Abstract
The fouling behavior of Alloy 6061 aluminum in artificial seawater in the presence of Boswellia serrata extract was investigated. Fouling is one of the major issues that occurred commonly in the marine environment. Experiments were conducted to develop a model for fouling propensity. A response surface method was used to develop a model to predict fouling behavior. The model predicts the effect of the variables on fouling in artificial seawater medium in the presence of B. serrata extract. The experimental variables chosen were temperature, flow rate and time. Performance of the regression model calculated by the response surface methodology was verified with the analysis of variance technique. The model equation that was proposed using the response surface methodology showed good agreement with the experimentally obtained data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Besides corrosion, fouling is a serious problem encountered by the industries. Corrosion and fouling result in tremendous economic implications. Materials when exposed to water suffer from a common phenomenon of fouling which is the formation of a film covering the surface that is in contact with water. Reduced flow area in pipelines and decreased heat transfer in boilers and condensers are consequence of scale build up. Corrosion fouling also results in decreased mechanical resistance of the equipment which is in contact with water [1].
Scale deposition on the interior walls of domestic heat exchangers, geothermal power plants, cooling water systems, pipes, etc., may result in severe technical and economic problems linked to a necessity for maintenance and the need to replace equipment [2]. Aluminum and its alloys are extensively used in industry because they combine good physical properties, adequate mechanical performance, and quite good resistance to uniform corrosion. Aluminum alloys are widely applied in aircraft, automotive, railway, marine and construction industry [3]. In industrial sector, fouling mitigation is greatly essential and indispensable requirement [4].
The most common adopted method to control fouling in industrial pipelines is chemical inhibition [5]. An inhibitor is a chemical substance or combination of substances which when added in very low concentrations effectively prevents or reduces fouling. Inhibitors play a vital role in closed environmental systems that have good circulation so that an adequate and controlled concentration of inhibitor is ensured [6].
A lot of synthetic inhibitor study for fouling mitigation may be found in the literature. But the effect of green inhibitor on fouling propensity has rarely been reported. This study is an effort to reveal the effect of green inhibitor on fouling through response surface methodology.
The study aims at the prediction of optimum operating conditions for minimum fouling of Alloy 6061 aluminum under dynamic conditions in artificial seawater slurry in the presence of a corrosion inhibitor.
Materials and Methods
Experimental Setup
Figure 1 exhibits the equipment for measuring the influence of process parameters on fouling in seawater in the presence of an anticorrosive agent on Alloy 6061 aluminum. The experimental rig shown consisted of a reservoir tank, a pump, flow rate controller, temperature controller, thermocouple, and valves.
Alloy 6061 aluminum sample was placed on a sample holder through which the slurry was driven by a centrifugal pump. Details of the study are in another reference [7]. The elemental composition of Alloy 6061 aluminum is provided in Table 1. The heater placed in the tank heated the slurry to the required experimental temperature.
Artificial Seawater
The testing solution, which consisted of the artificial seawater, was prepared as per ASTM standards [8]. The composition of artificial water consisted of sodium chloride—24.53%, magnesium chloride-5.20%, sodium sulfate—4.09%, calcium chloride—1.16%, potassium chloride—0.695%, sodium bicarbonate—0.201%, potassium bromide—0.101%, boric acid—0.027%, strontium chloride—0.025%, sodium fluoride—0.003%. The suspended solids in the slurry consisted of sea sand of size 300 μm. Throughout the experiments, a 0.3% sand concentration was maintained.
Boswellia Serrata
Boswellia Serrata is a plant. Boswellia serrata (family Burseraceae) is commonly found in many regions of the world, such as South Asia, Northern Africa, and the Middle East. Boswellia serrata extract contains terpenes, amino acids, resin, phenols, β-boswellic acid and polysaccharides [8]. It is reported to be a good anticorrosive agent under static conditions [9, 10]. In the present investigation, the gum portion of plant Boswellia serrata was used. It contains various polysaccharides such as arabinose, xylose, galactose and D-glucuronic acid [11]. Boswellia serrata extract was procured from Ms Revinto Life sciences Pvt. Ltd., Karnataka Structure of boswellic acid is shown in Fig. 2 [12].
