Abstract
The effect of chamber pressure on the microstructure and ablation behavior of ZrB2 coatings deposited by low-pressure plasma spraying was investigated. The results showed that as the spray chamber pressure further was reduced to less than 50 kPa, the porosity of the coating deposited at the same distance decreased with the chamber pressure, and the coating prepared under 100 Pa presented the lowest porosity of about 0.89%. The ablation performance test subjected to high-temperature plasma jet revealed that the linear ablation rate of ZrB2 coating increased with the porosity of the coating. As a result, among the ZrB2 coatings deposited at chamber pressures of 100 Pa, 5 kPa, 10 kPa and 50 kPa, the dense coating deposited at 100 Pa showed the lowest ablation rate of 0.33 µm/s. The dense ZrB2 coating with a thickness of about 100 μm was able to withstand 300 s ablation by a plasma flame with a net power of 25 kW resulting in an ablating coating surface temperature of about 2000 °C. The ablation mechanism of the coating was also examined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon fiber-reinforced carbon (C/C) composites are considered to be potential high-temperature structural materials in aerospace field due to their high thermal conductivity (Ref 1), low density (Ref 2, 3), high specific strength (Ref 2,3,4) and excellent high-temperature mechanical properties (Ref 5). However, C/C composites would be oxidized in an environment containing oxygen when temperature reaches higher than 500 °C, and the oxidation rate increases rapidly with the increase in temperature, which limits its applications (Ref 6). For example, C/C composite parts, such as nozzle-throat in solid rocket motor and wing leading edges of hypersonic vehicles, are usually subjected to high-temperature oxidation and airflow. Improvement in ablation resistance of C/C composites is in great demand in such applications. At present, one of the methods for improving the ablation performance of C/C composites is to apply ultra-high-temperature ceramic (UHTC) coatings on C/C composite surface. The main preparation methods include pack cementation (Ref 7,8,9), CVD (Ref 10,11,12,13) and plasma spraying (Ref 14,15,16,17,18). Among these methods, plasma spraying is an efficient method to prepare UHTC coatings, which can quickly deposit coatings on large-area workpieces for commercial production.
The refractory boride ZrB2 has a high melting point of 3245 °C, and its oxidation also leads to the formation of high melting point oxide ZrO2, being 2680 °C. It is known that ZrB2 has a slow oxidation rate at high temperatures. Even if it is oxidized during ablation, the generated ZrO2 scale could also provide protection for the C/C composites. Thus, ZrB2 has been proven to have outstanding ablation resistance. Atmospheric plasma spraying (APS) and supersonic atmosphere plasma spraying (SAPS) are widely used for depositing ZrB2-based coatings. Aliasgarian et al. deposited ZrB2-20 vol.% SiC coating with agglomerated powder on SiC-coated graphite by shrouded plasma spraying (Ref 19). Wang et al. prepared mullite-modified ZrB2-80 vol.% MoSi2 coating for C/C composites by SAPS (Ref 20). Hu et al. have deposited ZrB2/MoSi2 laminated coating on SiC-coated C/C composites by SAPS (Ref 21). Niu et al. fabricated ZrB2-20 vol.% MoSi2 composite coatings by APS and vacuum plasma spraying (VPS) and reported that the 1500 °C oxidation behaviors of these two coatings were quite different (Ref 22). It can be considered that their difference can be attributed to microstructure difference of the coatings deposited by two different plasma spray methods
Up to now, it is believed that addition of silicon-containing dopants such as SiC to ZrB2-based coating could improve the ablation performance. It is because the formed SiO2 due to oxidation of silicide is usually at liquid state and it could penetrate into the coating layer and seal the pores. However, because SiC decomposes into gaseous phase at temperatures higher than 2800 °C, the introduction of SiC into the Zr-based ceramic coating is usually made by using the agglomerated SiC-ZrB2 composite powders along with control of particle heating to a semi-molten state during plasma spraying. Accordingly, partial of the SiC could be retained into the coating. Since ZrB2 has a high melting point of 3245 °C and the heat transfer from one submicrometer ZrB2 to another is usually poor due to the limited connection, the agglomerated composite particles are usually very difficult to be fully melted in plasma jet. Subsequently, such composite powder particles are usually deposited in a partially molten state at relatively low particle velocities in plasma spray. Consequently, a large number of pores would be formed in the coating due to the poor spreading of partially molten particles along with the pores retained from those in porous unmelted powder. Therefore, based on the powder characteristics and requirement to retain SiC in the coating during plasma spray deposition, it is difficult to deposit sufficiently dense ZrB2-based coating. The porous feature of resultant composite coating requires sealing of in situ service at high temperature to prevent oxygen penetration into ZrB2 coating. Moreover, for coatings deposited under ambient atmosphere, the spray particles are oxidized, because air could get involved into the plasma jet (Ref 15,16,17, 19). The incorporation of oxides resulting from oxidation of spray particles during spraying reduces the content of ablation-resistant pure ZrB2 phase and thus deteriorates the overall ablation resistance. Therefore, it can be considered that if a sufficiently dense ZrB2-based coating can be deposited, ZrB2 coating without self-sealing dopants could also perform well under high temperature.
