Abstract
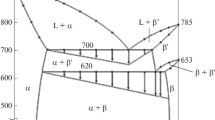
The quasi-binary section AgGaS2–PbS in the ternary system Ag2S–Ga2S3–PbS was studied by physicochemical analysis methods (differential thermal, X-ray powder diffraction, and metallographic analyses). The state diagram of the system was constructed, and the quaternary compound AgPb2GaS4 was detected to form by a peritectic reaction at 1225 K. Its formation conditions were identified, and its physicochemical properties were investigated. The compound AgPb2GaS4 was determined to crystallize in the orthorhombic system with the unit cell parameters a = 8.20 Å, b = 6.84 Å, c = 6.62 Å, and space group Pmn21. From the physicochemical analysis data, the existence of narrow ranges of solid solutions based on the initial components was determined.


Similar content being viewed by others
REFERENCES
Zhao Beijun, Zhu Shifu, Li Zhenghui, et al., Chin. Sci. Bull. 46, 2009 (2001).
D. S. Chemla, P. J. Kapacek, D. S. Robertson, et al., Opt. Commun. 3, 29 (1991).
J. P. Noblanc, J. Loudette, G. Duraffourg, et al., Appl. Phys. Lett. 20, 257 (1972).
N. A. Goryunova, Complex Diamondlike Semiconductors (Sovetskoe radio, Moscow, 1968) [in Russian].
N. Kh. Abrikosov and L. E. Shelimova, IV–VI Semiconductor Materials (Nauka, Moscow, 1975) [in Russian].
A. K. Kushwaha, R. Khenata, A. Bouhemadou, et al., J. Electron. Mater. 46, 4109 (2017). https://doi.org/10.1007/s11664-017-5290-6
Uematsu Taro, Doi Toshihiro, Torimoto Tsukasa, et al., J. Phys. Chem. Lett. 1, 3283 (2010). https://doi.org/10.1021/jz101295w
H. Karaagac and M. Parlak, J. Solid Films 519, 2055 (2011). https://doi.org/10.1016/j.tsf.2010.10.027
N. Karunagaran and P. Ramasamy, Mater. Sci. Semicond. Process 41, 54 (2016). doi. https://doi.org/10.1016/j.mssp.2015.08.012
Baojun Chen, Shifu Zhu, Beijun Zhao, et al., J. Cryst. Growth 310, 635 (2008). https://doi.org/10.1016/j.jcrysgro.2007.10.067
E. F. Sinyakova, V. I. Kosyakov, and K. A. Kokh, Inorg. Mater. 45, 1217 (2009). https://doi.org/10.1134/S002016850911004112
S. I. Chykhrij, O. V. Parasyuk, and V. O. Halka, J. Alloys Compd. 312, 189 (2000). https://doi.org/10.1016/ S0925-8388(00)01145-213
N. A. Rudnev, I. V. Melikhov, and A. M. Tuzova, Zh. Anal. Khim. 28, 635 (1973).
I. D. Olekseyuk, O. V. Parasyuk, V. O. Halka, et al., J. Alloys Compd. 325, 167 (2001). https://doi.org/10.1016/S0925-8388(01)01361-5
N. B. Singh, R. H. Hopkins, and J. D. Feichiner, J. Mater. Sci. 21, 837 (1986).
C. C. Cary and B. David, MRS Bull., No. 7, 28 (1998).
B. Y. Zhaq, S. F. Zhu, and F. L. Yu, Cryst. Res. Technol. 33, 943 (1998).
Xiu Zhiliang, Liu Suwen, Jiaoxian Yu, et al., J. Alloys Compd. 457, L9 (2008). https://doi.org/10.1016/j.jallcom.2007.03.060
O. I. Vlasenko, S. M. Levitskii, and Ts. A. Kriokov, Fiz. Khim. Tverd. Tela 7, 660 (2006).
G. Brand and V. Kramer, Mater. Res. Bull., No. 11, 1381 (1976).
V. B. Lazarev, Z. Z. Kish, E. Yu. Peresh, et al., Complex Chalcogenides in the A I –B III –C VI System (Metallurgiya, Moscow, 1993) [in Russian].
Ya. A. Ugai, Introduction to the Chemistry of Semiconductors (Vysshaya shkola, Moscow, 1975) [in Russian].
T. N. Komarova, Izv. Fiz.-Khim. Nauchno-Issled. Inst. Irkutsk. Gos. Univ. 27, 23 (1986).
G. V. Samsonov and S. V. Drozdova, Sulfides (Metallurgiya, Moscow, 1972) [in Russian].
M. I. Zargarova, A. N. Mamedov, D. S. Azhdarova, et al., Inorganic Substances Synthesized and Studied in Azerbaijan (Elm, Baku, 2004) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Jahangirova, S.K., Mammadov, S.H., Ajdarova, D.S. et al. Investigation of the AgGaS2–PbS and Some Properties of Phases of Variable Composition. Russ. J. Inorg. Chem. 64, 1169–1171 (2019). https://doi.org/10.1134/S0036023619090092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619090092