Abstract
Phase equilibria in the TiO2–SiO2 system have been studied experimentally using DTA and SEM/EDX. The thermodynamic parameters for the TiO2–SiO2 system have been assessed considering new experimental data of the present work and from the literature. Moreover, the miscibility of the liquid in the Al2O3–TiO2–SiO2 system has been studied at 2013 K in air and shrinkage of the miscibility gap at 3.8 mol% Al2O3 has been demonstrated. The experimental data obtained in the present work and literature as well as the thermodynamic databases for the binary Al2O3–TiO2, TiO2–SiO2, and Al2O3–SiO2 systems have been used to derive the thermodynamic description of the Al2O3–TiO2–SiO2 system using the CALPHAD approach. Solid phases have been modeled using the compound energy formalism. The liquid phase has been described by the two-sublattice partially ionic liquid model. A set of self-consistent parameters have been proposed, which resulted in a reasonably good agreement between the calculated and experimental data on the phase equilibria, liquid immiscibility, and thermodynamic properties in the Al2O3–TiO2–SiO2 system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Alumina-based materials are widely used as refractory and filter materials in metal production. Research and development of ceramic filter materials for metal melt filtration need availability of thermodynamic databases to model the interactions of a liquid metal alloy containing oxide inclusions with a filter that can be coated with various ceramic coatings. Moreover, possible interactions of ceramic filters with coatings can be modeled to improve efficiency of the complex ceramic filter system. In practice, Al2O3-based filter systems are currently being developed for aluminum-based melt filtration.[1,2] Oxide films and Al2O3 particles formed during melting and alloying can be present as a suspension on the surface of the melt or inside the melt because of flow turbulence. Spinel inclusions appear due to the presence of magnesium ions in the aluminum melt, mainly coming from the refractories of the furnace. Magnesium entering the molten aluminum also leads to the formation of non-metallic inclusions other than spinel. TiO2 coating deposited on corundum is supposed to filter actively spinel MgAl2O4 from Al-based molten alloy. The reduction of TiO2 can then lead to the formation of Al3Ti or (Al,Si)3Ti.[3] Therefore, to model Al melt filtration process, one should consider the Al–Mg–Ti–Si–O system. As a part of the mentioned complex system, the Al2O3–TiO2–SiO2 sub-system was recently investigated experimentally.[4] Being a continuation of the study of the oxide system, the aim of the present work is a critical evaluation of available data on phase relations as well as thermodynamic modeling of the ternary system and assessment of thermodynamic parameters in the Al2O3–TiO2–SiO2 system to derive a self-consistent thermodynamic database using the CALPHAD approach by applying the Thermo-Calc software.[5]
2 Literature Review
2.1 Binary Al2O3–TiO2 System
The Al2O3–TiO2 system is characterized by the formation of an intermediate compound Al2TiO5, also known as tialite, which has a pseudobrookite-type Fe2TiO5 structure with the lattice parameters of a = 0.3605, b = 0.9445, and c = 0.9653 nm.[6] According to the authors own experimental data,[7] the Al2TiO5 phase forms by the peritectic reaction L + Al2O3 ↔ Al2TiO5 at 2112 K and decomposes into Al2O3 and TiO2 at temperature of 1552 K, and also participates in a eutectic reaction L ↔ TiO2 + Al2TiO5 at 1999 K and 14.9 mol% of alumina. Al2TiO5 is nearly stoichiometric compound with a negligible homogeneity range. The mutual solubility of Al2O3 and TiO2 is negligible too. A recent thermodynamic assessment of the Al2O3–TiO2 system[8] was accepted in the present work. The calculated phase diagram agrees well with the experimental data.[7] Considering the disordering of Al+3 and Ti+4 cations between the octahedral sites M1 and M2, the Al2TiO5 phase was modeled using the model (Al+3,Ti+4)1M1(Al+3,Ti+4)2M2(O−2)5.
2.2 Binary Al2O3–SiO2 System
Mullite Al6Si2O13 is the only stable crystalline phase in the Al2O3–SiO2 system at atmospheric pressure. The character of mullite melting as congruent or peritectic is under discussion in the literature. However, the data from Fabrichnaya et al.[9] showing the peritectic reaction L + Al2O3 ↔ Al6Si2O13 at 2166 K and a eutectic reaction L + SiO2 ↔ Al6Si2O13 at 1855 K and 97.64 mol% of SiO2 were accepted in this work. The compound Al6Si2O13 has a mullite-type structure with a = 0.7452, b = 0.7581, and c = 0.2851 nm.[10] The solid solution homogeneity range of mullite bends towards higher Al2O3 contents with temperature increase. The mutual solubility of Al2O3 and SiO2 is negligible. The thermodynamic assessment of the Al2O3–SiO2 system presented by Dumitrescu and Sundman[11] was accepted in the present work after verification by Fabrichnaya et al.[9]
2.3 Binary TiO2–SiO2 System
First studies[12,13,14] were carried out in a reducing atmosphere, which caused the formation of Ti2O3 and Ti3O5 in addition to TiO2. Nevertheless, Umezu and Kakiuchi[12] detected the formation of immiscible liquids in melts (35.7–75.1 mol% TiO2 at 1853 K) and determined a eutectic reaction at 15.0 mol% TiO2 and 1773 K. In air, Bunting[15] observed the eutectic at 8.0 mol% TiO2 and 1813 K and did not report liquid immiscibility since the study was limited to 15.8 mol% TiO2. Rickers and Hummel[16] reported a solid solution of (SiO2 + TiO2) and relocated the eutectic to 17.0 mol% TiO2 and 1813 K. Agamawi and White[17] confirmed the eutectic at 8.0 mol% TiO2[15] but at 1843 K. Later, DeVries et al.[18] specified the eutectic point (8.1 mol% TiO2 and 1823 ± 4 K) previously determined by Bunting,[15] and also confirmed the presence of stable immiscibility in the liquid phase extending from 15.0 to 90.8 mol% TiO2 at 2053 K. McTaggart and Andrews[19] reported the presence of two liquids existing between 15.9 and 92.2 mol% TiO2 above 2038 K. The presence of the two-liquid region was subsequently confirmed,[20,21] but without experimental details. Kirschen et al.[22] and Kirillova et al.[23] carried out the high temperature investigations (up to 2533 K), however contradictory data on the boundaries of the miscibility gap were reported. Despite a number of studies of the phase equilibria in the TiO2–SiO2 system, it was concluded that there are still several inconsistencies in the description of this system, and a new experimental study is necessary.