Experimental Procedure
In case of fouling studies, the installed metal coupon was weighed before and after an experimental run on an electronic weighing balance. The difference in the weight of the sample before the experiment and after the experiment yielded noticeable amounts of deposits on the coupon surface. The experimental procedure of the weight gain measurement is shown in Fig. 3.
Experimental Analysis with Response Surface Methodology
The parameters were analyzed by Box–Behnken design (BBD), with response surface methodology (RSM). The levels and the factors in the study are displayed in Table 2. The RSM technique aids in optimizing the process parameters with a least number of experiments and is suitable for analyzing the interaction of several affecting factors [13, 14]. The response surface technique consists of a collection of statistical and mathematical methods useful for developing the empirical model. RSM uses quantitative experimental data to develop regression model and to optimize the response that is effected by several independent variables.
Box Behnken Design for Optimization
Fouling is mostly affected by temperature, velocity of the flowing fluid, and therefore, they were selected as the variables for the study [15]. The independent variables viz. temperature, flow rate and time were optimized using RSM (response surface methodology) for minimum fouling conditions. The proposed model was fitted to the data for the minimum fouling conditions. The effects of temperature, flow rate and time were quantitatively assessed using response surface curves. In the present study, the experimental runs were determined with Box–Behnken design with 15 trials. The results of the experimental trials are summarized in Table 3.
The parameters and the response were modelled to optimize the process conditions for the desired response. Statistical parameters were calculated with ANOVA [16]. Optimization using RSM involves three main steps; (1) statistically designed experiments (2) estimation of coefficients in a model and response prediction and checking the model adequacy within the setup of the experiment [17]. The three independent variables chosen for the experiment design are as follows: Temperature (A, K), flow rate (B, L min-1) and time (C, h). According to the experimental designs, the range and levels of factors were varied [18].
Optical Photograph and Scanning Electron Microscopy
Optical photographs of 6061 aluminum alloy specimens were taken after the experiments, to evaluate gross changes in the metal surface at different conditions. Optical photographs were taken using Olympus BX53M microscope. Surface morphology studies were carried out using analytical scanning electron microscope (JEOL JSM-6380L).
Result and Discussions
Analysis of Variance
The statistical competence of the model was resolved through analysis of variance (ANOVA). Significance of regression model and each factor were tested with F and P values. The P value is a number, calculated from a statistical test that explains likeliness to have found a particular set of observations if the null hypothesis were true. The null hypothesis means that there is no relationship between the variables of interest. The F value determines whether the test is statistically significant. If these values of the model are large, the reliability of the regression model is high. Statistically, the polynomial model excellence was determined through the coefficient of determination (R2) and the adjusted R2. R2 or the coefficient of determination is a statistical measure in a regression model that determines the proportion of variance in the dependent variable that can be explained by the independent variable.
The significance of the parameters and the regression model was verified using analysis of variance (ANOVA) and was carried out to verify the significant level of the parameters and the regression model. The F value of the model implied that the model was significant. P < 0.05 indicates that the model term is significant. Lack of fit (simulation loss curve) points the probability that predicted model does not fit the actual value. Non-significant lack of fit is good. The P value was observed to be 0.427. A small probability value (P < 0.05), within the analysis advocates that the influence of the factor is significant [19]. Results indicate that the probability values for main effects, the quadratic effects and interaction effects are lower than 0.05. Second-order model is satisfactory as confirmed from the insignificant lack of fit for estimating the response. The correlation coefficient R2 of the model was 95.55%, indicating that the predicted and actual values fitted well. Therefore, this model can be applied to predict fouling propensity in the presence of Boswellia Serrata extract at a concentration of 500 ppm effectively. The analysis data presented above illustrate that these factors have significant influences on the objective function, Y.
Influence of Individual Parameters on the Weight Gain
Effect of Temperature
The effect of temperature, flow rate and time is reflected in Fig. 4. The results clarify that a positive correlation between the temperature and the fouling deposition. The corrosion rate intensifies with an increase in the temperature [20, 21]. The corrosion products deposit on the surface and increase the weight of the sample. The metallic oxides formed (corrosion product) cause corrosion by either of two ways (1) when the metal oxides themselves act as foulants, or (2) when metal products act as adsorbent nuclei and pull seawater constituents and then the combined organic material constitute and metal product act as the foulant [22]. The bulk temperature influences chemical reaction rate and polymerization rate [23]. Fouling attachment tends to restrict oxygen diffusion to the sample surface and the occurrence of fouling can accelerate localize corrosion on the alloys that are susceptible to oxygen concentration cells [24].