Plasma jet expands significantly at a very low-pressure atmosphere. Under a chamber pressure of 100 Pa, spray particle velocity becomes much higher. Thus, using very low-pressure plasma spraying technology an oxide-free dense coating can be deposited. In this work, ZrB2 coatings were deposited under a chamber pressure of 100 Pa. In order to examine the effect of chamber pressure, the coatings were also deposited under different chamber pressures from 100 Pa to 50 kPa. The phase compositions and microstructures of the coatings were characterized. The ablation performance of the coatings was tested using 25 kW net power plasma flame. The microstructure of coating after ablation was characterized.
Experimental Procedure
Materials and Coating Deposition Process
To investigate the effect of chamber pressure and spray distance on the coating microstructure, sintered YSZ buttons instead of C/C composites with dimensions of Φ20 mm × 1 mm were employed as the substrate for ZrB2 coating deposition. It is because the microstructure of ZrB2 coating is independent on the substrate material. For the samples used for the ablation test, the cylindrical specimens (Φ30 mm × 10 mm) were cut from 2.5D C/C composites with a density of 1.87 g/cm3 and used for the substrate material. They were sand-blasted using corundum sand to remove graphite near surface, then cleaned ultrasonically in alcohol bath and dried at 80 °C for 2 h.
The Si coating with a thickness from 200 to 400 m was prepared under a chamber pressure of <100 Pa on C/C composites. Subsequently, the samples were transferred into a high-temperature furnace, and then heat-treated at 1450 °C for 1 h in a vacuum environment in order to obtain a SiC buffer layer (Ref 23).
A commercially available ZrB2 powder (Shanghai No-Nano Tech Co., Ltd., China) with a particle size range of 5-30 µm and purity of 99.9% was used as feedstock material. Fig. 1 shows the morphology of ZrB2 powder. The coating deposition was carried out by a low-pressure plasma system equipped with a F4-VB torch (MF-P 1000 APS/VPS, GTV, Germany). The nozzle diameter is 10 mm, and the distance between the powder injection position and the nozzle exit is 20 mm. The plasma torch was fixed on a six-axis robot which was placed in a 10 m3 chamber with a controlled atmosphere. The spray parameters are summarized in Table 1.
Ablation Tests
The ablation performance of the coatings was tested with the plasma spray torch which was used for the preparation of the coatings. The gas flow rates of Ar and H2 were 50 and 6 slpm, respectively. The energy input to the plasma arc was 50 kW and the energy taken away by cooling water was 25 kW, yielding a net plasma jet power of 25 kW. The distance between the nozzle tip of plasma torch and the top surface of samples was 80 mm. The flame temperature was estimated to be around 2100-2200 °C at the position of sample surface. The samples were placed vertically to the flame for 30-300 s to perform the ablation. Mass and linear ablation rates were measured to evaluate the ablation resistance of the coatings. The mass and linear ablation rates were calculated as follows:
where Rm and Rd are the mass rate and linear ablation rate, respectively; m0 and m1 refer to the weights of the specimens before and after ablation, d0 the thickness of the original ZrB2 coating and d1 the unoxidized coating thickness at the center of specimens after ablation, and t the ablation time.
Coating Characterization
The phase compositions and crystalline structure of the coatings were analyzed by x-ray diffraction (XRD, D8 advance, Germany) with the Cu radiation. The surface morphology, fracture surface morphology, cross-sectional microstructure and element distribution of the coatings before and after ablation were examined by scanning electron microscopy (SEM, MIRA3 LMH, TESCAN, Czech Republic) along with an energy-dispersive spectroscopy (EDS). The porosity of the sprayed coatings was estimated by image analyzing using Image J software and the average from 10 pictures was taken for each sample.
Results and Discussion
Phase Composition and Microstructure
The XRD patterns of the starting powder and ZrB2 coatings deposited under a spray distance of 200 mm and different chamber pressures of 100 Pa, 5 kPa, 10 kPa and 50 kPa are shown in Fig. 2. ZrB2 diffraction peaks can be clearly observed. Weak peaks corresponding to ZrO2 were observed due to the impurity of backfill gas. It was observed from Fig. 2 that the intensity of such peaks in the coatings decreased with the chamber pressure and became undetectable at the chamber pressure of 100 Pa.