Taking into account the miscibility gap data, the TiO2–SiO2 system was first assessed by Kaufman[24] using the CALPHAD method. Next thermodynamic assessments were performed by DeCapitani and Kirschen[25] and Kirschen et al.[22] using the Margules-type excess polynomial model and assuming the interaction parameters to be either temperature independent or dependent. An optimized phase diagram using a sub-regular solution model was recently proposed.[23] Based on their own experimental data, the critical point of the miscibility gap (2416 K at 58.9 mol% TiO2) was calculated as the lowest in comparison with other calculations.[22,24,25] Since these assessments are incompatible with the ionic two-sublattice liquid model, a new thermodynamic description of the system needs to be developed. Using all up-to-date experimental data, the TiO2–SiO2 system was newly assessed by Boulay et al.[26] using the partially ionic model to describe the liquid phase. However, the Ti+4 cations introduced on the first sublattice were only considered in[26] rather than the mixing of Ti+2 and Ti+3 on the cationic sublattice and Ti+4 as a neutral TiO2 species on the anionic sublattice as accepted in the present work regarding the Ti–O system.[7,27] Therefore, their assessment[26] is not accepted in the present work.
2.4 Ternary Al2O3–TiO2–SiO2 System
A literature review and an extensive experimental study of solid–liquid phase equilibria in the Al2O3–TiO2–SiO2 system in air were carried out by the present authors.[4] The phase equilibria in the Al2O3–TiO2–SiO2 system were investigated in the temperature range from 1486 K to melting by energy-dispersive X-ray spectroscopy (EDX) and X-ray diffraction (XRD) to confirm phase assemblages in the annealed samples. The solid-state invariant reaction SiO2 + Al2TiO5 ↔ Al6Si2O13 + TiO2 was found at 1743 K. On the liquidus projection, three invariant reactions were observed: eutectic L ↔ TiO2 + SiO2 + Al2TiO5 at 1750 K and transitional type reactions L + Al6Si2O13 ↔ SiO2 + Al2TiO5 at 1779 K and L + Al2O3 ↔ Al6Si2O13 + Al2TiO5 at 2007 K. No ternary phases were observed in the system.[4] Data on the crystal structures of all solid phases in the Al2O3–TiO2–SiO2 system are given in Table 1. Experimental investigation of the miscibility gap extending into the ternary system from the TiO2–SiO2 side was carried out by Kirschen et al.[22] A substantial contraction of the miscibility gap was found at an Al2O3 content of 4.0 mol% at 2073 K and a single-phase liquid at 10 mol% above 1970 K.
The thermodynamic description of the Al2O3–TiO2–SiO2 system was first undertaken by Kaufman[24] using the CALPHAD approach. The calculated isothermal sections in the temperature range of 1000–2800 K indicated extended miscibility gap in the liquid (up to about 25% Al2O3). Kaufman also indicated the tie-lines change at low temperatures resulting in a reaction at 1707 K: Al6Si2O13 + TiO2 ↔ SiO2 + Al2TiO5. Wherein, three-phase equilibria (SiO2 + TiO2 + Al6Si2O13) and (Al6Si2O13 + TiO2 + Al2TiO5) are stable above 1707 K, and (SiO2 + Al6Si2O13 + Al2TiO5) and (SiO2 + Al2TiO5 + TiO2) are stable below this temperature. However, these data contradict the experimental ones[4,33] at which the opposite stable equilibria were found above and below the reaction temperature. The liquidus projection of the Al2O3–TiO2–SiO2 system was also calculated[22] with emphasis on the immiscibility in the liquid using the thermodynamic description derived on the basis of their own experimental data.
3 Experimental Part
Two samples of the TiO2–SiO2 system were prepared by solid-state reaction from high-purity oxide powders (TiO2 99.990% and SiO2 99.995%, ~ 40 mesh, Alfa Aesar, Germany). Three samples of the Al2O3–TiO2–SiO2 system were prepared by co-hydrolysis followed by thermal decomposition of aqueous solutions.[33] The initial solutions were aluminium tri-sec-butoxide C12H27AlO3 (M = 246.32 g mol–1, ρ = 0.967 g cm–3, 97%), titanium(IV) isopropoxide C12H28TiO4 (M = 284.23 g mol–1, ρ = 0.955 g cm–3, 97%), and tetraethoxysilane C8H20SiO4 (M = 208.33 g mol–1, ρ = 0.934 g cm–3, 98%) produced by Alfa Aesar, Germany. Details of the sample preparation routine can be found elsewhere[4,33] The samples were heat treated at targeted temperatures in a muffle furnace (NABERTHERM, Germany) in order to achieve a pseudo-equilibrium state of the samples followed by furnace-cooling, and then examined.
X-ray diffraction study was carried out using an URD63 X-ray diffractometer (Seifert, FPM, Freiberg, Germany) with CuKα radiation (λ = 1.5418 Å). ICSD (Inorganic Crystal Structure Database, 2017, Karlsruhe, Germany)[34] was used for interpretation of the powder diffraction patterns. Qualitative and quantitative analyses of the XRD patterns were performed by Rietveld analysis using MAUD software.[35,36] Microstructure examination was done using LEO 1530 Gemini (Zeiss, Germany) equipped with an EDX detector (Bruker AXS Mikroanalysis GmbH, Germany). DTA measurements were performed on (i) TG-DTA SETSYS Evolution-1750 (SETARAM Instrumentation, France) in air using B-type tri-couple DTA rod (Pt/PtRh10% thermocouple) and open Pt crucibles and (ii) TG-DTA SETSYS Evolution-2400 (SETARAM Instrumentation, France) using tungsten DTA rod and open W crucibles and employing a permanent inert He flow. The heating and cooling curves were recorded at the rates of 10 and 30 K min-1, respectively. Temperature calibration of SETSYS Evolution-2400 was done using melting points of Al, Al2O3, and temperature of solid-state transformation in LaYO3[37]
4 Thermodynamic Models
4.1 Liquid
The ionic two-sublattice model[38,39] is applied to the Al2O3–TiO2–SiO2 system, using the formula (Al+3,Ti+2,Ti+3)P(O–2,Va,AlO3/2,O,SiO4–4,SiO2,TiO2)Q. P and Q are the number of sites on each sublattice that vary depending on the composition to keep electroneutrality. The same model can be used for both metallic and oxide melts. At a low oxygen content, the model becomes equivalent to the substitutional solution model considering interactions between metallic atoms. The liquid phase in the Al2O3–TiO2 system was reassessed in the previous work[7] using the formula (Al+3,Ti+2,Ti+3)P(O–2,Va,AlO3/2,O,TiO2)Q. Even as phase interactions with the metal were not considered in that study, the species Ti+2, Ti+3, Va, and O originated from the description of the Ti–O system. Since there is a continuous liquid solution in the Ti–O system from Ti to TiO2, vacancies are necessary to have consistent description of this binary system. The description of the liquid phase in the Al2O3–SiO2 system is accepted from the works of Dumitrescu and Sundman[11] and Fabrichnaya et al.[9] They both used a simple associated solution model considering interactions only between neutral species: (AlO3/2,SiO2). For the TiO2–SiO2 system, the ionic two-sublattice model is applied using the formula (Ti+2,Ti+3)P(O–2,Va,SiO4–4,SiO2,TiO2)Q. However, the species SiO4–4 cannot be formed in the TiO2–SiO2 system due to a wide liquid immiscibility, i.e. TiO2 does not participate in either destroying or sharing network of SiO2. In this case, the liquid TiO2 is described by the neutral species TiO2 and the liquid SiO2 by neutral SiO2. Thus, this model can be reduced to the (SiO2,TiO2) substitutional model and the corresponding interactions parameters were only assessed in this work.