Exposed Time Duration
Particles deposited on the metal surface undergoes an aging process through chemical or physical changes, which either weakens or strengthens the deposit [25]. For all types of fouling, a rough surface causes accelerated fouling and thus reduces the delay time. Surface roughness increases turbulence near the surface, which, in turn, increases the removal rate of fouling on the surface [23]. For a lesser time, the weight gain was the least, which could be explained as the initiation phase of fouling. As time elapsed, weight gain increased. For minimum and the maximum temperatures, the weight gain was the lowest in comparison to the mid temperature range. Some particles remained on the surface after deposition whereas few were carried away with fluid flow. The deposit removal rate was a function of deposition rate. Nevertheless, flow rate and temperature glaringly influenced the deposition rate. Shear forces increased as fluid rate increased which resulted in deposit removal.
Effect of Flow rate
The results indicated that the weight gain was the least at the mid flow rate and it was the highest at the low and high flow rates. The flow velocity was in a negative correlation with the fouling accumulation. A high flow velocity accelerates the fouling removal thus reducing deposit accumulation [26]. Alternatively at high flow rates species diffusion increases and may result in increased weight gain. In most cases, fouling decreases at higher fluid velocities. Shear stress rises with the increasing flow causing more fouling removal. Thus, resulting in lower fouling rates, and hence lower fouling resistance. For weak deposits such as (particulate fouling), higher flow velocity may eliminate fouling. For strongly adhering deposits, increasing the flow velocity beyond a particular point may not cause significant reduction in fouling. Further increase in velocity may not cause any effect on very strongly adhering deposits, while increasing the flow velocity may not have any effect at all [23].
Particulate deposition takes place in liquid system. Sand particles present in the artificial seawater slurry were responsible for particulate fouling. Calcium and bicarbonate ions in the seawater composition caused crystallization fouling. Artificial seawater consisting of chloride ions is the basis of corrosion fouling. Hence, composite fouling may be observed in artificial seawater slurry.
Response Surface and Contour Plots
Since fouling is affected by many variables not only the main effect of the parameters but also the interaction between the parameters is of prime importance. 3D surface plots were produced to illustrate the relationships between the response and the levels of independent variables [27]. The cross-interaction between any two variables is reflected in the contour map by keeping the other variable at constant and mid-level [28]. Response value analysis is also depicted in Fig. 5a. From the contours, it was found that the interactions between flow rate and temperature and time and flow rate were elliptical in nature indicating a significant interaction between each of the two factors. The round contour lines for time and temperature indicate a lack of interaction. Figure 5b shows the contours of fouling propensity with two independent variables as coordinates.
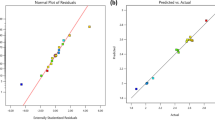
Normal Probability and Residuals Plots
Normal probability and residuals versus fitted values plots for fouling propensity in terms of weight gain are illustrated in Fig. 6. A residual is the difference between the observed value and the mean value that the model predicts for that observation. As shown in Fig. 6a, the normality assumption was moderately satisfied as per points in the plot form straight line. It could be shown from Fig. 6a that the predicted and experimental weight gain values are basically consistent. The clustered points along the diagonal line indicate the agreement of the analytical system. With the plot of residuals versurs fits model, realiablity was also examined. It is shown in Fig. 6b. As depicted in this figure, no increasing or decreasing points, patterns should be found. Hence, the model is adequate in describing fouling propensity by response surface methodology [18].
Optimum Conditions
The optimum level of each variable for minimal weight gain was resolved using the response optimizer tool of MINITAB 17 trial version software. Optimization plots were applied to assess the influence of designated variables individually and along with other variables on response. In this case, the target was to minimize the response. The best factorial blend and as was shown by a desirability value that is ~ 1.0000 was achieved using temperature—333 K, flow rate—12 L min-1 and time—0.5 h.
Visual Observation
Figure 7 corresponds to the images of the specimen after it was subjected to fouling tests in artificial seawater slurry with Boswellia Serrata under dynamic conditions. Dense deposit was found at the point where the specimen was fixed to the sample holder (Fig. 7b). This condition is attributed to the presence of dead zones at the junction of the sample holder. The deposition was less dense where the slurry impinged on the specimen (Fig. 7c). This is due to the shear effects causing the removal of the deposit under continuous impingement of the slurry.
