Abstract
This paper utilizes a statistical approach, the response surface optimization methodology, to determine the optimum conditions for the Acid Black 172 dye removal efficiency from aqueous solution by electrocoagulation. The experimental parameters investigated were initial pH: 4–10; initial dye concentration: 0–600 mg/L; applied current: 0.5-3.5 A and reaction time: 3–15 min. These parameters were changed at five levels according to the central composite design to evaluate their effects on decolorization through analysis of variance. High R2 value of 94.48% shows a high correlation between the experimental and predicted values and expresses that the second-order regression model is acceptable for Acid Black 172 dye removal efficiency. It was also found that some interactions and squares influenced the electrocoagulation performance as well as the selected parameters. Optimum dye removal efficiency of 90.4% was observed experimentally at initial pH of 7, initial dye concentration of 300 mg/L, applied current of 2 A and reaction time of 9.16 min, which is close to model predicted (90%) result.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effluents from industries, such as textile, leather, plastics, paper, food and cosmetics contain many coloring substances, which can be toxic, carcinogenic and mutagenic [1–3]. In addition, some synthetic dyes cause allergy and skin irritation [4]. The dye-containing wastewater, are not only aesthetic pollutants, but also may prevent light penetration in water, and thereby damage water sources and ecosystem [5–7].
Electrocoagulation (EC) treatment process has been widely used due to its simplicity and efficiency [8–10]. In this process, generation of coagulants (iron or aluminum ions) by electrodissolution of the sacrificial anode(s) leads to formation of particles that entrap the pollutants [11–13]. The main reactions for dye removal using aluminum electrodes are as follows:
At the anode:
At the cathode:
In the solution:
Response surface methodology (RSM) is a collection of mathematical and statistical techniques for modeling and analysis of problems in which a response of interest is influenced by set of independent variables [14, 15]. Main advantages of optimization by RSM to conventional method are reduction of experimental trials in providing sufficient information for statistically valid results and evaluation of the relative significance of parameters and their interactions [16, 17].
In recent years, the area of optimization dye removal efficiency by electrocoagulation has received enormous attentions [6, 18–20]. However, according to our knowledge, application of RSM design in decolorization by EC rarely presented in scientific papers [21–24]. On the other hand, up to now there is no research available on treatment of diazo and metal-complex Acid Black 172 dye in aqueous media except by biological procedures.
The aim of the present study was to optimize Acid Black 172 dye removal from aqueous solution by electrocoagulation process using RSM. For this purpose, central composite design (CCD) was used to develop a mathematical correlation between Acid Black 172 dye removal efficiency and four selected independent parameters including initial pH, initial dye concentration, applied current and reaction time.
Materials and methods
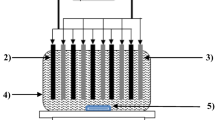
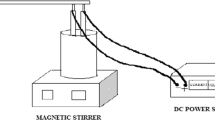
Synthetic wastewater was prepared by dissolving Acid Black 172 which was provided by Alvan Sabet Company (Iran) in distilled water. The general properties and chemical structure of the selected dye is presented in Figure 1. A plexiglass cell with effective volume of 2.5 liters and four aluminum plates with total effective area of 240 cm2 were used; the thicknesses of aluminum plates were 3 mm and the distances between electrodes was kept constant at 3 cm. Electrodes were connected to a DC power supply (Micro, PW4053R, 0-5A, 0–40 V) in a monopolar mode. For preparing a mixed solution in EC cell, a magnetic stirrer (Velp, Scientifica, Italy) was used.
For preparation of stock solutions of the synthetic wastewater, Acid Black 172 dye as dissolved in deionized water and then diluted to obtain the desired concentrations. Sodium chloride (NaCl) was used to increase the conductivity of the solutions containing Acid Black 172 as the supporting electrolyte. The solution initial pH was adjusted before experiments by NaOH and H2SO4 and controlled using pH meter (340i, WTW, Germany). All the experiments were performed at room temperature. A total of 30 samples were taken from the cell at the end of experiments and centrifuged by a centrifuge device (Hettich, EBA 21, USA) at 5000 rpm for 5 min and then analyzed. Dye concentration was measured at a wavelength corresponding to the maximum absorbance (λmax) by UV-visible spectrophotometer (HACH, DR4000, USA).