The ionic two-sublattice model was developed within the framework of the compound energy formalism (CEF)[40] widely used in CALPHAD assessments. CEF is proposed to describe thermodynamic models of phases with two or more sublattices that exhibit a variation in composition. For a two-sublattice model, the Gibbs energy is defined as
where \({y}_{i}^{s}\) and \({y}_{j}^{t}\) are the mole fractions of constituents i and j on the sublattices s and t, respectively; \({\alpha }_{s}\) is the number of sites on sublattice s per mole of formula unit of a phase. The excess Gibbs energy term associated with the different sublattices is described with Redlich–Kister equation as
The binary interaction parameters \({L}_{i,k}^{s}\) between i and k species on the sublattice s are signified as
Hereinafter, a colon is used to separate species on different sublattices, and a comma is used to separate the species on the same sublattice.
4.2 Mullite
Mullite is a part of the orthorhombic aluminosilicates system of general composition Al2(Al2+2xSi2−2x)O10−x. The structure of mullite (space group: Pbam) can be derived from the closely related but structurally simpler sillimanite (Al2SiO5, with x = 0; space group: Pbnm). In sillimanite, edge-connected AlO6 octahedra form chains running parallel to the crystallographic c-axis and are cross-linked by double chains with alternating AlO4 and SiO4 tetrahedra. In mullite, part of Si+4 is replaced by Al+3, which also leads to the formation of oxygen vacancies. The formation of O vacancies is accompanied by a displacement of the tetrahedral positions next to the bridging O atoms to new distorted tetrahedral positions that form triclusters. Thus, three different Al sites were described in mullite: one octahedral (O), one tetrahedral (T) as in sillimanite, and a distorted tetrahedral (T*) site assigned to Al in the tetrahedral triclusters.[41,42] Considering this, mullite phase was modeled on the basis of a sublattice description within CEF using the formula \({({\mathrm{Al}}^{+3})}_{1}^{O}{({\mathrm{Al}}^{+3})}_{1}^{{T}^{*}}{({\mathrm{Al}}^{+3},{\mathrm{Si}}^{+4})}_{1}^{T}{({\mathrm{O}}^{-2},\mathrm{Va})}_{5}\).[9,11] The description is accepted in this work.
According to Schneider,[43,44,45] Ti+4 is incorporated into the mullite structure at the Al+3 positions in the AlO6 octahedra. The resulting model for mullite in the Al2O3–TiO2–SiO2 system is then \({({\mathrm{Al}}^{+3},{\mathrm{Ti}}^{+4})}_{1}^{O}{({\mathrm{Al}}^{+3})}_{1}^{{T}^{*}}{({\mathrm{Al}}^{+3},{\mathrm{Si}}^{+4})}_{1}^{T}{({\mathrm{O}}^{-2},\mathrm{Va})}_{5}.\)
This model gives a new neutral end-member \({({\mathrm{Ti}}^{+4})}_{1}^{O}{({\mathrm{Al}}^{+3})}_{1}^{{T}^{*}}{({\mathrm{Al}}^{+3})}_{1}^{T}{({\mathrm{O}}^{-2})}_{5}\), a hypothetical Al2TiO5 with the mullite-type structure. The Gibs energy of this end-member is adjusted as
where \({^\circ G}_{\mathrm{CORUND}}\) is the Gibbs energy of corundum Al2O3,[46] \({^\circ G}_{{\mathrm{TiO}}_{2}}\) is the Gibbs energy of rutile TiO2,[27] and \({a}_{1}\) is the optimized parameter.
The remaining three parameters in the mullite system (in bold) are evaluated using the reciprocal reactions (Eqs. (5) to (7)):
All these reactions are assumed to have \(\Delta G=0\).
4.3 Pseudobrookite
Aluminum titanate Al2TiO5 (tialite) has a pseudobrookite structure (space group: Cmcm). For the Al2O3–TiO2 system, the three-sublattice model for pseudobrookite Al2TiO5 was applied[8] and the description is accepted in this work. In brief, the cation disordering in Al2TiO5 was described with the formula \({({\mathrm{Ti}}^{+4},{\mathrm{Al}}^{+3})}_{1}^{\mathrm{M}1}{({\mathrm{Ti}}^{+4},{\mathrm{Al}}^{+3})}_{2}^{\mathrm{M}2}{\mathrm{O}}_{5}.\) In normal pseudobrookite structure, the M1 sites are fully occupied by the Ti+4 cations and the M2 sites by Al+3. The degree of inversion is then determined as occupancy of M1 by Al+3. According to experimental data,[6,47,48] the degree of cation disorder is close to 0.667, which corresponds to complete disorder. In the presence of SiO2, the substitution of the Al+3 cations by Si+4 in Al2TiO4 is expected,[49,50] although no experimental details on the crystal structure were found. Also considering the ionic radii concept (Al+3 = 0.054 nm, Si+4 = 0.041 nm, and Ti+4 = 0.061 nm), the structural incorporation of Si+4 into both the M1 and M2 sublattices is proposed in this work. Thus, the formula for the pseudobrookite phase is extended accordingly \({({\mathrm{Ti}}^{+4},{\mathrm{Al}}^{+3},{\mathrm{Si}}^{+4})}_{1}^{\mathrm{M}1}{({\mathrm{Ti}}^{+4},{\mathrm{Al}}^{+3},{\mathrm{Si}}^{+4})}_{2}^{\mathrm{M}2}{\mathrm{O}}_{5}.\)