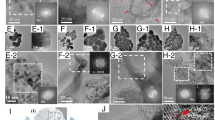
The surface morphology of the coatings is shown in Fig. 3. Coating surfaces were relatively rough, including smooth splat surface and sub-micrometer particles produced by splashing. A lot of semi-molten particles were observed on the surface of the coating prepared at 50 kPa. No obvious crack or defect was observed on the coating surfaces.
The cross-sectional microstructures of the coatings with the same spray passes of 15 times are shown in Fig. 4. At the same chamber pressure, the coating thickness increased with decreasing spray distance. It might be attributed to the improved deposition efficiencies with decreasing spray distance and the reduced area where spray particles are deposited on. The coatings were dense and the interfaces between splats were barely visible when the chamber pressure became less than 5 kPa. The fracture surface morphology of the typical coatings shown in Fig.5 presents no evident lamellar structure which is typical for thermal spray ceramic coating. This fact indicates that sintered bulk-like coatings were formed at low pressure as shown by marked areas. Thermally sprayed ceramic coatings usually show a lamellar porous microstructure with limited bonding between splats. The bonding ratio between adjacent splats always plays a key role in determining the physical barrier properties including ablation, oxidation and corrosion resistance. However, with the increase in particle velocity under low chamber pressure, high impact velocity could enhance infiltration of the spreading molten particles into surface cavities by increasing dynamic contact pressure. This helps to get a dense coating. On the other hand, at lower chamber pressure the plasma jet could directly heat the substrate surface to higher temperature which has been demonstrated helpful to chemical bonding formation between splats. In our previous study, it has been proven that as the interface temperature between splat and previous deposited coating layer is heated up a temperature higher than its glass transition temperature, the mobility of the surficial atoms in the melt at the interface is greatly enhanced to form the bond with the splats underneath (Ref 24). The interface between splats was still visible in the coatings deposited at 10 kPa due to the high critical bonding temperature of ZrB2, which can be estimated to be about 900 °C based on the reported theory (Ref 24). Under a chamber pressure of 50 kPa, due to the shorter plasma jet and heating distance, many pores are present in the coating and the coating contained a large number of semi-molten particles. The formation of open pores by shielding effect of semi-molten particles and incomplete stacking of particles is possible based on the surface morphology of the coating prepared at 50 kPa, which are difficult to be fully filled by semi-molten particles with poor flowability, thus resulting in a coating with high porosity.
The porosity of the sprayed coatings was also measured as shown in Fig. 6. The coatings deposited under 100 Pa presented the lowest porosity for each different spray distance. Furthermore, the porosity of the coating increased as the spray distance increased under the same chamber pressure.
The coatings for ablation test with a thickness of about 100 µm were prepared on SiC-coated C/C composites under the same spray distance of 150 mm, which were marked as ZB100, ZB5k, ZB10k and ZB50k according to the chamber pressure. The porosity levels of ZB100, ZB5k, ZB10k and ZB50k were about 0.89%, 1.48%, 4.06% and 9.47%, respectively, which are also shown in Fig. 6. The test results showed that the porosity of the coatings deposited at 150 mm was consistent with the results described above. The cross-sectional microstructure of ZrB2/SiC coatings deposited on C/C composites under different chamber pressures and the element distributions of Zr and Si are shown in Fig. 7. It can be seen from the Si element distribution in Fig. 7(a3, b3, c3, d3) that the molten Si penetrated into the gap between C fiber and graphite of the substrate and reacts with C to form SiC after heat treatment (Ref 23), forming a gradient expansion coefficient transition layer near the surface of C/C composites. As seen from Fig. 7(a1, b1, c1, d1), there were no obvious cracks and pores at the interface between SiC layer and ZrB2 layer, which infers a good bonding between them. The coefficient of thermal expansion (CTE) of SiC is about 4.6×10-6/K, a value between those of the C/C composites (1-2×10-6/K) and ZrB2 (6.9×10-6/K), and thus the SiC layer effectively contributes to the relaxation of the thermal mismatch between C/C composites and ZrB2.
Ablation properties of ZrB2 coatings
No spalling occurred during ablation process to all samples after 120 s ablation. The temperature/time curves of ZB100 sample during ablation test are illustrated in Fig. 8. The surface temperature rose rapidly and then became stable after about 1 minute. The final temperatures were 2115, 1980, 1870, 1860 and 1900 °C, for the C/C, ZB100, ZB5k, ZB10k and ZB50k samples, respectively.
Figure 9 shows the XRD patterns of the coatings after ablation for 120s. Only monoclinic zirconia (m-ZrO2) phase was observed on the surface of oxidized ZrB2 coatings, indicating that tetragonal zirconia (t-ZrO2) formed during high-temperature ablation test was completely converted into m-ZrO2 during cooling. No peaks of B2O3 phase were detected on the surface of coatings, which might be ascribed to its low melting point and high vapor pressure.
