by Fedorova and Epova.[86] The calculated heat capacity of the three compounds in the As-S system is shown in Fig. 4. Blachnik et al.[87] performed heat capacity measurements, but the evaporation of their samples during the experiments caused uncertainties. The most reliable measurements are again those by Chattopadhyay et al.[157]. As seen in Fig. 4, the heat capacity of the liquid is high, and a possible explanation is its tendency to polymerize.
For the ternary Ag-As-S system, the thermodynamic properties of \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) and \({\mathrm{AgAsS}}_{2}\) are available in the literature and were optimized first to reproduce the vapor pressure measurements presented in Fig. 6. For \({\mathrm{Ag}}_{7}{\mathrm{AsS}}_{6}\), the enthalpy of formation and standard entropy were set to the values suggested by Gasanova et al.[172] since they are the only thermodynamic properties based on measurements available for this compound. The present calculations confirm the decomposition reactions 10 and 11 proposed by Godovikov et al.[175] and Fedorova et al.[176].
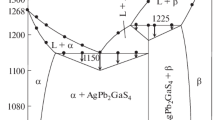
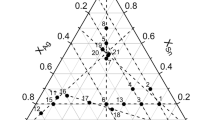
The liquid phase was then modeled and five ternary parameters, presented in Table 18, were used to reproduce more accurately the experimental data. Since three large positive terms are used to describe the three miscibility gaps in the Ag–S and As-S binary systems, three corrective ternary parameters have been added to balance these positive terms to avoid the formation of large ternary miscibility gaps and thus obtain a more precise interpolation. As there is no thermodynamic information available for \({\mathrm{Ag}}_{2}{\mathrm{AsS}}_{2}\) and \(\mathrm{AgAsS}\) and their decomposition temperatures are still uncertain, the Neumann–Kopp rule was used as a first approximation for these two compounds. Their Gibbs energies were then adjusted to reproduce as best as possible the available phase diagram data. A group of calculated phase diagrams together with the experimental data are shown in Fig. 7, 8, 9 and 10. Some phases are present at equilibrium in minor quantities and are identified in the figures as such. The quasi-binary \({\mathrm{Ag}}_{2}\mathrm{S}\)-\({\mathrm{As}}_{2}{\mathrm{S}}_{3}\) is the most important in the Ag-As-S ternary system and it is well reproduced as seen from Fig. 7(b). The two quasi-binaries \({\mathrm{Ag}}_{7}{\mathrm{AsS}}_{6}\)-\({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\) and \({\mathrm{Ag}}_{7}{\mathrm{AsS}}_{6}\)-\({\mathrm{Ag}}_{2}\mathrm{S}\) are also well reproduced as shown in Fig. 7(c) and (d). There is however a negligeable amount of a second liquid calculated, which was not detected by the measurements of Blachnik and Wickel.[164] The four cross sections measured by Kovaleva[163] along with the calculations from this work are presented in Fig. 8. Also, the predictions of Roland[139, 165,166,167] along with the calculations are presented in Fig. 9. There is a good agreement between the predictions and the calculations of \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\)-\(\mathrm{S}\) and \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\)-\(\mathrm{As}\). However, a miscibility gap was predicted for \({\mathrm{AgAsS}}_{2}\)-\({\mathrm{As}}_{4}{\mathrm{S}}_{4}\), but is not present in this work. Figure 10 shows the calculated \({\mathrm{Ag}}_{2}\mathrm{S}\)-\(\mathrm{As}\) and \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\)-\(\mathrm{Ag}\) along with the experimental data. Eutectic compositions and temperatures deviate slightly from measured values. Certain differences to what was measured for \({\mathrm{Ag}}_{3}{\mathrm{AsS}}_{3}\)-\(\mathrm{Ag}\) diagram were expected since Schmid-Fetzer et al.[202] already noted that in what was suggested by Chaus et al.[169] some phase diagram features were not considered. A summary of some invariant reactions involving four-phase intersection points with liquid in the ternary \(\mathrm{Ag}\)-\(\mathrm{As}\)-\(\mathrm{S}\) system is presented in Table 19.
5 Conclusion
The increasing use of complex ores in smelters causes many problems due to their high arsenic content. To predict the behaviour of arsenic during the smelting process and help elaborate solutions, the development of thermodynamic models and their associated databases is essential. In this work, the thermodynamic information available in the literature was critically evaluated and was used to optimize the descriptions of the Ag-As, Ag–S and As-S binary systems. The MQM was applied to model the liquid phase while the CEF was utilized for solid solutions. The optimization of the binary As-S system presented a great challenge due the lack of complete thermodynamic information. The calculated phase diagrams for the three assessed binary systems provide an accurate description of all reliable experimental data. Using the Kohler-Toop interpolation, the Ag-As-S ternary system was assessed for the first time, and the calculations also provide a satisfactory description of the ternary data.
References
S. Northey, S. Mohr, G.M. Mudd, Z. Weng, and D. Giurco, Modelling Future Copper Ore Grade Decline Based on a Detailed Assessment of Copper Resources and Mining, Resour. Conserv. Recycl., 2014, 83, p 190–201.
A. Elshkaki, T.E. Graedel, L. Ciacci, and B.K. Reck, Copper Demand, Supply, and Associated Energy Use to 2050, Glob. Environ. Change, 2016, 39, p 305–315.
G.A. Flores, C. Risopatron, and J. Pease, Processing of Complex Materials in the Copper Industry: Challenges and Opportunities Ahead, JOM, 2020, 72, p 3447–3461.
M. Valcke, G. Ponce, M.-H. Bourgault, Évaluation du risque cancérigène attribuable aux concentrations d’arsenic et de cadmium dans l’air de la ville de Rouyn-Noranda, Avis scientifique, Santé publique du Québec, Québec, 2022, pp. 37.
C.C. Patterson, Native Copper, Silver, and Gold Accessible to Early Metallurgists, Am. Antiq., 1971, 36, p 286–321.
J.M. Mazurków, A. Kusior, A. Mikuła, and M. Radecka, Transition Metal Sulfides for Electrochemical Applications: Controlled Chemical Conversion of CuS to Ag2S, Appl. Surf. Sci., 2022, 606, p 154984.