The Gibbs energy of normal pseudobrookite Al2TiO5 is described by the end-member \({({\mathrm{Ti}}^{+4})}_{1}^{\mathrm{M}1}{({\mathrm{Al}}^{+3})}_{2}^{\mathrm{M}2}{\mathrm{O}}_{5}\) (or \({^\circ G}_{{\mathrm{Ti}}^{+4}:{\mathrm{Al}}^{+3}};\) here and further in the discussion of pseudobrookite, the oxygen index is omitted). To model the degree of inversion in Al2TiO5, the end-member \({({\mathrm{Al}}^{+3})}_{1}^{\mathrm{M}1}{({\mathrm{Ti}}^{+4})}_{2}^{\mathrm{M}2}{\mathrm{O}}_{5}\) (or \({^\circ G}_{{\mathrm{Al}}^{+3}:{\mathrm{Ti}}^{+4}}\)) is used. The values for the \({^\circ G}_{{\mathrm{Ti}}^{+4}:{\mathrm{Al}}^{+3}},\) \({^\circ G}_{{\mathrm{Al}}^{+3}:{\mathrm{Ti}}^{+4}},\) \({^\circ G}_{{\mathrm{Al}}^{+3}:{\mathrm{Al}}^{+3}}\) (or \({^\circ G}_{{\mathrm{Al}}_{3}{\mathrm{O}}_{5}}^{\mathrm{tialite}}\)), and \({^\circ G}_{{\mathrm{Ti}}^{+4}:{\mathrm{Ti}}^{+4}}\) (or \({^\circ G}_{{\mathrm{Ti}}_{3}{\mathrm{O}}_{5}}^{\mathrm{tialite}}\)) were taken from the previous assessment.[8]
A new neutral end-member \({({\mathrm{Si}}^{+4})}_{1}^{\mathrm{M}1}{({\mathrm{Al}}^{+3})}_{2}^{\mathrm{M}2}{({\mathrm{O}}^{-2})}_{5},\) a hypothetical Al2SiO5 with the pseudobrookite structure, is found in the pseudobrookite system. To define corresponding parameters, a simplified model \({({\mathrm{Al}}^{+3},{\mathrm{Si}}^{+4})}_{1}^{\mathrm{M}1}{({\mathrm{Al}}^{+3},{\mathrm{Si}}^{+4})}_{2}^{\mathrm{M}2}{\mathrm{O}}_{5}\) can be considered in this case. Similarly to the description of Al2TiO5, the four thermodynamic parameters for new end-members can be expressed only by two independent parameters related to normal and inverse structures, respectively, \({^\circ G}_{{\mathrm{Si}}^{+4}:{\mathrm{Al}}^{+3}}\) and \({^\circ G}_{{\mathrm{Al}}^{+3}:{\mathrm{Si}}^{+4}}:\)
where \({^\circ G}_{\mathrm{ANDAL}}\) is the Gibbs energy of andalusite,[51] \({^\circ G}_{{\mathrm{Al}}_{3}{\mathrm{O}}_{5}}^{\mathrm{tialite}}\) (or \({^\circ G}_{{\mathrm{Al}}^{+3}:{\mathrm{Al}}^{+3}}\)) is the Gibbs energy of a fictive compound Al3O5 with the pseudobrookite structure,[8] and \({a}_{2}\) and \({a}_{3}\) are the optimized parameters.
The fourth parameter \({^\circ G}_{{\mathrm{Si}}^{+4}:{\mathrm{Si}}^{+4}}\) (or \({^\circ G}_{{\mathrm{Si}}_{3}{\mathrm{O}}_{5}}\)) is determined by the reciprocal reaction
where \(\Delta {G}_{1}=0\).
The remining two parameters in pseudobrookite model, \({^\circ G}_{{\mathrm{Ti}}^{+4}:{\mathrm{Si}}^{+4}}\) and \({^\circ G}_{{\mathrm{Si}}^{+4}:{\mathrm{Ti}}^{+4}},\) are related to the mentioned parameters by the following reciprocal reaction
where \(\Delta {G}_{2}\) is the non-zero value optimized in this work (\(\Delta {G}_{2}=\Delta {G}_{3}+\Delta {G}_{4}\)), which could be defined by Eqs. (12) and (13):
4.4 Rutile, Corundum and SiO2-Based Phases
The corundum phase is the Al2O3-rich solid solution, which crystallizes with trigonal symmetry in the space group \(R\overline{3 }c.\) Since no reliable information on any solubility in rutile, corundum, and SiO2-based phases is reported, these phases are described as stoichiometric. The description of corundum is taken from Hallstedt,[46] the description of rutile is from Hampl and Schmid–Fetzer,[27] and the descriptions of Q-SiO2, C-SiO2, and T-SiO2 are all from Hillert et al.[51]
5 Results and Discussion
5.1 The Binary TiO2–SiO2 System
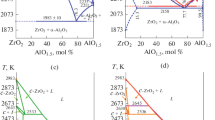
According to DTA measurements up to 1923 K in air, the eutectic temperature in the SiO2-rich side of the TiO2–SiO2 system was determined to be 1818 K (Fig. 1a). The non-intensive and long-drawn-out thermal effect observed for the sample TS-2 can probably be explained by the viscosity of the sample. The temperature obtained in air is in a good agreement with most published data, however this value is more than 60 K higher than that reported by Kirillova et al.[23] although the experimental conditions were similar. DTA measurement of the sample TS-1 in He indicated a decrease in the temperature of eutectic (1798 K; Fig. 1a). In Fig. 2a, SEM/EDX revealed the eutectic composition measured for the sample TS-2 after melting in DTA, which was equal to 8.7 mol% TiO2. The microstructure examination of the sample TS-1 after DTA melting (up to 1923 K) in air did not reveal the eutectic formation, probably due to the glass formation. However, a SiO2 enriched matrix containing 7.0 mol% of TiO2 and practically pure TiO2 grains were observed, which indicates the presence of a two-phase field (L1-SiO2 + TiO2) discussed below. The temperature and composition of the eutectic reaction are summarized along with the literature data in Table 2.