After ablation for 120 s, the linear loss rate of 15.2 µm/s and mass-loss rate of 31.66 mg/s were observed for C/C composites, and in the meantime, the tests yielded the mass-loss rates of 0.75±0.08 mg/s, 1.23±0.15 mg/s, 1.42±0.18 mg/s, and 2.67±0.29 mg/s for ZB100, ZB5k, ZB10k and ZB50k coatings, respectively. It is clear that the coated samples presented much lower mass-loss rate compared with bare C/C composite. Nevertheless, the samples mass should increase when ZrB2 was oxidized to ZrO2 if the oxide layer did not peel off. However, after 120 s ablation, as shown above by the mass-loss rates the mass of all samples decreased, which indicates that the peeling of ZrO2 occurred partially since it will be shown that most of ZrO2 was still attached to C/C composite. Therefore, as the linear ablation rate, the linear oxidation rate of the coating was used to characterize the ablation resistance of the coatings in the present study. The linear ablation rate will be discussed in the next section.
Surface and Cross Section Analysis after Ablation
Figure 10 illustrates the surface morphology of ZrB2 coatings after ablation for 120 s at ablation center area. A significant change in morphology occurred to the surface of the coated samples. The oxidation products of a flaky structure were observed on the surface after ablation and the density of platelets increased with the increase in the porosity of the coatings. Similar oxide sheets were also observed in the oxidation experiments of ZrB2-SiC-WB composite coatings reported by Niu et al (Ref 25). As can be seen from the EDS result shown in Fig. 11, the platelets are B2O3 crystals. It can be inferred that those boria platelets were formed during the high-speed cooling process when ablation plasma arc turned off. More platelets formed on the surface of the coatings with higher porosity during cooling. This can be attributed to faster ablation rates and higher concentration of B2O3 vapor near the surface. Since the maximum thickness of the B2O3 platelets was only 0.5 µm, and the ZrO2 layer near the surface did not contain boria because the surface temperature during ablation was higher than the melting point of boria, no peak of B2O3 was detected in the XRD patterns of the ablated samples as shown in Fig. 9 due to limited amount of boria.
Figure 12 shows the cross-sectional microstructures of the coatings after ablation for 120 s, at both the ablation central and edge areas. The SiC inner coating remained intact and bonded with the C/C composites closely. After ablation, the coatings were divided into two distinct layers. The outer ZrB2 coating was oxidized into fine ZrO2, while the inner coatings remained unoxidized and bonded with the SiC coating. The linear ablation rate of ZrB2 coatings is shown in Fig. 13. It can be clearly observed that the linear ablation rate of coating decreases with the decrease in the coating porosity. When the coating consisted entirely of columnar crystals formed across the interfaces of multiple splats as shown in Fig. 5(a, b) the dense coating would present the same ablation resistance as the bulk. Thus, the coating showed excellent ablation resistance with low porosity because such dense coating hardly allows the permission of oxygen into it. As result, it can be considered that the oxidation of ZrB2 coating will proceed towards the inner gradually at the same ablation rate dependent on the local temperature.
The microstructures of ZrO2 grains near the ZrO2-ZrB2 interface are shown in Fig. 14. The ZrO2 produced from the oxidation of ZB100 coating presented equiaxed crystals, similar to the structure of oxide layer generated by oxidation of the ZrB2 bulk at 1500 °C (Ref 26). This fact reveals that the ablation of the coating occurs in the same manner as that of ZrB2 bulk. On the other hand, it was observed that the grains of crystalline zirconia from all other coatings exhibited an elongated columnar-like structure. The formation of columnar-like ZrO2 has been reported upon oxidation of ZrB2 (Ref 27) and ZrB2-SiC composites (Ref 28,29,30). The oriented growth of the oxide crystallites was most likely due to the faster oxidation rate of coatings with higher porosity, since the gasification of B2O3 promotes the growth of zirconia in a direction parallel to the discharge of the gas by-products. Therefore, as the oxidation rate increases, the discharge rate of B2O3 gas increases, which promotes the ZrO2 grain to show an obvious columnar structure.