For optimization of Acid Black 172 dye removal efficiency using CCD, 31 experiments consisting of 16 factorial points, 8 axial points (α = 2) and seven replicates at the center point were designed. Levels of selected parameters are shown in Table 1. As presented in Table 1, each independent variable was coded in 5 levels (−2, -1, 0, 1 and 2) as xi according to Equation 4:
where X0 is value of the Xi (selected parameters) at the center point and ΔX presents the step change. Acid Black 172 removal efficiency was taken as the response of the experiments according Equation 5:
where Yi is the percentage of dye removal efficiency
b0= the constant coefficient
bi = the regression coefficients for linear effects
bii = the quadratic coefficients
bij = the interaction coefficients
and xi, xj are the coded values of the parameters.
The statistical software “Minitab”, version 15.1.1.0 was used for the regression and graphical analyses of the experimental data obtained. The accuracy of the fitted model was justified through analysis of variance (ANOVA) and the coefficient of R2.
Results
Development of regression model equation and validation of the model
The design matrix with experimental and predicted Acid Black 172 removal efficiencies are listed in Table 2. The final model is expressed by:
Estimated P values of the parameters for Acid Black 172 removal efficiency (%) are illustrated in Figure 2. As depicted in Figure 2, the amounts of P (P = 0.00) for all independent parameters confirms that four selected factors are significant. However, it was found that all square and interaction terms except x1 2, x2 2, x1x2, x1x3, x1x4 and x3x4 (P ≥ 0.05) were significant to the response. The analysis of variance (ANOVA) for the Acid Black 172 dye removal efficiency is given in Table 3, According to this table, the P value of 0 (P ≤ 0.05) justifies the reliability of the fitted polynomial model through ANOVA with 95% confidence level. Furthermore, parity plot for the experimental and predicted value of Acid Black 172 removal efficiency (%) is demonstrated in Figure 3. High R2 value of 94.48% validates the statistical significance of the model for the selected dye removal.
In addition, normal probability and residuals versus fitted values plots for Acid Black 172 removal efficiency are illustrated in Figure 4. As seen in Figure 4(a), the normality assumption was relatively satisfied as the points in the plot form fairly straight line. The reliability of the model was also examined with the plot of residuals versus fits in Figure 4(b). As illustrated in this figure, no series of increasing or decreasing points, patterns such as increasing residuals with increasing fits and a predominance of positive or negative residuals should be found. As a result, Figure 4 shows that the model is adequate to describe Acid Black 172 removal efficiency by response surface methodology.
Effects of operating parameters
The main effect of each parameter on the Acid Black 172 removal efficiency is shown in Figure 5. For a better explanation, 3D plots are also presented in Figure 6. As illustrated in Figure 5, by decreasing in initial pH and initial dye concentration, and increasing in applied current and reaction time, dye removal efficiency improved. For instance, Acid Black 172 removal efficiencies decreased from 96.6% to 73.4% with the increase in initial pH from 4 to 10, respectively. In this investigation, according to Figure 5(a), best performances of EC system for dye removal were obtained at initial pH of 4.
Surface plots as a function of: (a) initial dye concentration and applied current; (b) initial dye concentration and initial pH; (c) initial pH and applied current (d) initial pH and reaction time; (e) reaction time and initial dye concentration; (f) reaction time and applied current. Hold values: (initial pH =7, initial dye concentration =300 mg/L, applied current =2 A, and reaction time = 9 min).