S.C. Sharath, D. Gayathri, S.R. Mannopantar, and M.N. Kalasad, Growth and Characterization of Ag2S Semiconductor Nanoparticles, Mater. Today Proc., 2022, 67, p 276–279.
A.D. Pelton, S.A. Degterov, G. Eriksson, C. Robelin, and Y. Dessureault, The Modified Quasichemical Model I—Binary Solutions, Metall. Mater. Trans. B, 2000, 31, p 651–659.
A.D. Pelton, and P. Chartrand, The Modified Quasi-Chemical Model: Part II. Multicomponent Solutions, Metall. Mater. Trans. A, 2001, 32, p 1355–1360.
B. Sundman, and J. Ågren, A Regular Solution Model for Phases with Several Components and Sublattices, Suitable for Computer Applications, J. Phys. Chem. Solids, 1981, 42, p 297–301.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R.B. Mahfoud, J. Melançon, and S. Petersen, FactSage Thermochemical Software and Databases, Calphad, 2002, 26, p 189–228.
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, K. Hack, L.-H. Jung, Y.-B. Kang, J. Melançon, A.D. Pelton, C. Robelin, and S. Petersen, FactSage Thermochemical Software and Databases—Recent Developments, Calphad, 2009, 33, p 295–311.
C. Shi, Y. Du, H. Biao, B. Yang, Y. Pan, F. Guo, S. Liu, and Q. Du, Thermodynamic Descriptions of the Ag-X (X = S, As, Lu) Systems, Calphad, 2018, 62, p 207–214.
V. Prostakova, D. Shishin, and E. Jak, Thermodynamic Optimization of the As–S System, Calphad, 2021, 72, p 102235.
A. Descamps, De la formation des arséniures métalliques, Compt. rend., 1878, 86, p 1022–1023.
K. Friedrich, and A. Leroux, Silber und Arsen, Metallurgie, 1906, 3, p 192–195.
M. Hansen, and K. Anderko, Constitution of Binary Alloys. McGraw-Hill, New York, 1958.
V.W. Heike, and A. Leroux, Das Erstarrungsbild der Silber-Arsen-Legierungen, Z. Anorg. Chem., 1915, 92, p 119–126.
S.J. Broderick, and W.F. Ehret, An X-Ray Study of the Alloys of Silver with Bismuth, Antimony and Arsenic. Part II, Phys. Chem., 1931, 35, p 3322–3329.
E.A. Owen, and V.W. Rowlands, Solubility of Certain Elements in Copper and in Silver, J. Inst. Metals, 1940, 66, p 361–378.
E.A. Owen, and D.P. Morris, The Application of X-Ray Methods to the Determination of Phase Boundaries in Metallurgical Equilibrium Diagram, J. Inst. Metals, 1949, 76, p 145–168.
G.A. Eade, and W. Hume-Rothery, The Constitution of Silver-Rich Silver-Arsenic Alloys, Z. Metallkd., 1959, 50, p 123–126.
H.W. King, and T.B. Massalski, Lattice Spacing Relationships and the Electronic Structure of H.C.P. ζ Phases Based on Silver, Philos. Mag., 1961, 6, p 669–682.
H.T. Hall, The Systems Ag-Sb-S, Ag-As-S, and Ag-Bi-S: Phase Relations and Mineralogical Significance Brown University, Providence, 1966, p185
D. Lüdecke, C. Lüdecke, and T. Hehenkamp, Thermodynamic Activity from Quasi-isopiestic Measurements in α-Ag-As and α-Cu-As, Acta Metall., 1983, 31, p 95–100.
C. Jeannot, P. Perrot, and G. Tridot, Sur les équilibres argent-soufre, C. R. Acad. Sc. Paris. Ser. C, 1969, 268, p 2177–2180.
F.C. Kracek, Phase Relations in the System Sulfur—Silver and the Transitions in Silver Sulfide, Trans. Am. Geophys. Union, 1946, 27, p 364–374.
K. Friedrich, and A. Leroux, Silber und Schwefelsilber, Metallurgie, 1906, 3, p 361–367.
N. Barbouth, and J. Oudar, Étude de la solubilité du soufre dans l’argent Comptes rendus hebdomadaires des séances de l’Académie des sciences, Série C, Sciences chimiques, 1967, 264, p 2120–2122.
E. Raub, and E. Röschel, Die Löslichkeit von Schwefel, Selen und Tellur in festem Silber (in German), Metall., 1971, 25, p 7–10.
K. Fueki, K. Ota, and K. Kishio, Solubility and Diffusion Coefficient of Sulfur in Silver, Bull. Chem. Soc. Jpn., 1978, 51, p 3067–3068.
C.C. Bissett, The Systen Silver-Silver Sulfide, J. Chem. Soc., 1914, 105, p 1223–1228.
F.M. Jaeger, and H.S. van Klooster, Studien über natürliche und künstliche Sulfoantimonite und Sulfoarsenite, Zeitschrift für anorganische, Chemie, 1912, 78, p 245–268.
T. Rosenqvist, A Thermodynamic Investigation of the System Silver-Silver Sulphide, JOM, 1949, 1, p 451–460.
N. Fukatsu, and Z. Kozuka, Measurement of the Thermodynamic Quantities for the Ag-S System by the EMF Method (in Japanese), J. Jpn. Inst. Met., 1981, 45, p 1263–1270.
C. Wagner, Investigations on Silver Sulfide, J. Chem. Phys., 1953, 21, p 1819–1827.
H. Rau, Defect Equilibria in Silver Sulphide, J. Phys. Chem. Solids, 1974, 35, p 1553–1559.
G. Bonnecaze, A. Lichanot, and S. Gromb, Propriétés électroniques et électrogalvaniques du sulfure d’argent α domaine d’existence, J. Phys. Chem. Solids, 1978, 39, p 813–821.
G. Bonnecaze, A. Lichanot, and S. Gromb, Propriétés électrogalvaniques et électroniques du sulfure d’argent β: Domaine d’existence, J. Phys. Chem. Solids, 1978, 39, p 299–310.
A.V. Ditman, and I.N. Kulikova, Investigation of the Dissociation of Solid and Fused Silver Sulphide by the Dew Point Method, Zh. Fiz. Khim., 1979, 53, p 260–261.