Microstructures of the samples (a) TS-2 after DTA heating up to 1973 K in air; (b) TS-1 after DTA heating up to 2273 K in He, fine TiO2 solids are distributed in SiO2-rich L1; (c) TS-1 after heat treatment at 2013 K in air; (d) ATS-3 after heat treatment at 2013 in air; (e) ATS-7 after heat treatment at 2013 in air; and (f) ATS-8 after heat treatment at 2013 in air
To study liquid immiscibility in the TiO2–SiO2 system, the sample TS-1 was heated up both in air and in an He atmosphere. The sample exhibiting the miscibility gap resulted in the separation of the melt into two liquids: one rich in SiO2 called L1 and the other rich in TiO2 called L2 (including the TiO2 grains and the surrounding matrix). Merely on visual examination, the sample TS-1 heat treated at 2013 K in air followed by furnace-cooling was partially melted, however the temperature of the monotectic reaction L1 ↔ L2 + TiO2 was not reached, revealing the presence of the two-phase field (L1-SiO2 + TiO2). As supporting evidence, no certain L2 spherulites were observed for the sample TS-1 melted in air (Fig. 2c), while the grains of practically pure TiO2 and SiO2-rich L1 were only detected (Table 3). The experiments in air above 2023 K are not possible in the current work. Therefore, the sample TS-1 was also heated up to 2273 K in a He atmosphere using DTA. In this case, the solidified microstructure of the sample is characterized by the appearance of TiO2-rich spheres (L2) embedded in the SiO2-rich matrix (L1) as illustrated in Fig. 2b, which indicates that the monotectic temperature was reached. Considering these data and literature data, this indicates a decrease in temperature of equilibria in the He atmosphere as compared to air. A similar behavior was observed for the MnO-containing system.[52] When compared with the literature data, a decrease in the temperature of a monotectic reaction to 1962 K was found (Fig. 1b; Table 2). DeVries et al.[18] discussed the effect of the atmosphere on the results of phase equilibria in the TiO2–SiO2 system: Umezu and Kakiuchi[12] got substantially lower temperatures of eutectic and monotectic reactions in a strongly reducing atmosphere than DeVries et al. in air. However, the temperature of the monotectic reaction obtained by Kirillova et al.[23] in a protective gas atmosphere (He) was not substantially different from the results[18,19] obtained in air. Possible reasons for the fact that the equilibria temperatures in this work were lower than those reported in the literature could be both the effect of the oxygen partial pressure and the accuracy of the temperature measurement. Since faster diffusion occurs in the liquid state than in solid, it can be assumed that the compositions L1 and L2 correspond to the temperature of the monotectic equilibrium. However, the samples were heated to higher temperatures, and therefore the compositions of the two liquids could correspond to the temperatures higher than the monotectic temperature. Thus, there is uncertainty in indicated liquid compositions, since the temperature corresponding to the measured compositions L1 and L2 is not exactly known. On the other hand, the compositions L1 and L2 observed for the samples melted in a He atmosphere may differ from those obtained under air conditions. Since the experimental study of the sample in air at a higher temperature (above 2023 K) was not possible in the current work, the temperature of the monotectic reaction of 2053 K available from the literature[18,23] is accepted. The compositions of the coexisting liquids L1 and L2 measured by EDX are given in Table 3.
5.2 Liquid Immiscibility in the Al2O3–TiO2–SiO2 System
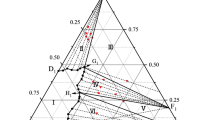
The microstructures of the samples ATS-3, -7, and -8 heat treated at 2013 K in air are shown in Fig. 2. One should keep in mind that the bulk sample compositions differ slightly from the nominal ones, therefore, only the sample compositions measured using EDX are considered (see Table 3). As discussed above, the temperature of the monotectic reaction in the TiO2–SiO2 boundary is about 2053 K. However, a tendency to the separation of the melt into two liquids was observed at 2013 K for the samples ATS-7 and -7a (Fig. 2e): the globular particles enriched in TiO2 for the liquid rich in TiO2 (L2) and the liquid rich in SiO2 (L1) with TiO2-rich inclusions. The samples ATS-7 and -7a contain 2.1-2.2 mol% Al2O3. In contrast, typical dendritic microstructures without separation were observed for the samples ATS-3 and -8 (Fig. 2d, f), which contain a slightly higher amount of Al2O3, 3.8 and 4.9 mol% Al2O3, respectively. This suggests that the miscibility gap extends into the ternary system by no more than 3.8 mol% Al2O3. It can be assumed that a clearer separation of two liquids would be observed at high temperatures (above 2053), but this cannot be done in the present work. The compositions of the coexisting liquids L1 and L2 measured by EDX are given in Table 3. The results obtained are in a good agreement with those proposed by Kirschen et al.,[22] Fig. 3.
Experimental miscibility gap with data by Kirschen et al.[22] Solid triangles and spheres point to the compositions of the quenched coexisting liquids, open symbols denote the quench of a homogeneous liquid
5.3 Thermodynamic Optimization and Calculations
The Thermo-Calc software[5] was applied to optimize thermodynamic parameters using the PARROT module within the CALPHAD approach,[53] and the further calculations of the phase diagram were carried out in the POLY-3 module. During optimization, individual weights were assigned to every type of experimental data considering uncertainties of each experimental method. Thermodynamic descriptions of the binary subsystems Al2O3–TiO2 and Al2O3–SiO2 were accepted from the works of Ilatovskaia et al.[7,8] and Dumitrescu and Sundman,[11] respectively. Thermodynamic data for the solid phases in the TiO2–SiO2 system originated from Hampl and Schmid–Fetzer[27] and Hillert et al.[51] were accepted, and the liquid phase of the system was only re-assessed in this work. The thermodynamic parameters for the Al2O3–TiO2–SiO2 system were assessed in this work based on the phase equilibria data obtained by XRD, SEM/EDX, and DTA in the previous work[4] and available in the literature.[17,22,54,55]
5.3.1 The Binary TiO2–SiO2 System
The calculated phase diagram of the TiO2–SiO2 system is shown in Fig. 4a. Due to the large uncertainties in the reported liquid immiscibility data, different weights were assigned to the corresponding data points during optimization. Since a critical assessment of the available data was provided and the modeling was amended by their own experimental measurements at high temperatures, larger weight was given to the data of Kirillova et al.[23] The critical point of the miscibility gap calculated by Kirillova et al.[23] is at 2416 K and 58.9 mol% TiO2. In this work, it is calculated to be at 2325 K and 46.25 mol% TiO2. The eutectic point calculated in this work is at 1833 K and 4.0 mol% TiO2. These results are compared with the experimental data in Table 2, showing reasonable agreement.
It should be noted that the data of Kirschen et al.[22] are shown in Fig. 4a for comparison, but they were not accounted quantitatively in this work. Although Kirschen et al. performed very good levitation-quenching experiments for the TiO2–SiO2 binary system, the experimental temperatures were probably chosen too high (one should keep in mind that the uncertainty of the temperature measurement by optical pyrometer used in[22] is about ± 50 K) as evidenced by the microstructure provided in their work. When the molten sample is quenched from above the critical temperature of miscibility gap, the homogeneous melt could decompose into two separated liquid phases, resulting in an interconnected structure but not well-dispersed droplets. That, in turn, makes it more difficult to interpret the results obtained. Such a behavior was observed by Kirillova et al.[23] for the sample containing 50 mol% TiO2 quenched from 2458 to 2533 K.