Process optimization
In order to determine the optimum point by electrocoagulation process, the desired objective in terms of Acid Black 172 removal efficiency was defined as target to achieve 90% removal efficiency. Table 4 shows the optimum values for Acid Black 172 removal from aqueous solution. The first row of this table is optimal conditions without any starting value. The optimum points from second to fifth rows in Table 4 was obtained with consideration of 4, 0.5 A and 3 min, as starting values for initial pH, applied current and reaction time, respectively. The initial dye concentrations of 150, 300, 450 and 600 mg/L were selected for second, third, fourth and fifth rows as starting values, correspondingly. As reported in Table 4, the experimental dye removal efficiencies and RSM predictions are in close agreement.
Dye removal kinetic
The influence of reaction time on dye removal at different initial concentrations is illustrated in Figure 7(a). Second order kinetic model according to Equation 7 is:
where Ct, Co, and k are dye concentrations at any time t, initial dye concentration, and kinetic constant, respectively. Plots of (1/Ct-1/C0) with time are shown in Figure 7(b) for various initial dye concentrations (from 50 to 600 mg/L), at initial pH of 7 and applied current of 2 A. As demonstrated in this figure, reaction rate follows second order kinetic and its values increases from 0.001/min to 0.041/min when initial dye concentration decreased from 600 to 50 mg/L in the solutions, respectively.
Discussion
According to the obtained results, the most and the least important independent parameters were initial dye concentration and initial pH, respectively. Similar to our results, Aleboyeh et al. [22], Alinsafi et al. [21] and Arslan-Alaton et al. [23] study groups reported that initial pH was the least important parameter in comparison with the other variables. In addition, Durango-Usuga et al. [25] and Srivastava et al. [26] expressed that initial dye concentration is one of the most important factors in decolorization optimization respectively by Factorial and Taguchi designs, which is similar to our results.
Percentages of dyes removal in treatment by electrocoagulation process under optimized conditions through design of experiment methods (RSM, Taguchi and Factorial designs) are compared in Table 5. Present study shows 90.4% Acid Black 172 removal efficiency using electrocoagulation process through RSM at optimum point. As reported in Table 5, Alinsafi et al. [21] and Yildiz [27] achieved over 90% dyes removal efficiency at much higher reaction time and lower current density, respectively in comparison with the present study.
Many Researchers have examined the impact of different parameters including initial pH, initial dye concentration, current density and reaction time on the dye removal efficiency in complex electrocoagulation process. Some study groups showed that the increase in current density and reaction time and the decrease in initial dye concentration improved the decolorization efficiency [6, 19, 22, 28], which is similar to our results. However, optimum initial pH reported for different types of anionic dyes removal in electrocoagulation process was different. For example, optimum initial pH was reported 7, 5–9 and 4–6.5 by Aleboyeh et al. [22], Aoudj et al. [6] and Basiri Parsa et al. [20] study groups, respectively. Lower optimum initial pHs were also obtained by other researchers [26, 27, 29].
According to our knowledge, up to now there is no research available on treatment of Acid Black 172 in aqueous media by electrocoagulation procedure. Therefore, the observed data from our results have been compared with the other treatment methods of Acid Black 172. For instance, Du research group obtained 86% Acid Black 172 removal by Pseudomonas sp. DY1 at their optimum conditions through response surface methodology [30], which is close to our results.
Conclusions
According to the results of this investigation, RSM is a powerful statistical optimization tool for Acid Black 172 removal using electrocoagulation process. The RSM results revealed that four selected parameters as well as some of their squares and interactions influenced the electrocoagulation performance. High R2 value of 94.48% through ANOVA, verified that the accuracy of the Minitab proposed polynomial model is acceptable. The optimum Acid Black 172 removal efficiency were found at initial pH of 7, initial dye concentration of 300 mg/l, applied current of 2 A and reaction time of 9.16 min. An experiment was performed in optimum conditions which confirmed that the model and experimental results are in close agreement (90.4% compared to 90% for the model).
References
Rezaee A, Ghaneian MT, Khavanin A, Hashemian SJ, Moussavi GH, Ghanizadeh GH, Hajizadeh E: Photochemical oxidation of reactive blue 19 dye (RB19) in textile wastewater by UV/K2S2O8 process. Iran J Environ Health Sci Eng. 2008, 5 (2): 95-100.