The Alloy 6061 aluminum sample is presented after the completion of the fouling tests. (a) Deposits along the inner diameter of the tube are evident. (b) Deposits that were present at the bottom portion of the tube, as oriented in the test, are shown. (c) The deposits that were present at the top portion of the tube, as oriented in the test, are shown (d) the surface of the alloy before study
Optical Microscope
Figure 8 corresponds to Alloy 6061 aluminum surface exposed to fouling experiments where Boswellia Serrata extract in artificial seawater slurry was the test medium. The optical microscope image shows the fouling deposit formed on the alloy surface. Scratches due to polishing are visible. Wide pits and cracks were not evident on the surface indicating that the Boswellia Serrata extract was to be a good inhibitor for corrosion.
Fouling phenomena may occur due to slurry particles, salts present in the seawater solution or the interaction between the inhibitor and the seawater slurry. The interaction between the salts and the metal may result in corrosion fouling. Due to the flow of the fluid and the shear effects, there may be a removal of the deposit taking place from the surface of the metal. Therefore, fouling deposit and removal will be continuously occurring during the flow of the fluid. The deposit on the surface may be the corrosion product, ingredients of the slurry or the primary/secondary inhibitor complex. The deposition is schematically represented in Fig. 9.
SEM/EDX
The EDX corresponding to the SEM image (at a magnification of 100X) depicted in Fig. 10 indicates that the weight percent of the elements present on the surface of Alloy 6061 aluminum. The surface morphology exhibited uneven deposits on the surface of Alloy 6061 aluminum. Elemental analysis revealed the presence of Al and O indicating the formation of corrosion product, an oxide film on the surface of Alloy 6061 aluminum. This film responsible for the corrosion protection of alloy, while its corrosion results from the dissolution of aluminum in artificial seawater. Boswellia serrata extract contains terpenes, amino acids, resin, phenols, β-boswellic acid and polysaccharides. Carbon is found in most of these contents of the extract. Traces of carbon inhibitor were found in the EDX analysis indicating the adsorption of the corrosion inhibitor, B. Serrata.
Conclusions
-
RSM was used to investigate the main and interaction effects of important independent variables effecting fouling.
-
A second-order response surface model was suitable for predicting the relationship between weight gain and operation parameters.
-
The depositional behavior of seawater fouling in presence of Boswellia Serrata was presented in the model with the clear physical significance.
-
The regression model, designed with Box Behnken design, agreed well with experimental case, which could be proved with the R2 value of 0.9555.
-
The optimal conditions of operation in the presence of Boswellia Serrata extract with minimal weight gain was 333 K—temperature, 12 L min-1—flow rate and 0.50 h time and the desirability was 1.0.
References
F.-A.N. SettaAnne, Efficiency assessment of inhibitors on CaCO3 precipitation kinetics in the bulk and deposition on a stainless steel surface (316 L). Desalination. 281, 340–347 (2011)
C. Gabrielli, G. Maurin, R. Poindessous, Rosset, Nucleation and growth of calcium carbonate by an electrochemical scaling process. J. Cryst. Growth. 200, 236–250 (1999)
T. Zhang, J. Wang, G. Zhang, H. Liu, The corrosion promoting mechanism of Aspergillus niger on 5083 aluminum alloy and inhibition performance of miconazole nitrate. Corros. Sci. 176, 108930 (2020)
M. Awais, A. Arafat, Recent advancements in impedance of fouling resistance and particulate depositions in heat exchangers. Int. J. Heat Mass Trans. 141, 580–603 (2019)
A. M. Pritchard, The economics of fouling. In: Fouling Science and Technology. Fouling Science and Technology, pp. 31–45 (1988)
G. Palanisamy, Corrosion Inhibitorsin Corrosion inhibitors. In tech open (2019)
M. Lavanya, R. Padmalatha, M.V. Ramachandra, S. Subbalaxmi, Parametric study of aluminium alloy fouling in marine environment using RSM technique. Tribol. Mater. Surf. Interfaces. 14(2), 110–118 (2019)
ASTM D1141-98, Standard Practice for the Preparation of Substitute Ocean Water, (2013)
M. Mobin, A.J. Basik, Boswellia serrata gum as highly efficient and sustainable corrosion inhibitor for low carbon steel in 1 M HCl solution: experimental and DFT studies. J. Mol. Liq. 263, 174–186 (2018)
J.R. Xavier, J. Raja, S.P. Beryl, B. Vinodhini, G. Janaki, Evaluation of mechanical properties and corrosion protection performance of surface modified nano-alumina encapsulated epoxy coated mild steel. J. Bio. Tribo. Corr. 7, 1–17 (2021)
M. Saraf, K. Natarajan, K. Mobin, Emerging robust heterostructure of MoS2–rGO for high-performance supercapacitors. Appl. Mater. Inter. 10(19), 16588–16595 (2018)
R.C. Gupta, Nutraceuticals in Arthritis. In Nutraceuticals (Academic Press, 2016), pp. 161–176.