As discussed above, the experiments in an He atmosphere revealed a decrease in temperatures. Thus, the calculations performed at p(O2) = 1·10–2.5 bar are compared with the obtained experimental results in Fig. 4a. The selection of oxygen partial pressure for calculations is based on the results of Dilner et al.,[52] where consistency between calculations and DTA measurements performed in the same device was found. According to the calculations, a decrease in the temperature of a monotectic reaction to 2025 K at p(O2) = 1·10–2.5 bar was found. The eutectic temperature calculated at p(O2) = 1·10–2.5 bar is also 23 K lower than that calculated in air. A similar temperature shift (20 K) for heating in air and in He was observed experimentally (see Fig. 1a). The agreement between calculations and the DTA results in this case is good.
Stolyarova and Lopatin[56] measured the activity of SiO2 in the BaO–TiO2–SiO2 system at 1900 K using high-temperature differential mass spectrometry. The activity of SiO2 in the TiO2–SiO2 system at 1900 K for TiO2–SiO2 1:1 is only available. The calculated activity of SiO2 (in relation to the liquid state) in the TiO2–SiO2 system at 1800 and 1900 K at p(O2) = 0.21 bar is compared with the experimental data in Fig. 4b. The experimental activity at 1900 K is well reproduced in this work.
5.3.2 The Ternary Al2O3–TiO2–SiO2 System
Using the assessed thermodynamic parameters from the binary systems, an interpolation was first performed. However, the interaction parameters were introduced into the mullite, pseudobrookite, and liquid descriptions to fit experimental data.[4,17,22,54,55] The derived database is presented in Table 4. Using the derived Al2O3–TiO2–SiO2 thermodynamic description, the calculated isothermal sections of the Al2O3–TiO2–SiO2 system at 1486, 1722, 1747, and 1792 K are shown in Fig. 5 along with the experimental results[4] indicating a good consistency despite the below-mentioned shortcomings.
Isothermal section of the Al2O3–TiO2–SiO2 system determined experimentally[4](left) and calculated (right) at p(O2) = 0.21 bar and a 1722 K, b 1747 K, and c 1792 K
Data on the solid-state transformation and invariant equilibria on the liquidus of the Al2O3–TiO2–SiO2 system measured by DTA and SEM/EDX[4] were accounted. Assuming that there is no solubility of other components in corundum Al2O3, the phase was modeled as stoichiometric. Limited solubilities in rutile TiO2 and cristobalite SiO2 were observed in the experimental work,[4] however they were not modeled in this work due to the lack of reliable data. The evident solubility in C-SiO2 may be explained by the experimental difficulty of reaching equilibrium in the SiO2-rich region of the ternary system caused by the high viscosity. Parameters describing ternary extension of mullite and pseudobrookite were optimized based on the composition measurements[4,55] However, an attempt to get a wider ternary extension of the homogeneity range of mullite leads to the solid-state reaction C-SiO2 + Al2TiO5 ↔ Al6Si2O13 + TiO2 becoming inconsistent with the experimental results.[4] It should also be noted that even if the ternary extensions of mullite and pseudobrookite are not considered in the calculations, the solid-state reaction does not occur, and the low-temperature assemblages are stable up to the solidus temperatures that leads to invariant reactions on liquidus inconsistent with the experimental data. Therefore, the Gibbs energy of the pseudobrookite phase was adjusted to be more stable than mullite to keep the solid-state reaction. This was the reason for the widespread extension of pseudobrookite into the ternary system and the negligible or even absent extension of mullite when pseudobrookite Al2TiO5 is thermally stable (above 1552 K[7]). It should be noted that such discrepancy with the experimental data could be explained by the fact that the used thermodynamic descriptions of the binary Al2O3–SiO2 and Al2O3–TiO2 systems are not mutually consistent. Since some thermodynamic parameters describing the Al2O3–SiO2 and Al2O3–TiO2 systems were accepted unchanged, the conditions for optimization of newly obtained parameters were limited. In this case, a possible way to improve the reproduction of experimental data is to re-assess the parameters for the Al2O3–SiO2 and/or Al2O3–TiO2 systems. However, changing of these binary systems will most likely lead to inconsistencies in previous descriptions in which these systems have already been used. Therefore, the current thermodynamic description is used, however further re-optimization is considered to be necessary.
The complex description of the liquid phase was necessary to fit the parameters of melting relations determined recently.[4] The results on the liquid immiscibility obtained in this work were also considered. Given the experimental data on liquidus, the ternary mixing parameters were optimized for the liquid phase (see Table 4). The calculated liquidus surface is presented in Fig. 6, whereas the calculated temperatures and compositions of invariant reactions are presented in Table 5 along with the experimental data. The agreement with the experimental data[4] is relatively good and the calculations indicate the same character of the invariant reactions. The calculated critical point of the miscibility gap is at the composition 5.2Al2O3–62.1TiO2–32.7SiO2 (in mol%), which reproduces well the experimentally observed value of 3.8 mol% Al2O3 discussed above.
6 Conclusion
Phase equilibria in the TiO2–SiO2 system were studied using DTA and SEM/EDX, and the miscibility gap in liquid was observed. Moreover, the miscibility of the liquid in the Al2O3–TiO2–SiO2 system was studied at 2013 K in air, which demonstrated shrinkage of the miscibility gap at 3.8 mol% Al2O3.
The CALPHAD approach was applied to derive the thermodynamic descriptions of the TiO2–SiO2 and Al2O3–TiO2–SiO2 systems which are suitable for calculation of the phase diagrams in air conditions. However, including Ti+3 in the description of solid phases requires further experimental studies at low oxygen partial pressures, whereas Ti+3 was only considered in the liquid phase. The mullite and pseudobrookite phases were modeled within the compound energy formalism. For the liquid phase, the two-sublattice partially ionic model was applied. The present description reproduces the recent experimental data for the solid-state reaction C-SiO2 + Al2TiO5 ↔ Al6Si2O13 + TiO2 and liquid immiscibility, although reproducing of the solid-state reaction required the introduction of a rather extended ternary solubility in the Al2TiO5 phase which was not observed experimentally. The calculated isothermal sections and liquidus projection are reasonably consistent with the experimental data.
References
C. Voigt, L. Ditscherlein, E. Werzner, T. Zienert, R. Nowak, U. Peuker, N. Sobczak, and C.G. Aneziris, Wettability of AlSi7Mg Alloy on Alumina, Spinel, Mullite and Rutile and Its Influence on the Aluminum Melt Filtration Efficiency, Mater. Des., 2018, 150, p 75–85. https://doi.org/10.1016/j.matdes.2018.04.026
E. Werzner, M. Abendroth, C. Demuth, C. Settgast, D. Trimis, H. Krause, and S. Ray, Influence of Foam Morphology on Effective Properties Related to Metal Melt Filtration, Adv. Eng. Mater., 2017, 19(9), p 1700240. https://doi.org/10.1002/adem.201700240
A. Salomon, C. Voigt, O. Fabrichnaya, C.G. Aneziris, and D. Rafaja, Formation of Corundum, Magnesium Titanate, and Titanium(III) Oxide at the Interface Between Rutile and Molten Al or AlSi7Mg0.6 Alloy, Adv. Eng. Mater., 2017, 19(9), p 1700106. https://doi.org/10.1002/adem.201700106
M. Ilatovskaia, F. Barthel, and O. Fabrichnaya, Phase Relations in the Al2O3-TiO2-SiO2 System, Ceram. Int., 2020, 46(18), p 29402–29412. https://doi.org/10.1016/j.ceramint.2020.05.103
J.O. Andersson, T. Helander, L. Höglund, P.F. Shi, and B. Sundman, Thermo-Calc and DICTRA, Computational Tools for Materials Science, Calphad, 2002, 26, p 273–312.