H. Reye, Zustandsdiagramm Ag—S im Bereich der Verbindung Ag2±δS, Z. Phys. Chem. Neue Folge (Wiesbaden), 1980, 119, p 251–254.
R.C. Sharma, and Y.A. Chang, The Ag-S (Silver-Sulfur) system, Bull. Alloy Phase Diagr., 1986, 7, p 263–269.
A.J. Frueh, The Crystallography of Silver Sulfide, Ag2S, Z. Kristallogr. Cryst. Mater., 1958, 110, p 136–144.
L.A. Taylor, The Significance of Twinning in Ag2S, Am. Mineral. J. Earth Planet. Mater., 1969, 54, p 961–963.
B.J. Skinner, The System Cu-Ag-S, Econ. Geol., 1966, 61, p 1–26.
M. Bellati, and S. Lussana, Sui Calori Specifici E Di Transformazione Dei Solfuri E Seleniuri Di Argento E Di Rame (in Italian), Atti, Classe di scienze fisiche, matematiche e naturali, 1889, 6, p 1051–1059.
W. Truthe, Über das Verhalten der Sulfide von Ph, Cu., Ag und des Cu2 in den Schmelzen der zugehörigen Chloride, Zeitschrift für anorganische Chemie, 76 (1912) 161–173.
R.C. Emmons, C.H. Stockwell, and R.H.B. Jones, Argentite and Acanthite, Am. Miner., 1926, 11, p 326–328.
R. Roy, A.J. Majumdar, and C.W.H. Hulbe, The Ag2S and Ag2Se Transitions as Geologic Thermometers, Econ. Geol., 1959, 54, p 1278–1280.
C.M. Perrott, and N.H. Fletcher, Heat Capacity of Silver Sulfide, J. Chem. Phys., 1969, 50, p 2344–2350.
K.P. Mamedov, M.F. Gadzhiev, Z.J. Suleimanov, and Z.D. Nurieva, X-ray and Thermographic Study of Phase Transition α→ β in Ag2S, Russ. J. Inorg. Mater., 1980, 16, p 241–243.
W.T. Thompson, and S.N. Flengas, Drop Calorimetric Measurements on Some Chlorides, Sulfides, and Binary Melts, Can. J. Chem., 1971, 49, p 1550–1563.
F. Grønvold, and E.F. Westrum, Silver (I) Sulfide: Ag2S Heat Capacity from 5 to 1000 K, Thermodynamic Properties, and Transitions, J. Chem. Thermodyn., 1986, 18, p 381–401.
J. Jang, K. Cho, S.H. Lee, and S. Kim, Synthesis and Electrical Characteristics of Ag2S Nanocrystals, Mater. Lett., 2008, 62, p 1438–1440.
D. Živković, V. Ćosović, Ž Živković, N. Štrbac, M. Sokić, N. Talijan, B. Boyanov, and A. Mitovski, Kinetic Investigation of Silver Sulfide Phase Transformations, Mater. Sci. Semicond. Process., 2013, 16, p 217–220.
S.I. Sadovnikov, A.I. Gusev, and A.A. Rempel, An In Situ High-Temperature Scanning Electron Microscopy Study of Acanthite–Argentite Phase Transformation in Nanocrystalline Silver Sulfide Powder, Phys. Chem. Chem. Phys., 2015, 17, p 20495–20501.
A.I. Gusev, and S.I. Sadovnikov, Effect of Small Size of Particles on Thermal Expansion and Heat Capacity of Ag2S Silver Sulfide, Thermochim. Acta, 2018, 660, p 1–10.
A.A. Valeeva, S.I. Sadovnikov, and A.I. Gusev, Polymorphic Phase Transformations in Nanocrystalline Ag2S Silver Sulfide in a Wide Temperature Interval and Influence of Nanostructured Ag2S on the Interface Formation in Ag2S/ZnS Heteronanostructure, Nanomaterials, 2022, 12, p 1668.
H. Okazaki, and A. Takano, The Specific Heat of Ag2S in α-phase, Z. Naturforsch. A, 1985, 40, p 986–988.
H. Pélabon, Sur le sulfure, le séléniure et le tellure d’argent, Comptes rendus, 1906, 143, p 294–296.
K.K. Kelley, Heats of Fusion of Inorganic Substances, US Government Printing Office, 1936.
J. Koh, and K. Itagaki, Measurements of Thermodynamic Quantities for Molten Ag2S–Sb2S3 and Cu2S–Ni3S2 Systems by Quantitative Thermodynamic Analysis, Trans. Jpn. Inst. Met., 1984, 25, p 367–373.
P.V. Gultyaev, and A.V.P. Petrov, The Specific Heats of a Number of Semiconductors, Sov. Phys. Solid State, 1959, 1, p 330.
P.N. Walsh, and A.E.W.D. White, The Heat Capacity of the Silver Chalcogenides, Ag1.99S, Ag1.99Se, and Ag1.88Te from 16 to 300° K, J. Phys. Chem., 1962, 66, p 1546–1549.
W. Jost, and P. Kubaschewski, Spezifische Wärmen von Silber-und Kupfer (I)-Chalkogeniden von− 70° C bis zu 550° C, Z. Phys. Chem., 1968, 60, p 69–78.
S.L. Sadovnikov, and A.I. Gusev, Thermal Expansion and the Heat Capacity of Nanocrystalline and Coarse-Crystalline Silver Sulfide Ag2S, Phys. Solid State, 2017, 59, p 1887–1894.
F.G. Keyes, and W.A. Felsing, The Equilibrium Conditions of the Reaction Between Silver Sulfide and Hydrogen, J. Am. Chem. Soc., 1920, 42, p 246–251.
K. Jellinek, and J. Zakowski, Ueber die Affinitat der Metalle zum Schwefe, Z. Anorg. Allg. Chem., 1925, 142, p 1–53.
H. Reinhold, Über die Bestimmung der Bildungsaffinität des Silbersulfids (-Selenids-Tellurids) durch elektrometrische Messungen an festen Ketten (in German), Zeitschrift für Elektrochemie und angewandte physikalische Chemie, 1934, 40, p 361–364.