A. López, W. Nicholls, M.T. Stickland, W.M. Dempster, Reynolds averaged navier-stokes simulations of the airflow in a centrifugal fan using open FOAM. Phys. Commun. 197, 88–95 (2015)
M. Zhong, K.L. Huang, J.G. Zeng, S. Li, Rapid and high yield extraction method for saponins from safed musli. J. Sep. Sci. 33, 2160–2167 (2010)
S.J. Pugh, G.F. Hewitt, H. Müller-Steinhagen, Fouling during the use of seawater as coolant: the development of a user guide. Heat Transfer Eng. 30(10–11), 851–858 (2009)
L. Yu-Xuan, X. Qi-Yan, R.-T. Guo, Z.-Y. Wang, Removal of NO by using sodium persulfate/limestone slurry: Modeling by response surface methodology. Fuel. 254, 115612 (2019)
S.K. Behera, H. Meena, S. Chakraborty, B.C. Meikap, Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Min. Sci. Technol. 28(4), 621–629 (2018)
M. Taheri, R.A.M. Mohammad, A. Mokhtar, Optimization of acid black 172 decolorization by electrocoagulation using response surface methodology. Iran. J. Environ. Healt. 9, 23 (2012)
H. Majdi, E. Ja, M. Mohebbi, Optimization of convective drying by response surface methodology. Comput. Electron. Agr. 156, 574–84 (2019)
M. Lavanya, V.R. Murthy, P. Rao, Electrochemical investigation of erosion-corrosion behavior of 6061 aluminum alloy in marine environment. Tribol. Ind. 4, 40 (2018)
M. Cao, L. Liu, L. Fan, Z. Yu, Y. Li, E.E. Oguzie, F. Wang, Influence of temperature on corrosion behavior of 2A02 Al alloy in marine atmospheric environments. Materials. 11(2), 235 (2018)
E.F. Somerscales, Fundamentals of corrosion fouling. Exp. Therm. Fluid. Sci. 14, 335–355 (1997)
N. Kazi Salim, Particulate Matter: Interfacial Properties, Fouling, and its Mitigation, Mineral Scales and Deposits, (2015) pp. 41–168.
S.N. Kazi, Heat Exchangers-Basics Design Applications. Intechopen (2012)
A. Muhammad, A.A. Bhuiyan, Recent advancements in impedance of fouling resistance and particulate depositions in heat exchanger. Int. J. Heat Mass Tran. 141, 580–603 (2019)
D. Yang, J. Liu, E. Xiaoxue, L. Jiang, Model for seawater fouling and effects of temperature, flow velocity and surface free energy on seawater fouling. Chin. J. Chem. Eng. 24, 658–664 (2016)
T.Y. Yun, R.J. Feng, D.B. Zhou, Optimization of fermentation conditions through response surface methodology for enhanced antibacterial metabolite production by Streptomyces sp. 1–14 from cassava rhizosphere. PloS one. 13, e0206497 (2018)
J. Lai, H. Wang, D. Wang, T. Wu, Ultrasonic extraction of antioxidants from Chinese sumac (Rhus typhina L.) fruit using response surface methodology and their characterization. Molecules. 19(7), 9019–9032 (2014)
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lavanya, M., Rao, P. & Murthy, V.R. Assessing the Effect of Boswellia Serrata Extract on Fouling of Alloy 6061 Aluminum in Artificial Seawater Slurry. J Fail. Anal. and Preven. 21, 1704–1713 (2021). https://doi.org/10.1007/s11668-021-01221-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-021-01221-8