S.T. Norberg, S. Hoffman, M. Yoshimura, and N. Ishizawa, Al6Ti2O13, a New Phase in the Al2O3-TiO2 System, Acta Cryst., 2005, C61, p i35–i38. https://doi.org/10.1016/S0364-5916(02)00037-8
M. Ilatovskaia, G. Savinykh, and O. Fabrichnaya, Thermodynamic Description of the Ti-Al-O System Based on Experimental Data, J. Phase Equilib., 2017, 38, p 175–184. https://doi.org/10.1007/s11669-016-0509-4
M. Ilatovskaia, and O. Fabrichnaya, Thermodynamic Assessment of the Al2O3-MgO-TiO2 System, J. Alloys Compd., 2019, 790, p 1137–1148. https://doi.org/10.1016/j.jallcom.2019.03.046
O. Fabrichnaya, A. Costa e Silva, and F. Aldinger, Assessment of Thermodynamic Functions in the MgO-Al2O3-SiO2 System, Z. Metallkunde, 2004, 95, p 793–805.
P.E. Kalita, A. Cornelius, K. Lipinska, S. Sinogeikin, R.X. Fischer, H. Lührs, and H. Schneider, High Pressure Behavior of 7:4 Mullite and Boron-Substituted Mullite: Compressibility and Mechanisms of Amorphization, J. Am. Ceram. Soc., 2014, 97(9), p 2980–2989. https://doi.org/10.1111/jace.13027
L. Dumitrescu, and B. Sundman, A Thermodynamic Reassessment of the Si-Al-O-N System, J. Eur. Ceram. Soc., 1995, 15, p 239–247.
S. Umezu, and F. Kakiuchi, Investigation on Iron Blast Furnace Slags Containing Titanium, Nippon Kogyo Kwaishi, 1930, 46, p 366–377.
R. Rieke, Melting Influence of Titanic Acid on Silica, Alumina and Kaolin, Sprechsaal, 1908, 41, p 405. , in German
D. Bogatzkii, Investigation of the System TiO2-SiO2, Metallurgist, 1938, 11, p 59–67.
E.N. Bunting, Phase Equilibria in the Systems TiO2, TiO2-SiO2 and TiO2-Al2O3, Bur. Stand. J. Res., 1933, 11, p 719–725.
R. Rickers, and F. Hummel, Reaction in the System TiO2-SiO2: Revision of the Phase Diagram, J. Am. Ceram. Soc., 1951, 34, p 271–279. https://doi.org/10.1111/j.1151-2916.1951.tb09129.x
Y.M. Agamawi, and J. White, The System Al2O3-TiO2-SiO2, Trans. Br. Ceram. Soc., 1952, 51(5), p 293–325.
R.C. DeVries, R. Roy, and E.F. Osborn, The System TiO2-SiO2, Trans. Br. Ceram. Soc., 1954, 53(9), p 525–540.
G. McTaggart, and A. Andrews, Immiscibility Area in the System TiO2-ZrO2-SiO2, J. Am. Ceram. Soc., 1957, 40, p 167–170. https://doi.org/10.1111/j.1151-2916.1957.tb12596.x
F. Massazza, and E. Sirchia, The System MgO-SiO2-TiO2 Note I: Review of Binary Systems, Chim. Ind., 1958, 40, p 376–380. , in Italian
F. Galakhov, M. Vavilonova, and V. Averyanov, Determination of the Limits of the Metastable Liquidation in the Silica-Rich Region of the System TiO2-SiO2, Bull. Acad. Sci. USSR Div. Inorg. Mater., 1974, 10, p 153–154.
M. Kirschen, C. DeCapitani, F. Millot, J.-C. Rifflet, and J.-P. Coutures, Immiscible Silicate Liquids in the System SiO2-TiO2-Al2O3, Eur. J. Min., 1999, 11, p 427–440. https://doi.org/10.1127/ejm/11/3/0427
S.A. Kirillova, V.I. Almjashev, and V.V. Gusarov, Phase Relationships in the SiO2-TiO2 System, Russ. J. Inorg. Chem., 2011, 56(9), p 1464–1471. https://doi.org/10.1134/S0036023611090117
L. Kaufman, Calculation of Multicomponent Ceramic Phase Diagrams, Physica B, 1988, 150, p 99–114.
C. DeCapitani, and M. Kirschen, A Generalized Multicomponent Excess Function with Application to Immiscible Liquids in the System CaO-SiO2-TiO2, Geochim. Cosmochim. Acta, 1998, 62, p 3753–3763.
E. Boulay, J. Nakano, S. Turner, H. Idrissi, D. Schryvers, and S. Godet, Critical Assessment and Thermodynamic Modeling of BaO-SiO2 and SiO2-TiO2 Systems and Their Extension into Liquid Immiscibility in the BaO-SiO2-TiO2 System, Calphad, 2014, 47, p 68–82. https://doi.org/10.1016/j.calphad.2014.06.004
M. Hampl, and R. Schmid-Fetzer, Thermodynamic Description of the Ti-O System, Int. J. Mater. Res., 2015, 106(5), p 439–453. https://doi.org/10.3139/146.111210
J. Lewis, D. Schwarzenbach, and H.D. Flack, Electric Field Gradients and Charge Density in Corundum, Alpha-Al2O3, Acta Cryst., 1982, A38, p 733–739. https://doi.org/10.1107/S0567739482001478
D.M. Tobaldi, A. Tucci, A.S. Škapin, and L. Esopsito, Effect of SiO2 Addition on TiO2 Crystal Structure and Photocatalytic Activity, J. Eur. Ceram. Soc., 2010, 30(12), p 2481–2490. https://doi.org/10.1016/j.jeurceramsoc.2010.05.014
S.M. Antao, I. Hassan, J. Wang, L.P. Lee, and B.H. Toby, State-of-the-Art High-Resolution Powder X-ray Diffraction (HRPXRD) Illustrated with Rietveld Structure Refinement of Quartz, Sodalite, Tremolite and Meionite, Can. Miner., 2008, 46, p 1501–1509. https://doi.org/10.3749/canmin.46.5.1501
R.T. Downs, and D.C. Palmer, The Pressure Behavior of Alpha-Cristobalite, Am. Miner., 1994, 79, p 9–14.