H.A. Zeumer, and W.A. Roth, Die Bildungswarme einiger Sulfide (in German), Z. physik. Chem (A), 1935, 173, p 365.
J.R. Goates, A.G. Cole, E.L. Gray, and N.D. Faux, Thermodynamic Properties of Silver Sulfide, J. Am. Chem. Soc., 1951, 73, p 707–708.
E. Kordes, and B. Rackow, Bestimmung der Schwefeltensionen von Schwermetall-sulfiden durch Beobachtung des Anlaufens von Metallen im H2/H2S-Gasgemisch (in German), Z. Phys. Chem., 1952, 200, p 129–157.
K. Kiukkola, and C. Wagner, Measurements on Galvanic Cells Involving Solid Electrolytes, J. Electrochem. Soc., 1957, 104, p 379.
H. Bielen, Bestimmungen von Dissoziationsdampfdrucken fester Verbindungen nach der Anlaufmethode. IV. Schwefeltensionen einiger Schwermetallsulfide (in German), Z. Anorg. Allg. Chem., 1965, 338, p 185–194.
L.T. Bryndzia, and O.J. Kleppa, Standard Molar Enthalpies of Formation of Sulfosalts in the Ag-As-S System and Thermochemistry of the Sulfosalts of Ag with As, Sb, and Bi, Am. Miner., 1989, 74, p 243–249.
V.M. Glazov, and N.M. Korenchuk, Vapour Pressure and Thermodynamic Properties of Copper and Silver Chalcogenides Russ, J. Phys. Chem., 1971, 45, p 1520–1521.
Y.I. Dutchak, I.A. Tishchenko, and N.M. Korenchuk, Mass Spectrometric Study Vapor Pressures over Solid and Liquid Silver Halides, Izv. Akad. Nauk SSSR Neorg. Mater., 1977, 13, p 1980–1983.
V. Piacente, P. Scardala, and D. Ferro, Study on the Thermal Decomposition of Ag2S, J. Mater. Sci. Lett., 1990, 9, p 365–367.
P.J. Smith, and W.W. Smeltzer, A Method for Long-Term Sulfidation of Metal at Low Sulfur Pressures and Its Application to Sulfidation of an Fe-20 at.% Al Alloy at 1023 K, Oxid. Met., 1987, 28, p 291–308.
A. Yazawa, F. Sehnale’k, and K. Koike, Thermodynamic Studies on the Liquid Silver-Sulphur System, J. Min. Metall. Inst. Jpn., 1965, 81, p 395–398.
W. Jonker, Untersuchungen über das System: Schwefel und Arsen, Z. anorg. Chem., 1909, 62, p 89–107.
L. Clark, The Fe-As-S System: Phase Relations and Applications, Econ. Geol., 1960, 55, p 1345–1381.
P.B. Barton, Themochemical Study of the System Fe-As-S, Geochim. Cosmochim. Acta, 1969, 33, p 841–857.
N.V. Timofeeva, G.Z. Feklichev, E.M. Apollonov, and V.N. Kalashniko, Arsenic-Sulfur and Arsenice-Selenium Glass Forming Systems at High Pressures and Temperatures, Sovremennye Problemy Fizicheskoi Khimii, 1971, 6, p 234–259.
R. Ceolin, B. Legendre, and P. Khodadad, Sur le système arsenic-soufre: étude, entre 500 et 950 °C, des équilibres de phases dans le secteur limité par l’arsenic et le sulfure As2S, Comptes rendus hebdomadaires des séances de l’Académie des sciences, Série C Sciences chimiques, 1977, 284, p 495–498.
Z. Fedorova, and E. Epova, Thermal Study of an Arsenic-Sulfur System Issled po Eksperim Mineral, Novosibirsk, 1979, p12–18
R. Blachnik, A. Hoppe, and U. Wickel, Die Systeme Arsen-Schwefel und Arsen-Selen und die thermodynamischen Daten ihrer Verbindungen, Z. Anorg. Allg. Chem., 1980, 463, p 78–90.
Z.N. Fedorova, I.V. Osipova, and V.V. Gurov, Behaviour of Proustite at the Annealing in the As2S3 Vapours (in Russian), Izv. Akad. Nauk SSSR Neorg. Mater., 1985, 21, p 14–16.
P. Espeau, J. Tamarit, M. Barrio, D. López, M. Perrin, H. Allouchi, and R. Céolin, Solid State Studies on Synthetic and Natural Crystalline Arsenic(III) Sulfide, As2S3(Orpiment): New Data for an Old Compound, Chem. Mater., 2006, 18, p 3821–3826.
P. Debenedetti, S.A. Rice, and A.R. Dinner, Liquid Polymorphism, Liquid Polymorphism. Advances in Chemical Physics Wiley, New York, 2013, p2013
M. Frumar, A.P. Firth, and A.E. Owen, Optically Induced Crystal-to-Amorphous-State Transition in As2S3, J. Non-Cryst. Solids, 1995, 192, p 447–450.
J. Doupovec, I. Thurzo, and B.D.V.P. Shilo, Anisotropy of Some Physical Parameters in As-Prepared As2S3 Bulk Glass, Mater. Res. Bull., 1977, 12, p 119–125.
J. Málek, Dilatometric Study of Structural Relaxation in Arsenic Sulfide Glass, Thermochim. Acta, 1998, 311, p 183–198.
F. Zhang, Y. Chen, R. Wang, X. Shen, J. Wang, and T. Xu, Study of Glass Transition Kinetics of As2S3 and As2Se3 by Ultrafast Differential Scanning Calorimetry, Chin. Phys. B, 2019, 28, p 047802.
S.A. Dembovskii, Y.A. Polyakov, and A.A. Vaipolin, Prepation and Some Properties of Arsenic Trisulfide Single Crystals, Izv. Akad. Nauk SSSR Neorganicheskiye. Mater., 1968, 4(5), p 767–768.
M. Myers, and E. Felty, Heats of Fusion of the A2V B3VI Compounds As2S3, As2Se3, As2Te3, and Sb2S3, J. Electrochem. Soc., 1970, 117, p 818–820.
B. Voigt, and B. Jacob, Zum Kristallisations- und Schmelzverhalten von Auripigment und den strukturanalogen Verbindungen Claudetit und Arsenselenid, Monatshefte Für Chemie Chem. Mon., 1982, 113, p 895–906.