Sadri Moghaddam S, Alavi Moghaddam MR, Arami M: Decolorization of an acidic dye from synthetic wastewater by sludge of water treatment plant. Iran J Environ Health Sci Eng. 2010, 7 (5): 437-442.
Ehrampoush MH, Ghanizadeh GH, Ghaneian MT: Equilibrium and kinetics study of reactive Red 123 dye removal from aqueous solution by adsorption on eggshell. Iran J Environ Health Sci Eng. 2011, 8 (2): 101-108.
Mohammadian Fazli M, Mesdaghinia AR, Naddafi K, Nasseri S, Yunesian M, Mazaheri Assadi M, Rezaie S, Hamzehei H: Optimization of reactive blue 19 decolorization by ganoderma sp. using response surface methodology. Iran J Environ Health Sci En. 2010, 7 (1): 35-42.
Hasani Zonoozi M, Alavi Moghadam MR, Arami M: Removal of acid red 398 dye from aqueous solutions by coagulation/flocculation pocess. J Environ Eng & Manage. 2008, 7 (6): 695-699.
Aoudj S, Khelifa A, Drouiche N, Hecini M, Hamitouche H: Electrocoagulation process applied to wastewater containing dyes from textile industry. Chem Eng Process. 2010, 49 (11): 1176-1182. 10.1016/j.cep.2010.08.019.
Yang Y, Wang G, Wang B, Li Z, Jia X, Zhou Q, Zhao Y: Biosorption of acid black 172 and Congo Red from aqueous solution by nonviable penicillium YW 01: kinetic study, equilibrium isotherm and artificial neural network modeling, bioresour. Technol. 2011, 102 (2): 828-834.
Malakootian M, Yousefi N: The efficiency of electrocoagulation process using aluminum electrodes in removal of hardness from water. Iran J Environ Health Sci Eng. 2009, 6 (2): 131-136.
Murugan AA, Ramamurthy T, Subramanian B, Kannan CS, Ganesan M: Electrocoagulation of textile effluent: RSM and ANN modeling. Int J Chem React Eng. 2009, 7: 10.2202/1542-6580.1942.
Bhatti MS, Kapoor D, Kalia RK, Reddy AS, Thukral AK: RSM and ANN modeling for electrocoagulation of copper from simulated wastewater: Multi objective optimization using genetic algorithm approach. Desalination. 2011, 274 (1–3): 74-80.
Bazrafshan E, Mahvi AH, Nasseri S, Shaieghi M: Performance evaluation of electrocoagulation process for diazinon removal from aqueous environments by using iron electrodes. Iran J Environ Health Sci Eng. 2007, 4 (2): 127-132.
Daneshvar N, Khataee AR, Amani Ghadim AR, Rasoulifard MH: Decolorization of C.I. Acid Yellow 23 solution by electrocoagulation process: Investigation of operational parameters and evaluation of specific electrical energy consumption (SEEC). J Hazard Mater . 2007, 148 (3): 566-10.1016/j.jhazmat.2007.03.028.
Behbahani M, Alavi Moghaddam MR, Arami M: Techno-economical evaluation of fluoride removal by electrocoagulation process: Optimization through response surface methodology. Desalination. 2011, 271 (1–3): 209-218.
Montgomery DC: Design and analysis of experiments. 2000, USA: John Wiley and Sons, 5
Khuri AI: (Ed): Response surface methodology and related topics. 2006, Singapore: World Scientific
Ölmez T: The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. J Hazard Mater. 2009, 162 (2–3): 1371-1378.
Sadri Moghaddam S, Alavi Moghaddam MR, Arami M: Response surface optimization of acid red 119 dye from simulated wastewater using Al based waterworks sludge and polyaluminium chloride as coagulant. J Environ Manage. 2011, 92 (4): 1284-1291. 10.1016/j.jenvman.2010.12.015.