In the ablation processes, the coatings are eroded by complex corrosion processes, such as mechanical scour, chemical and physical corrosions. The main chemical and physical changes of the ZrB2 coating are shown as following:
Due to the evaporation and discharge of B2O3, the ZrO2 layer is porous and could not restrict the penetration of oxygen. At 120 s, the temperatures of the surface and backside of the samples were about 2000 and 850 °C, respectively, and the thickness of ZrO2 layer was about 40 µm. Supposing that the thermal conductivities of the oxide scale, the unoxidized ZrB2 coating, and C/C composites were 1.5, 30 and 700 W·m-1·K-1 (Ref 31,32,33), respectively, and ignoring the effect of thin SiC layer, the temperature of the ZrO2-ZrB2 interface would be estimated to be about 1700 °C, which is close to the boiling point of B2O3 of 1860 °C. Although ZrO2 could act as a thermal barrier layer to lower the temperature of inner layer, B2O3 still could not provide sufficient protection due to the high temperature during ablation. This was also evidenced by the EDS analysis of the scale shown in Fig. 15, where the B content near the ZrB2-scale interface was at a value close to that of the upper region. The low ablation resistance of the coating with high porosity is mainly due to the existence of pores in ZrB2 coating near the ZrB2-scale interface. Such pores will allow oxygen to penetrate into the coating, which increases the contact area of the coating with oxygen and allows multiple layers of splats to contact with oxygen simultaneously. The higher the porosity of the coating, the deeper the oxygen penetrates in the coating and the faster the ablation of the coating.
Ablation Properties of ZB100 Coatings for Different Times
After being ablated for 30, 60, and 300 s by the plasma flame, the mass and linear rates of ZB100/SiC-coated composites are shown in Table 2. The coating mass increased after 30 s and 60 s ablation. However, since the linear ablation rate remained constant during 60 s, the result of a smaller mass increase rate at 60 s indicates that oxide layer had started to peel off at 60 s due to the stress introduced by the volume expansion of the transformation from ZrB2 to ZrO2 and phase transformation of ZrO2 during cooling process. Moreover, it is evident from the cross sections of ablated samples that the delamination of ZrO2 was attributed to buckling of near surface ZrO2 layer. Such buckling is caused by the compressive stress in the ZrO2 scale, being originated from the volume expansion.
Figure 16 shows the surface morphologies of ZB100 coatings after ablation for different times. After the 30 s ablation (Fig. 16a), oxide platelets began to appear, and the splats with smooth surface transformed into equiaxed crystals of ZrO2. At the same time, it can be inferred that the concentration of B2O3 vapor near the coating surface was higher during the cooling process due to further oxidation after longer ablation test, and therefore the more and larger B2O3 platelets appeared on the surface for longer ablation time. Figure 16(d) shows the surface morphology of the sample ablated for 300 s. It was considered that liquid phase appeared during ablation process. From the EDS results (Fig. 16), it can be inferred that the grey glassy regions were mainly distinguished as SiO2 and the bright solid regions were composed of ZrO2. The linear ablation rate of the coatings for 300 s ablation became a little lower than the 120 s results. This may be possibly due to the self-sealing effect of liquid boria in the ZrO2 scale near the ZrB2/ZrO2 interface.
The cross sections SEM images of the ZB100 coatings after ablation are exhibited in Fig. 17. The thickness of oxide layers generated by ablation for 30, 60, and 300 s is 10, 20, and 75 µm, respectively. Because of the lack of a heat input standard for ablation tests, the present work compares the ablation resistance of coatings from other investigators using the ablation rate of C/C composites as the standard. The linear ablation rate of C/C composites under the ablation parameters selected in this work was 15.2 µm/s. Li et al. prepared ZrC-SiC coatings by solid-phase infiltration and the linear ablation rate under the ablation test conditions with a linear ablation rate of 17.5 µm/s for C/C composite and reported the lowest linear ablation rate of 10.5 µm/s for coatings during 20 s ablation (Ref 34). Shi et al. prepared SiC-ZrC coatings by slurry infiltration, the linear ablation rate of C/C composites under the ablation test was also 17.5 µm/s and the linear ablation rate of the coating was 2.42 µm/s (Ref 35). It is evident that the dense ZrB2 coating deposited by very low-pressure plasma spray presents an excellent ablation resistance. As shown in Fig. 13(a), the thickness of oxide layer after ablation for 120 s is about 35 µm. The results show that for dense ZrB2 coating, the thickness of ZrO2 layer is approximately proportional to ablation time. The ZrO2 formed by the ablation of ZB100 coatings for different times presents equiaxed grains. In addition, due to the fluctuation of substrate surface, the thickness of ZrB2 coating was not completely uniform. Therefore, after 300 s ablation, although most regions still have unoxidized coating with thickness of 10 µm (Fig.17c), at a few locations the coating with a relatively thin thickness was completely oxidized. As a result, a fraction of SiC inner layer below was also oxidized, resulting in the appearance of holes in the SiC layer as shown in Fig 17(d). The oxidation of SiC was the reason why SiO2 was observed on the coating surface. Meanwhile, even after 300 s ablation, the oxide layer formed by the oxidation of ZrB2 was still attached to the substrate, thus the geometric linear ablation rate would be significantly smaller than the linear oxidation rate defined above.