Z. Černošek, E. Černošková, and L. Beneš, Crystalline Arsenic Trisulfide: Preparation, Differential Scanning Calorimetry and Raman Scattering Measurements, Mater. Lett., 1999, 38, p 336–340.
E.V. Britzke, A.F. Kapustinskii, and L.G. Chentzova, A Study of the Thermal Dissociation of the Disulfide and Trisulfide of Arsenic, Zhurnal Khimicheskoi Promyshlennosti, 1931, 8, p 1–7.
D.D. Wagman, W.H. Evans, V.B. Parker, R.H. Schumm, L. Halow, S.M. Bailey, L. Kenneth, and R. Nuttal, Selected Values for Inorganic and C1 and C2 Organic Substances in SI Units, J. Phys. Chem. Ref. Data, 1982, 11, p 37–38.
K.C. Mills, Thermodynamic Data for Inorganic Sulphides, Selenides and tellurides, 1974.
V.G. Neverov, G.A. Rybakova, and V.R. Panus, Thermochemical Study of Compounds in the Arsenic-Sulfur System, Izv. Akad. Nauk. SSSR Neorganicheskiye Mater., 1989, 25, p 713–716.
G. Johnson, G. Papatheodorou, and C. Johnson, The Enthalpies of Formation and High-Temperature Thermodynamic Functions of As4S4 and As2S3, J. Chem. Thermodyn., 1980, 12, p 545–557.
P.A.G. O’Hare, Calorimetric Measurements of the Specific Energies of Reaction of Arsenic and of Selenium with Fluorine. Standard Molar Enthalpies of Formation ΔfHom at the Temperature 298.15 K of AsF5, SeF6, As2Se3, As4S4, and As2S3. Thermodynamic Properties of AsF5 and SeF6 in the Ideal-Gas State. Critical Assessment of ΔfHom (AsF3, l), and the Dissociation Enthalpies of As-F Bonds, J. Chem. Thermodyn., 1993, 25, p 391–402.
L. Bryndzia, and O. Kleppa, Standard Molar Enthalpies of Formation of Realgar (α-AsS) and Orpiment (As2S3) by High-Temperature Direct-Synthesis Calorimetry, J. Chem. Thermodyn., 1988, 20, p 755–764.
M. Babanly, G. Muradova, T. Il’yasly, and D. Babanly, Thermodynamic Properties of Arsenic Sulfides Studied by EMF Measurements, Inorg. Mater., 2011, 47, p 227–230.
D.K. Nordstrom, and D.G. Archer, Arsenic Thermodynamic Data and Environmental Geochemistry. Springer, New York, 2003.
V.A. Romanovsky, and V.V. Tarasov, Low-Temperature Specific Heat and Entropy of Sulfides of the Group V Elements at 298.15 K, Solid State Phys. USSR, 1960, 11, p 1294–1299.
M. Hattori, K. Nagaya, S. Umebachi, and M. Tanaka, Heat Capacities of As-S Glasses, J. Non-Cryst. Solids, 1970, 3, p 195–204.
K. Kageyama, M. Imaoka, H. Suga, S. Seki, Fourth Japan Calorimetry Meeting, quoted by Hattori et al., 1970, 1968.
V.V. Tarasov, V.M. Zhdanov, Zhurnal Fizichesko. Khimii, 44 (1970) 2384, quoted in Hattori et al., 1970.
J.S. Haggerty, A.R. Cooper, J.H. Heasley, Heat Capacity of Three Inorganic Glasses and Liquids and Supercooled Liquids, Phys. Chem. Glass, 9 (1968) 47–51, quoted in Hattori et al., 1970.
U.E. Schnaus, C.T. Moynihan, R.W. Gammon, P.B. Macedo, Heat Capacities of glassy and liquid As2S3 and As2Se3, Catholic Univ. of America Washington, 1969.
G.D. Mironova, A.V. Zotov, and N.I. Gul’ko, Determination of the Solubility of Orpiment in Acid Solutions at 25–150° C, Geochem. Int., 1984, 21, p 53–59.
G.W.C. Kaye, T.H. Laby, Tables of Physical and Chemical Constants (in Russian), Fizmatgiz1962.
M.K. Karapet'yants, M.L. Karapet'yants, Fundamental Thermodynamic Constants of Inorganic Matter (in Russian), Khimiya1962.
A. Kubaschewski, and C.B. Alcock, Metallurgical Thermochemistry, 5th edn. Elsevier, New York, 1979.
L.E. Eary, The solubility of amorphous As2S3 from 25 to 90 C, Geochim. Cosmochim. Acta, 1992, 56, p 2267–2280.
C.-S. Lu, and J. Donohue, An Electron Diffraction Investigation of Sulfur Nitride, Arsenic Disulfide (Realgar), Arsenic Trisulfide (Orpiment) and Sulfur, J. Am. Chem. Soc., 1944, 66, p 818–827.
A. Rogstad, High-Temperature Laser Raman Spectroscopic Study on Mixtures of Arsenic and Sulphur Vapours, J. Mol. Struct., 1972, 14, p 421–426.
M. Janai, P.S. Rudman, and A. Mandelbaum, Mass spectrometric analysis of arsenic trisulfide, J. Non-Cryst. Solids, 1978, 27, p 67–73.
V.S. Ban, and B.E. Knox, Mass-Spectrometric Study of the Laser-Induced Vaporization of Compounds of Arsenic and Antimony with the Elements of Group VIa, J. Chem. Phys., 1970, 52, p 248–253.
F.M. Faure, M.J. Mitchell, and R.W. Bartlett, Thermodynamics of Vaporization of Sulfides of Antomony and Arsenic, High Temp. Sci., 1973, 5, p 128.
R.D. Brittain, K.H. Lau, R.H. Lamoreaux, D.L. Hildenbrand, Studies of the Vaporisation Chemistry of Arsenic in Sulfide Smelting Phase Advances in Sulfide Smelting, 1983, pp. 197–215.