Balla W, Essadki AH, Gourich B, Dassaa A, Chenik H, Azzi M: Electrocoagulation/electroflotation of reactive, disperse and mixture dyes in an external-loop airlift reactor. J Hazard Mater. 2010, 184 (1–3): 710-716.
Mollah MYA, Gomes JAG, Das KK, Cocke DL: Electrochemical treatment of orange II dye solution—use of aluminum sacrificial electrodes and floc characterization. J Hazard Mater. 2010, 174 (1–3): 851-858.
Basiri Parsa J, Rezaei Vahidian H, Soleymani AR, Abbasi M: Removal of acid brown 14 in aqueous media by electrocoagulation: optimization parameters and minimizing of energy consumption. Desalination. 2011, 278 (1–3): 295-302.
Alinsafi A, Khemis M, Pons MN, Leclerc JP, Yaacoubi A, Benhammou A, Nejmeddine A: Electro-coagulation of reactive textile dyes and textile wastewater. Chem Eng Process. 2005, 44 (4): 461-470.
Aleboyeh A, Daneshvar N, Kasiri MB: Optimization of C.I. Acid Red 14 azo dye removal by electrocoagulation batch process with response surface methodology. Chem Eng Process. 2008, 47 (5): 827-832. 10.1016/j.cep.2007.01.033.
Arslan-Alaton I, Kobya M, Akyol A, Bayramoğlu M: Electrocoagulation of azo dye production wastewater with iron electrodes: process evaluation by multi-response central composite design. Color Technol. 2009, 125 (4): 234-241. 10.1111/j.1478-4408.2009.00202.x.
Nourouzi MM, Chuah TG, Choong TSY: Optimisation of reactive dye removal by sequential electrocoagulation–flocculation method: comparing ANN and RSM prediction. Water Sci Technol. 2011, 63 (5): 985-995. 10.2166/wst.2011.280.
Durango-Usuga P, Guzmán-Duque F, Mosteo R, Vazquez MV, Peñuela G, Torres-Palma RA: Experimental design approach applied to the elimination of crystal violet in water by electrocoagulation with Fe or Al electrodes. J Hazard Mater. 2010, 179 (1–3): 120-126.
Srivastava VC, Patil D, Srivastava KK: Parameteric optimization of dye removal by electrocoagulation using Taguchi methodology. Int J Chem React Eng. 2011, 9: 10.1515/1542-6580.2299.
Yildiz YS: Optimization of bomaplex Red CR-L dye removal from aqueous solution by electrocoagulation using aluminum electrodes. J Hazard Mater. 2008, 153 (1–2): 194-200.
Sengil IA, Ozacar M: The decolorization of C.I. Reactive black 5 in aqueous solution by electrocoagulation using sacrificial iron electrodes. J Hazard Mater. 2009, 161 (2–3): 1369-1376.
MartÍnez-Huitle CA, Brillas E: Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal, B Environ. 2009, 87 (3–4): 105-145.
Du LN, Yang YY, Li G, Wang S, Jia XM, Zhao YH: Optimization of heavy metal-containing dye acid black 172 decolorization by pseudomonas sp. DY1 Using statistical designs. Int Biodeter Biodeg. 2010, 64 (7): 566-573. 10.1016/j.ibiod.2010.06.009.
Acknowledgements
The authors are grateful to the Amirkabir University of Technology (AUT) research fund for the financial support. In addition, the authors wish to express thanks to Mr. Masoud Asadi Habib and Mr. Mohsen Behbahani (former MSc students of Amirkabir University of Technology), and Ms. Lida Ezzedinloo for their assistance during experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors also declare that they have no competing interests.
Authors’ contributions
All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Taheri, M., Moghaddam, M.R.A. & Arami, M. Optimization of Acid Black 172 decolorization by electrocoagulation using response surface methodology. J Environ Health Sci Engineer 9, 23 (2012). https://doi.org/10.1186/1735-2746-9-23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1735-2746-9-23