K. Kihara, T. Matsumoto, and M. Imamura, High-Order Thermal-Motion Tensor Analyses of Tridymite, Z. Kristallogr., 1986, 177, p 39–52.
E. Fidancevska, and V. Vassilev, Obtaining of Powders by Controlled Hydrolysis and Sintering of Materials from the TiO2-Al2O3-SiO2 System, J. Univ. Chem. Technol. Metall., 2010, 45(4), p 421–430.
M. Hellenbrandt, The Inorganic Crystal Structure Database (ICSD)—Present and Future, Crystallogr. Rev., 2004, 10, p 17–22. https://doi.org/10.1080/08893110410001664882
L. Lutterotti, S. Matthies, and H.R. Wenk, MAUD: A Friendly Java Program for Material Analysis Using Diffraction, CPD Newslett., 1999, 21, p 14–15.
L. Lutterotti, M. Bortolotti, G. Ischia, I. Lonardelli, and H.R. Wenk, Rietveld Texture Analysis from Diffraction Images, Z. Kristallogr. Supl., 2007, 26, p 125–130. https://doi.org/10.1524/9783486992540-020
O. Fabrichnaya, M. Kriegel, J. Seidel, G. Savinykh, L. Ogorodova, I. Kiseleva, and H. Seifert, Calorimetric Investigation of the La2Zr2O7, Nd2Zr2O7, Sm2Zr2O7 and LaYO3 Compounds and CALPHAD Assessment of the La2O3-Y2O3 System, Thermochim. Acta, 2011, 526, p 50–57. https://doi.org/10.1016/j.tca.2011.08.021
M. Hillert, B. Jansson, B. Sundman, and J. Ågren, A Two-Sublattice Model for Molten Solutions with Different Tendency for Ionization, Metal. Trans., 1985, A16, p 261–266. https://doi.org/10.1007/BF02816052
B. Sundman, Modification of the Two-Sublattice Model for Liquids, Calphad, 1991, 15, p 109–119. https://doi.org/10.1016/0364-5916(91)90010-H
M. Hillert, The Compound Energy Formalism, J. Alloys Compd., 2001, 320, p 161–176. https://doi.org/10.1016/S0925-8388(00)01481-X
R. Sadanaga, M. Tokonami, and Y. Takeuchi, The Structure of Mullite, 2Al2O3∙SiO2, and Relationship with the Structures of Sillimanite and Andalusite, Acta Cryst., 1962, 15, p 65–68. https://doi.org/10.1107/S0365110X62000134
R.J. Angel, and C.T. Prewitt, Crystal Structure of Mullite: A Re-Examination of the Average Structure, Am. Miner., 1986, 71, p 1476–1482.
H. Schneider, R.X. Fischer, and J. Schreuer, Mullite: Crystal Structure and Related Properties, J. Am. Ceram. Soc., 2015, 98(10), p 2948–2967. https://doi.org/10.1111/jace.13817
H. Schneider, Transition Metal Distribution in Mullite, Ceram. Trans., 1990, 6, p 135–158.
H. Schneider, Foreign cation incorporation in mullite, in Mullite. H. Schneider and S. Komarneni, Eds., Wiley-VCH Verlag, Weinheim, 2005, p 70–140
B. Hallstedt, Thermodynamic Assessment of the MgO-Al2O3 System, J. Am. Ceram. Soc., 1992, 75, p 1497–1507. https://doi.org/10.1111/j.1151-2916.1992.tb04216.x
B. Morosin, and R.W. Lynch, Structure Studies on Al2TiO5 at Room Temperature and at 600°C, Acta Cryst., 1972, B28, p 1040–1046. https://doi.org/10.1107/S0567740872003681
R.D. Skala, D. Li, and I.M. Low, Diffraction, Structure and Phase Stability Studies on Aluminium Titanate, J. Eur. Ceram. Soc., 2009, 29, p 67–75. https://doi.org/10.1016/j.jeurceramsoc.2008.05.037
M. Ishitsuka, T. Sato, T. Endo, and M. Shimada, Synthesis and Thermal Stability of Aluminum Titanate Solid Solutions, J. Am. Ceram. Soc., 1987, 70(2), p 69–71. https://doi.org/10.1111/j.1151-2916.1987.tb04931.x
R. Papitha, M.B. Suresh, D. Das, and R. Johnson, Mineral-Oxide-Doped Aluminum Titanate Ceramics with Improved Thermomechanical Properties, J. Ceram., 2013. https://doi.org/10.1155/2013/214974
M. Hillert, B. Sundman, and X. Wang, A Thermodynamic Evaluation of the Al2O3-SiO2 System, TRITA MAC, 1989, 204, p 1–23.
D. Dilner, D. Pavlyuchkov, T. Zienert, L. Kjellqvist, and O. Fabrichnaya, Thermodynamics of the Mg-Mn-O System: Modeling and Heat Capacity Measurement, J. Am. Ceram. Soc., 2017, 100, p 1661–1672. https://doi.org/10.1111/jace.14686
H.L. Lukas, S.G. Fries, and B. Sundman, Computational Thermodynamics. The Calphad Method. Cambridge University Press, New York, 2007.
F.Y. Galakhov, The Study of the Alumina-Rich Area of Aluminosilicate Systems. Report 3. The TiO2-Al2O3-SiO2 System, Izvest. Akad. Nauk SSSR, 1958, 5, p 529–534. , in Russian
C.R. Green, and J. White, Solid Solubility of TiO2 in Mullite in the System Al2O3-TiO2-SiO2, Trans. Br. Ceram. Soc., 1974, 73(3), p 73–75.
V.L. Stolyarova, and S.I. Lopatin, Mass Spectrometric Study of the Vaporization and Thermodynamic Properties of Components in the BaO-TiO2-SiO2 System, Glass Phys. Chem, 2005, 31(2), p 132–137. https://doi.org/10.1007/s10720-005-0034-8
Acknowledgements
The study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 169148856 – SFB920 (Subproject A03). The authors appreciate the technical assistance from the employees of the Institute of Materials Science (TU Freiberg), namely B. Wahl, Dr. C. Schimpf, and B. Bleiber.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ilatovskaia, M., Fabrichnaya, O. Liquid Immiscibility and Thermodynamic Assessment of the Al2O3–TiO2–SiO2 System. J. Phase Equilib. Diffus. 43, 15–31 (2022). https://doi.org/10.1007/s11669-021-00935-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-021-00935-4