N. Chakraborti, and D.C. Lynch, Thermodynamics of roasting arsenopyrite, Metall. Trans. B, 1983, 14, p 239–251.
S.A. Pashinkin, A.D. Molodyk, V.I. Belousov, S.S. Strel’chenko, and V.A. Fedorov, Composition of Arsenic(III) Sulfide Vapor, Izv. Akad. Nauk SSSR Neorg. Mater., 1974, 10, p 1600.
B. Brunetti, V. Piacente, and P. Scardala, Torsion Measurement of Orpiment Vapor Pressure, J. Chem. Eng. Data, 2007, 52, p 1343–1346.
C. Hsiao, and A. Schlechten, Volatility and Stability of Metallic Sulphides, J. Miner. Met. Mater. Soc., 1952, 4, p 65–69.
R.A. Isakova, and V.N. Nesterov, Vapor Pressure of Arsenic and Thallium Sulfides, Trudy Institua Metallurgii i Obogashcheniya, Akademiya Nauk Kazakhskoi SSR, 1962, 5, p 29–33.
R.A. Isakova, Vapor Pressure and Dissociation of Metal Sulfides. Nauka, Almaty, 1968.
G.P. Ustyugov, A.A. Kudryavtsev, and B.M. Kuadzhe, Saturated Vapor Pressure of Arsenic Sulfides, Izv. Akad. Nauk. SSSR Neorganicheskiye Mater., 1968, 4, p 1338–1339.
A.V. Novoselova, and A.S. Pashinkin, Vapor Pressure of Volatile Metal Chalcogenides. Nauka, Moscow, 1978.
G.G. Gospodinov, and A.S. Pashinkin, Saturated Vapor Pressure of Solid Arsenous Sulfide, Inorg. Mater. USSR, 1974, 10, p 1777–1778.
K. Henke, and A. Hutchison, Arsenic: Environmental Chemistry, Health Threats and Waste Treatment. Wiley, New York, 2009.
E.J. Porter, and G.M. Sheldrick, Crystal Structure of a New Crystalline Modification of Tetra-Arsenic Tetrasulphide (2, 4, 6, 8-Tetrathia-1, 3, 5, 7-Tetra-Arsatricyclo [3, 3, 0, 0 3, 7]-Octane), J. Chem. Soc. Dalton Trans., 1972, 13, p 1347–1349.
G.W. Roland, Concerning the Alpha-AsS<–> Realgar Inversion, Can. Mineral., 1972, 11, p 520–525.
L. Bindi, G. Pratesi, M. Muniz-Miranda, M. Zoppi, L. Chelazzi, G.O. Lepore, and S. Menchetti, From Ancient Pigments to Modern Optoelectronic Applications of Arsenic Sulfides: Bonazziite, the Natural Analogue of β-As4S4 from Khaidarkan deposit, Kyrgyzstan, Miner. Mag., 2015, 79, p 121–131.
A.A. Godovikov, Z.N. Fedorova, and V.V. Gurov, Study of Volatile Components in Systems Containing Sulfur and Arsenic, Akad. Nauk SSSR Sib. Otd. Inst. Geol. Geofiz, 1981, 45, p 26–32.
G.W. Roland, Phase Relations and Geological Application of the System Ag-As-S. Unpubl., Lehigh University, 1966, p. 191.
L.G. Berry, R.M. Thompson, X-ray powder data for ore minerals: The Peacok Atlas, Geological Society of America, 1962.
G. Street, and Z. Munir, The Structure and Thermal Properties of Synthetic Realgar (As4S4), J. Inorg. Nucl. Chem., 1970, 32, p 3769–3774.
Y.I. Gerasimov, A.N. Krestovnikov, Chemical Thermodynamics in Non-ferrous Metallurgy, Dep. Sci. Tech. Inf., 1934.
T.K. Chattopadhyay, E. Gmelin, and H.G.V. Schnering, Heat Capacity Study of the Phase Transitions in As4S3 and As4S4, Phys. Status Solidi (a), 1983, 76, p 543–551.
A.A. Godovikov, Z.N. Fedorova, V.V. Gurov, Study of Volatile Components in Systems Containing Sulfur and Arsenic, Akad. Nauk SSSR, Sib. Otd., Inst. Geol. Geofiz., (1981) 26–32.
A.D. Mah, Thermodynamic Data for Arsenic Sulfide Reactions Albany Research Center, Bureau of Mines, Albany, Oreg. (1982).
A.V. Steblevskii, A.S. Alikhanyan, V.I. Gorgoraki, and A.S. Pashinkin, Vapor Formation in the Arsenic-Selenium System, Zh. Neorg. Khim., 1986, 31, p 834–837.
W. Weller, K. Kelley, Low Temperature Heat Capacities and Entropies at 298.15 K of Sulfides of Arsenic, Germanium and Nickel, in: B.o.M. U.S. Department of the Interieur (Ed.), 1964.
A. Emelina, A. Alikhanian, A. Steblevskii, and E. Kolosov, Phase Diagram of the As-S System, Inorg. Mater., 2007, 43, p 95–104.
E. Szarvasy, and C. Messinger, Ueber die Molekulargrösse der Arsenamphid-Verbindungen, Chem. Ber., 1897, 30, p 1343–1347.
K. Castro, E. Balladares, O. Jerez, M. Pérez-Tello, and Á. Aracena, 457, Behavior of As/AsxSy in Neutral and Oxidizing Atmospheres at High Temperatures—An Overview, Metals, 2022, 12, p 457.
Z.A. Munir, G.B. Street, and H.F. Winters, Mass-Spectrometric and Vapor Pressure Studies on the Sublimation of Realgar (As4S4), J. Chem. Phys., 1971, 55, p 4520–4527.
B.A. Strathdee, and L.M. Pidgeon, Thermal Decomposition and Vpour Pressure Measurements on Arsenopyrite and an Arsenical Ore, Can. Min. Metall. Bull., 1961, 64, p 883–887.
A.S. Pashinkin, A.A. Izergin, R.A. Amirov, A.S. Alikhanyab, and A.V. Steblevskii, Saturarted Vapor Pressure and Heat of Formation in Arsenic Sulfide (As4S4), Izvestiya Akademii Nauk SSSR Neorganicheskie Materialy, 1982, 18, p 1253–1256.
H.J. Whitfield, Crystal structure of tetraarsenic trisulfide, J. Chem. Soc. A (1970) 1800–1803.