From the above ablation results obtained for the coatings with different porosity levels and different ablation times, the following ablation model for ZrB2 can be proposed to explain the ablation behavior of the ZrB2 coatings of different porosity levels. The coatings were primarily oxidized successively from the top surface during the ablation process. The surface morphology of ZB100 coating after 30s ablation (Fig. 18) shows that the splats on the original coating surface were firstly oxidized to submicron oxide particles. Due to the discharge of gaseous boria by-product, there are opening through-pores in the scale which allow oxygen to come into contact with the fresh unoxidized coating. Although boria is present in the scale, the amount was small and no significant presence of boria was observed in the ablated samples, thus not providing sufficient protection. Therefore, since the coating is sufficiently dense, the oxidation of ZrB2 coating primarily takes place at the ZrB2-ZrO2 scale interface. The main reason for the different ablation rates of coatings with different porosity levels is that the pores at the ZrB2-scale interface allow oxygen to penetrate into ZrB2 coating at certain depth being positively related to porosity in the coating. The higher the porosity, the deeper the oxygen penetrates in the coating. As a result, the oxidation of ZrB2 occurs in a bulk layer with a certain thickness rather than that just occurring from ZrB2/scale interface as for the dense bulk ZrB2. Accordingly, the increased porosity in the coating increases the reaction area between ZrB2 and oxygen, making the oxidation proceed faster for coatings with high porosity as shown in Fig. 19. Moreover, owing to the presence of pores, multiple layers of splat might be oxidized simultaneously in coatings with high porosity when the oxygen is sufficient. As a result of the increased velocity of the powder particles at very low pressure, the low porosity of less than 1% effectively reduced the linear oxidation rate of the coating.
The current method to improve the ablation resistance of ultra-high-temperature ceramic coatings such as ZrB2 and ZrC is mainly to add additives that form high boiling point products under ablation, and sufficient liquid phase filled in the scale could reduce the ablation rate of the ceramic coatings. With the single phase of ZrB2 as in the present study, at the early stage since the temperature in the oxide scale is sufficiently high to evaporate boria the self-sealing effect was not observed. However, with the increase in the ZrO2 thickness during ablation, its thermal barrier effect reduces the temperature near the ZrB2-scale interface. It is evident that the linear ablation rate after 60s tends to decrease as shown in Table 2. Therefore, through the coating materials design the UHTC coating with the self-sealing effect and thus excellent ablation resistance may be developed assisted with the deposition of dense coating by very low-pressure plasma spraying.
Conclusions
ZrB2 coatings were prepared by low-pressure plasma spray with chamber pressures varying from 50 kPa to 100 Pa. The coating microstructure was characterized by SEM and ablation resistance of the resultant coatings was tested by plasma flame with a net power of 25 kW. Following conclusions can be drawn:
-
1)
When the spray distance was kept the same, the porosity of the coatings increased with the chamber pressure. Among all coatings deposited, the coating prepared at 100 Pa and 150 mm spraying distance presented the lowest porosity of about 0.89%.
-
2)
The linear ablation rate of the plasma sprayed ZrB2 coating increased with the coating porosity. During 120 s ablation, the linear ablation rate of the coating deposited at 100 Pa was as low as 0.29±0.03 µm/s, suggesting the best ablation resistance as compared with the literature result so far as the author’s best knowledge. The coating deposited at 100 Pa with a thickness of 100 μm could withstand 300 s ablation with a plasma jet of 25 kW net power which leads to a high surface temperature of about 2000 °C.
-
3)
An ablation model for the present ZrB2 coatings was proposed to explain the effect of coating porosity on the ablation behavior. During the ablation, oxygen reaches the ZrB2-scale interface through the opening through-scale pores of ZrO2 layer and continues to oxidize the ZrB2 coating. For the dense coating deposited under a pressure of 100 Pa, oxygen could only react with ZrB2 coating at the ZrB2-scale interface owing to the widespread bulk-like structure. High porosity leads to the penetration of oxygen into ZrB2 coating and thus oxidation occurs in a bulk layer rather than the interface region. The coating with a high porosity oxidizes in a high volume of the coating which results in high linear ablation rate.
References
P. Chowdhury, H. Sehitoglu and R. Rateick, Damage Tolerance of Carbon-Carbon Composites in Aerospace Application, Carbon, 2018, 126, p 382-393.
J.P. Zhang, Q.G. Fu and Y.J. Wang, Interface Design and HfC Additive to Enhance the Cyclic Ablation Performance of SiC Coating for Carbon/Carbon Composites from 1750 °C to Room Temperature under Vertical Oxyacetylene Torch, Corr. Sci., 2017, 123, p 139-146.