H.J. Whitfield, Crystal structure of the b-form of tetraarsenic trisulfide, J. Chem. Soc. Dalton Trans. (1973) 1737–1738.
A. Gavezzotti, F. Demartin, C. Castellano, and I. Campostrini, Polymorphism of As4S3 (Tris-(μ2-sulfido)-tetra-arsenic): Accurate Structure Refinement on Natural α-and β-Dimorphites and Inferred Room Temperature Thermodynamic Properties, Phys. Chem. Miner., 2013, 40, p 175–182.
T. Chattopadhyay, C. Carlone, A. Jayaraman, and H. Schnering, Effect of Temperature and Pressure on the Raman Spectrum of As4S3, J. Phys. Chem. Solids, 1982, 43, p 277–284.
A. Pashinkin, V. Zharov, M. Sotnikova, and E. Zhukov, Pressure of Arsenic-Sulfide As4S3 Vapor, Zh. Neorg. Khim., 1987, 32, p 1701–1704.
S.M. Isabaev, A.S. Pashinkin, E.G. Milke, M.I. Zhambekov, Physical and chemical principles of sulfidation of arsenic-containing compounds, Alma-Alta, USSR1986.
C.L. Sullivan, Dissociation Energies of New Group Va Chalcogenide Molecules, Case Western Reserve University Cleveland Ohio, 1971, pp. 144.
M. Elli, and E.E. Giudici, Hydrothermal Processes VII: Synthesis of Silver-Sulfur-Arsenic Compounds (in Italian), Chim. Ind., 1966, 48, p 126–131.
F.H. Wehmeier, R.A. Laudise, and J.W. Shiever, The System Ag2S-As2S3 and the Growth of Crystals of Proustite, Smithite and Pyrargyrite, Mat. Res. Bull., 1968, 3, p 767–777.
I.S. Kovaleva, L.D. Popova, N.P. Luzhnaya, V.V. Sukhankina, and L.I. Antonova, Reactions in the System Ag-As-S in the Region of Crystallization of Ag3AsS3 and AgAsS2, Inorg. Mater. (Engl. Transl.), 1971, 7, p 1340–1344.
R. Blachnik, and U. Wickel, Phase Relations in the System Ag-As-S and Thermal Behaviour of Ag7MX6 Compounds, Zeitschrift für Naturforschung B, 1980, 35, p 1268–1271.
G.W. Roland, Phase Relations Below 575 degrees C in the System Ag-As-S, Econ. Geol., 1970, 65, p 241–252.
G.W. Roland, and R.G. Seidensticker, A Coprecipitation Mechanism to Explain Ag7AsS6 Inclusions in Proustite (Ag3AsS3) Crystals, J. Cryst. Growth, 1971, 10, p 213–217.
G. Petzow, and G. Effenberg, Ternary Alloys. A Comprehensive Compendium of Evaluated Constitutional Data and Phase Diagrams, Vol. 1. VCH, Weinheim, 1988.
B. Gather, and R. Blachnik, Temperature-Composition Diagrams in the Ag2(VIb)-(Vb) Sections of the Ternary Ag-(Vb)-(VIb) Systems, J. Less-Common Met., 1978, 58, p 7–12.
I.S. Chaus, N.M. Kompanichenko, V.G. Andreichenko, and A.G. Grishchuk, The Ag3AsS3-Ag System, Izv. Akad. Nauk SSSR Neorg. Mater., 1981, 17, p 2146–2149.
A.M. Gaudin, and D.W. McGlashan, Sulphide Silver Minerals; A Contribution to Their Pyrosynthesis and to Their Identification by Selective Iridescent Filming, Econ. Geol., 1938, 33, p 143–193.
H. Sommerlad, On Attempts to Prepare Sulphantimonites and Sulfarsenites of Silver, Copper and Lead by Dry Means, Z. Anorg. Chem., 1898, 18, p 420–447.
Z.T. Gasanova, L.F. Mashadieva, V.P. Zlomanov, and M.B. Babanly, Thermodynamic Study of the Ag2S-As2S3-S System by EMF Measurements with Ag4RbI5 as a Solid Electrolyte, Inorg. Mater., 2014, 50, p 6–9.
V.E. Gorbunov, K.S. Gavrichev, V.L. Zalukaev, and M.L. Golovei, Heat Capacity and Thermodynamic Properties of Proustite, Ag3AsS3, in the 10–350 K range, Zh. Fiz. Khim., 1982, 56, p 1121–1124.
V.M. Gurevich, V.E. Gorbunov, K.S. Gavrichev, and I.L. Khodakovsky, Low-Temperature Heat-Capacity of Proustite Ag3AsS3 and Smithite AgAsS2, Geokhimiya, 1989, 1, p 132–139.
A.A. Godovikov, N.A. Il'yasheva, R.G. Kuryayeva, S.N. Nenasheva, Z.N. Fedorova, Investigation of Dry Sulfide systems (in Russian), Novosibirsk, 1975.
Z.N. Fedorova, V.V. Gurov, R.G. Kuryaeva, and I.V. Sinyakov, Dissociation Pressures of Solid Proustite ans Smithite, Izv. Akad. Nauk SSSR Neorg. Mater., 1977, 13, p 2220–2223.
B. Lange, F. Scholz, H. Bautsch, F. Damaschun, and G. Wappler, Thermodynamics of the Xanthoconite-Proustite and Pyrostilpnite-Pyrargyrite Phase Transition as Determined by Abrasive Stripping Voltammetry, Phys. Chem. Miner., 1993, 19, p 486–491.
J. Wernick, New Semiconducting Ternary Compounds, J. Phys. Chem. Solids, 1957, 3, p 157–159.
J.H. Wernick, S. Geller, and K.E. Benson, Synthetic Proustite, Ag3AsS3, Anal. Chem., 1958, 30, p 303.
M.M. Faktor, R. Hanks, and T.H. Lemon, Thermal Analysis of Proustite, J. Inorg. Nucl. Chem., 1968, 30, p 2077–2080.
M.I. Golovei, G.N. Shpyrko, and M.I. Gurzan, Enthalpy of Melting of Ag3AsS3 and Ag3SbS3, Inorg. Mater. (Engl. Trans.), 1974, 10, p 307.
N.A. Il’yasheva, and B.G. Nenashev, Heat of Melting of Proustite (Ag3AsS3), Inorg. Mater. (Engl. Trans.), 1977, 13, p 888–889.
Z.N. Fedorova, V.V. Gurov, and B.G. Nenashev, Determination of the Saturated Vapor Pressure over AgAsS2 Melt, Izv. Akad. Nauk SSSR Neorg. Mater., 1985, 21, p 14–16.
R. Blachnik, and B. Gather, Enthalpies of Melting of Some Ternary ABX2-Compounds (A =Cu, Ag, B = As, Sb, Bi, X = S, Se, Te) (in German), Z. Naturforsch. B, 1972, B27, p 1417–1418.
The Open Quantum Materials Database. https://oqmd.org/. Accessed 22 December 2022.
The Materials Project. https://materialsproject.org/. Accessed 26 December 2022.
J.O. Andersson, T. Helander, L. Höglund, P. Shi, and B. Sundman, Thermo-Calc & DICTRA, Computational Tools for Materials Science, Calphad, 2002, 26, p 273–312.
G.V. Belov, G.F. Voronin, and A.L. Emelina, Convex Envelope Algorithm for Phase Diagram Calculations and Its Software Implementation, Mat. Model, 2006, 18, p 67–78.
F. Liu, Q. Xu, H. Liang, H. Wan, C. Zhong, X. Min, and L. Zhang, Stabilization Mechanism of Arsenic-Sulfide Slag by Density Functional Theory Calculation of Arsenic-Sulfide Clusters, J. Hazard. Mater., 2021, 410, p 124567.
S. Tsuchihashi, and Y. Kawamoto, Properties and Structure of Glasses in the System As-S, J. Non-Cryst. Solids, 1971, 5, p 286–305.
A.T. Dinsdale, SGTE Data for Pure Elements, Calphad, 1991, 15, p 317–425.
D.R. Stull, and H. Prophet, JANAF Thermochemical Tables. U.S. Department of Commerce, Washington, 1985.
S.A. Degterov, A.D. Pelton, and J.D. L’Ecuyer, Thermodynamic Optimization of the Selenium-Arsenic (Se-As) System, J. Phase Equilib., 1997, 18, p 357–368.
I. Barin, Thermochemical Data of Pure Substances, 3rd edn. VCH Verlag GmbH, Weinheim, 1995.
I. Barin, O. Knacke, and O. Kubaschewski, Thermochemical Properties of Inorganic Substances. Springer, Germany, 1977.
D.D. Wagman, W.H. Evans, I. Halow, V.B. Parker, S.M. Bailey, R.H. Schumm, Selected Values of Chemical Thermodynamic Properties, National Bureau of Standards, U.S. Department of Commerce, Washington, 1968–1971.
A.D. Pelton, A general “Geometric” Thermodynamic Model for Multicomponent Solutions, Calphad, 2001, 25, p 319–328.
R. Sadanaga, and S. Sueno, X-ray Study on the α-β Transition of Ag2S, Mineral. J., 1967, 5, p 124–143.
S. Banerjee, S. Bhattacharya, and D. Chakravorty, Resistivity Hysteresis of Ag2S Nanocomposites, J. Phys. Chem. C, 2007, 111, p 13410–13413.
A.I. Gusev, and S.I. Sadovnikov, Acanthite–argentite transformation in nanocrystalline silver sulfide and the Ag2S/Ag nanoheterostructure, Semiconductors, 2016, 50, p 682–687.
P. Waldner, Gibbs Energy Modeling of Digenite and Adjacent Solid-State Phases, Metall. Mater. Trans. B, 2017, 48, p 2157–2166.
R. Schmid-Fetzer, V. Tomashik, A. Watson, O. Bodak, W. Chong, in Ternary Alloy Systems: Phase Diagrams, Crystallographic and Thermodynamic Data critically evaluated by MSIT Non-Ferrous Metal Systems. Part 1, Springer, Berlin, 2006.
D.V. Malakhov, and T. Balakumar, Undulate Phase Boundaries on Binary T–x Diagrams, Calphad, 2008, 32, p 89–93.
R.P. Elliott, Constitution of Binary Alloys, First Supplement. McGraw-Hill, New York, 1965.
M.R. Baren, The Ag-As (Silver-Arsenic) System, Bull. Alloy Phase Diag., 1990, 11, p 113–116.
K.K. Kelley, G.S. Parks, and H.M. Huffman, A New Method for Extrapolating Specific Heat Curves of Organic Compounds below the Temperatures of Liquid Air, J. Phys. Chem., 1929, 33, p 1802–1805.
I. Barin, Thermochemical Data of Pure Substances. VCH Publishers, Weinheim, 1989.
Z.K. Liu, Y. Wang, S.L. Shang, Zentropy Theory for Positive and Negative Thermal Expansion, J. Phase Equilib. Diffus. (2022) 1–8.
A.T. Ward, Raman Spectroscopy of Sulfur, Sulfur-Selenium, and Sulfur-Arsenic Mixtures, J. Phys. Chem., 1968, 72, p 4133–4139.
F.C. Lin, and S.M. Ho, Chemical Durability of Arsenic-Sulfur-Iodine Glasses, J. Am. Ceram. Soc., 1963, 46, p 24–28.
M.J. Stashick, and R.A. Marriott, Viscoelastic Behavior Corresponding to Reptative Relaxation Times Across the λ-Transition for Liquid Elemental Sulfur, J. Chem. Phys., 2020, 152, 044503.
N.I. Kopylov, Y.D. Kaminsky, G.A. Tolstikov, Arsenic (in Russian), Siberia, 2004.
A.V. Steblevskii, A.S. Alikhanyan, A.S. Pashinkin, and V.A. Malyusov, Vapor Composition in the Arsenic-Sulfur System, Zh. Neorg. Khim., 1986, 31, p 2451–2456.
Acknowledgments
The authors would like to thank the financial support from NSERC and CRC programs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kidari, O., Chartrand, P. Thermodynamic Evaluation and Optimization of the Ag-As-S system. J. Phase Equilib. Diffus. 44, 269–299 (2023). https://doi.org/10.1007/s11669-023-01040-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-023-01040-4