J. Ren, Y. Zhang, H. Hu, P. Zhang, T. Fei and L. Zhang, HfC Nanowires to Improve the Toughness and Oxidation Resistance of Si-Mo-Cr/SiC Coating for C/C Composites, Ceram. Int., 2016, 42, p 14518-14525.
Y. Chu, H. Li, Q. Fu, H. Wang, X. Hou, X. Zou and G. Shang, Oxidation Protection of C/C Composites with a Multilayer Coating of SiC and Si+SiC+SiC Nanowires, Carbon, 2012, 50, p 1280-1288.
Y. Chu, H. Li, L. Li and L. Qi, Oxidation Protection of C/C Composites by Ultra Long SiC Nanowire-Reinforced SiC-Si Coating, Corr. Sci., 2014, 84, p 204-208.
R. Djugum and K. Sharp, The Fabrication and Performance of C/C Composites Impregnated with TaC Filler, Carbon, 2017, 115, p 105-115.
X. Yao, H. Li, Y. Zhang, H. Wu and X. Qiang, A SiC-Si-ZrB2 Multiphase Oxidation Protective Ceramic Coating for SiC-coated Carbon/Carbon Composites, Ceram. Int., 2012, 38, p 2095-2100.
X. Yao, H. Li, Y. Zhang and Y. Wang, Oxidation and Mechanical Properties of SiC/SiC-MoSi2-ZrB2 Coating for Carbon/Carbon Composites, J. Mater. Sci. Technol., 2014, 30, p 123-127.
M.H. Hu, K.Z. Li, H.J. Li, B. Wang and H.L. Ma, Double layer ZrSi2-ZrC-SiC/SiC Oxidation Protective Coating for Carbon/Carbon Composites, Surf. Eng., 2014, 31, p 335-341.
Y.-J. Wang, H.-J. Li, Q.-G. Fu, H. Wu, D.-J. Yao and B.-B. Wei, Ablative Property of HfC-based Multilayer Coating for C/C Composites under Oxy-acetylene Torch, Appl. Surf. Sci., 2011, 257, p 4760-4763.
Y.-L. Wang, X. Xiong, X.-J. Zhao, G.-D. Li, Z.-K. Chen and W. Sun, Structural Evolution and Ablation Mechanism of a Hafnium Carbide Coating on a C/C Composite in an Oxyacetylene Torch Environment, Corr. Sci., 2012, 61, p 156-161.
P. Wang, Y. Qi, S. Zhou, P. Hu, G. Chen, X. Zhang and W. Han, Polycrystalline ZrB2 Coating Prepared on Graphite by Chemical Vapor Deposition, Phys. Status Solidi B, 2016, 253, p 1590-1595.
S.-L. Wang, K.-Z. Li, H.-J. Li, Y.-L. Zhang and T. Feng, Structure Evolution and Ablation Behavior of ZrC Coating on C/C Composites under Single and Cyclic Oxyacetylene Torch Environment, Ceram. Int., 2014, 40, p 16003-16014.
Y. Zhang, H. Wang, T. Li, Y. Fu and J. Ren, Ultra-high Temperature Ceramic Coating for Carbon/Carbon Composites Against Ablation Above 2000 K, Ceram. Int., 2018, 44, p 3056-3063.
Y. Yang, K. Li, G. Liu and Z. Zhao, Ablation-Resistant Composite Coating of HfC-TaC-SiC for C/C Composites Deposited by Supersonic Atmospheric Plasma Spraying, J. Ceram. Sci. Technol, 2016, 7, p 379-386.
Y.-J. Wang, H.-J. Li, Q.-G. Fu, H. Wu, L. Liu and C. Sun, Ablation Behaviour of a TaC Coating on SiC Coated C/C Composites at Different Temperatures, Ceram. Int., 2013, 39, p 359-365.
C. Sun, C.-MXu. Xu and W.-F. Cao, Effect of Mild Oxidation on Ablation Properties of ZrSiO4 Coating Prepared by Supersonic Atmospheric Plasma Spraying on Carbon/Carbon Composites, J. Therm. Spray. Tech., 2020, 29, p 1982-1990.
Luo, X., Yang, X., Huang, Q. et al.: Ablative Property and Mechanism of ZrC-TaC/ZrC-SiC Coatings on C/C Composites under Different Heat Fluxes. J. Therm. Spray. Tech., 2021, p 1-13
R. Aliasgarian, M. Naderi, S.E. Mirsalehi and S. Safi, The Ablation Behavior of ZrB2-SiC Coating Prepared by Shrouded Plasma Spray on SiC-Coated Graphite, J. Alloys. Compd., 2018, 742, p 797-803.
L. Wang, Q.G. Fu, N.K. Liu and Y.C. Shan, Supersonic Plasma Sprayed MoSi2-ZrB2 Antioxidation Coating for SiC-C/C Composites, Surf. Eng., 2016, 32, p 508-513.
D. Hu, Q. Fu, T. Liu and M. Tong, Structural Design and Ablation Performance of ZrB2/MoSi2 Laminated Coating for SiC Coated Carbon/Carbon Composites, J. Eur. Ceram. Soc., 2020, 40, p 212-219.
Y. Niu, Z. Wang, J. Zhao, X. Zheng, Y. Zeng and C. Ding, Comparison of ZrB2-MoSi2 Composite Coatings Fabricated by Atmospheric and Vacuum Plasma Spray Processes, J. Therm. Spray. Technol., 2016, 26, p 100-107.
C. Hu, Y. Niu, H. Li, M. Ren, X. Zheng and J. Sun, SiC Coatings for Carbon/Carbon Composites Fabricated by Vacuum Plasma Spraying Technology, J. Therm. Spray. Technol., 2011, 21, p 16-22.
S.-W. Yao, C.-J. Li, J.-J. Tian, G.-J. Yang and C.-X. Li, Conditions and Mechanisms for the Bonding of a Molten Ceramic Droplet to a Substrate After High-Speed Impact, Acta Mater., 2016, 119, p 9-25.
C. Li, Y. Niu, X. Zhong, T. Liu, X. Pan, M. Shi, X. Zheng and C. Ding, Effect of Heating-Cooling Rates on Microstructure Evolution of ZrB2-based Coatings During Oxidation, J. Eur. Ceram. Soc., 2019, 39, p 4565-4574.
W.G. Fahrenholtz, The ZrB2 Volatility Diagram, J. Am. Ceram. Soc., 2005, 88, p 3509-3512.
T.A. Parthasarathy, R.A. Rapp, M. Opeka and R.J. Kerans, A Model for the Oxidation of ZrB2, HfB2 and TiB2, Acta Mater., 2007, 55, p 5999-6010.
I.G. Talmy, J.A. Zaykoski, M.M. Opeka, and S. Dallek, Oxidation of ZrB2 ceramics modified with SiC and group IV-VI transition metal diborides, Elec. Chem. Soc. Proc, 2001, p 144-158
X.-H. Zhang, P. Hu and J.-C. Han, Structure Evolution of ZrB2-SiC During the Oxidation in Air, J. Mater. Res., 2011, 23, p 1961-1972.
J. Han, P. Hu, X. Zhang and S. Meng, Oxidation Behavior of Zirconium Diboride-Silicon Carbide at 1800 °C, Scr. Mater., 2007, 57, p 825-828.
Q. Mistarihi, M.-A. Umer, J.-H. Kim et al., Fabrication of ZrO2-based Nanocomposites for Transuranic Element-Burning Inert Matrix Fuel, Nucl. Eng. Des., 2015, 47, p 617-623.
H. Yuan, J. Li, Q. Shen and L. Zhang, Preparation and Thermal Conductivity Characterization of ZrB2 Porous Ceramics Fabricated by Spark Plasma Sintering, Int. J. Refract. Hard. Met., 2013, 36, p 225-231.
T.-Q. Li, Z.-H. Xu, Z.-J. Hu and X.-G. Yang, Application of a High Thermal Conductivity C/C Composite in a Heat-Redistribution Thermal Protection System, Carbon, 2010, 48, p 924-925.
Z. Li, H. Li, W. Li et al., Preparation and Ablation Properties of ZrC-SiC Coating for Carbon/Carbon Composites by Solid Phase Infiltration, Appl. Surf. Sci., 2001, 258, p 565-571.
X.-H. Shi, J.-H. Huo, J.-L. Zhu et al., Ablation Resistance of SiC-ZrC Coating Prepared by a Simple Two-Step Method on Carbon Fiber Reinforced Composites, Corr. Sci., 2014, 88, p 49-55.
Acknowledgments
The present project is financially supported by Research Funding for Innovation Team of Xi’an Jiaotong University (Grant No. xtr0118008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is an invited paper selected from presentations at the 2021 International Thermal Spray Conference, ITSC2021, that was held virtually May 25-28, 2021 due to travel restrictions related to the coronavirus (COVID-19) pandemic. It has been expanded from the original presentation)
Rights and permissions
About this article
Cite this article
Wang, D., Zhang, L., Zhang, SL. et al. Microstructure and Ablation Behavior of Low-Pressure Plasma Sprayed ZrB2 Coatings Down to 100 Pa. J Therm Spray Tech 31, 282–296 (2022). https://doi.org/10.1007/s11666-021-01290-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-021-01290-z