Abstract
Summary
Distinguishing oral bisphosphonates from other bone-sparing therapies, this retrospective observational study, first, characterized treated osteoporosis patients in the UK, and secondly, explored factors associated with the risk of discontinuation or switching between therapies. The latter should be considered when evaluating real-world data.
Purpose
This retrospective observational study evaluated the characteristics of women with postmenopausal osteoporosis, including comorbidities and determinants of treatment patterns with bone-sparing agents.
Methods
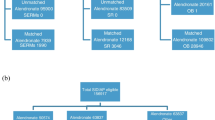
The UK Clinical Practice Research Datalink was used to identify postmenopausal women (aged ≥50 years) treated with a bone-sparing agent or diagnosed with osteoporosis between 1 January 1993 and 31 December 2008. Two non-mutually-exclusive subpopulations were defined: (1) patients active in the database on 31 December 2008; (2) patients treated with a bone-sparing agent since 1 January 1993. Subpopulation 1 was used to describe patient comorbidities and osteoporosis treatment history, and subpopulation 2 was used to explore the characteristics associated with bone-sparing treatment patterns use via multivariable regression for repeated multinomial responses.
Results
A total of 62,657 individuals met the inclusion criteria; subpopulation 1 comprised 38,469 women (61.4 %), of whom 21,687 received a bone-sparing agent in 2008 (99.7 % oral bisphosphonates and the remainder other agents). Those receiving other agents were more likely to have had previous treatment with bone-sparing agents, to have experienced previous fractures, and to have visited their doctor more frequently. Analyses also identified several comorbidities associated with an increased risk of discontinuation of bone-sparing agents, including heart disease, gastrointestinal disease, and renal failure. Anticonvulsant use was associated with a dramatic increase in the risk of switching.
Conclusions
Several patient characteristics were associated with discontinuation of, or switching between, bone-sparing treatments. Patients receiving bone-sparing medication other than oral bisphosphonates were more likely to have comorbid conditions and a history of fracture and to have taken an oral bisphosphonate previously.
Similar content being viewed by others
References
Bouillon R, Burkhardt P, Christiansen C, Fleisch HA, Fujita T, Gennari C, Martin TJ, Mazzuoli G, Melton LJ, Ringe JD (1991) Consensus development conference: prophylaxis and treatment of osteoporosis. Osteoporos Int 1:114–117
Scottish Intercollegiate Guidelines Network (2003) National Clinical Guideline 71: Management of osteoporosis. Available at: http://www.sign.ac.uk/pdf/sign71.pdf. Accessed July 2014
American Association of Clinical Endocrinologists Osteoporosis Task Force (2001) American Association of Clinical Endocrinologists 2001 medical guidelines for clinical practice for the prevention and management of postmenopausal osteoporosis. Endocr Pract 7:293–312
International Osteoporosis Foundation (2012) Europe guidelines. Available at: http://www.iofbonehealth.org/europe-guidelines. Accessed 30 April 2014
Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R, European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) (2008) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19:399–428
Cramer A, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ (2000) Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 27:687–694
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Reginster JY, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R, Vertebral Efficacy with Risedronate Therapy (VERT) Study Group (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 11:83–91
Hooper MJ, Ebeling PR, Roberts AP (2005) Risedronate prevents bone loss in early postmenopausal women: a prospective, randomized controlled trial. Climacteric 8:251–262
Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata Y, Balogh A, Lemmel EM, Pors-Nielsen S, Rizzoli R, Genant HK, Reginster JY (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21:1453–1460
McCombs JS, Thibaud P, McLaughlin-Miley C, Shi J (2004) Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 48:271–287
Netelenbos JC, Geusens PP, Ypma G, Buijs SJ (2011) Adherence and profile of non-persistence in patients treated for osteoporosis—a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int 22:1537–1546
Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T (2012) GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int 23:223–231
Martin KE, Yu J, Campbell HE, Abarca J, White TJ (2011) Analysis of the comparative effectiveness of 3 oral bisphosphonates in a large managed care organization: adherence, fracture rates, and all-cause cost. J Manag Care Pharm 17:596–609
Hoffmann F, Jung TI, Felsenberg D, Glaeske G (2008) Pattern of intravenous bisphosphonate use in outpatient care in Germany. Pharmacoepidemiol Drug Saf 17:896–903
Foster SA, Foley KA, Meadows ES, Johnston JA, Wang S, Pohl GM, Long SR (2007) Characteristics of patients initiating teriparatide for the treatment of osteoporosis. Osteoporos Int 19:373–377
Briesacher BA, Andrade SE, Harrold LR, Fouayzi H, Yood RA (2010) Adoption of once-monthly oral bisphosphonates and the impact on adherence. Am J Med 123:275–280
Abelson A, Ringe JD, Gold DT, Lange JL, Thomas T (2010) Longitudinal change in clinical fracture incidence after initiation of bisphosphonates. Osteoporos Int 21:1021–1029
Middleton ET, Steel SA, Doherty SM (2007) The effect of prior bisphosphonate exposure on the treatment response to teriparatide in clinical practice. Calcif Tissue Int 81:335–340
Lawson DH, Sherman V, Hollowell J (1998) The general practice research database for the scientific and ethical advisory group. Q J Med 91:445–452
Garcia Rodriguez LA, Perez Gutthann S (1998) Use of the UK general practice research database for pharmacoepidemiology. Br J Clin Pharmacol 45:419–425
Gallagher A, Rietbrock S, Olson M, van Staa TP (2008) Fracture outcomes related persistence and compliance with oral bisphosphonate. J Bone Miner Res 23:1569–1575
Rietbrock S, Olson M, van Staa TP (2009) The potential effects on fracture outcomes of improvements in persistence and compliance with bisphosphonates. Q J Med 102:35–42
Jick H, Terris BZ, Derby LE, Jick SS (1992) Further validation of information recorded on a general practitioner based computerized data resource in the United Kingdom. Pharmacoepidemiol Drug Saf 1:347–349
Walley T, Mantgani A (1997) The UK general practice research database. Lancet 350:1097–1099
Agresti A, Booth J, Hobert JP, Caffo B (2008) Random-effects modeling of categorical response data. Sociol Methodol 30:27–80
Penning-van Beest FJ, Goettsh WG, Erkens JA, Herings RM (2006) Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther 28:236–242
National Institute for Health and Care Excellence (2011) TA160 Osteoporosis – primary prevention. Available at: http://guidance.nice.org.uk/TA160. Accessed 30 April 2014
National Institute for Health and Care Excellence (2011) TA161 Osteoporosis – secondary prevention. Available at: http://www.nice.org.uk/guidance/ta161/resources/ta161-osteoporosis-secondary-prevention-including-strontium-ranelate-understanding-nice-guidance2. Accessed 30 April 2014
Watts NB, Worley K, Solis A, Doyle J, Sheer R (2004) Comparison of risedronate to alendonate and calcitonin for early reduction of nonvertebral fracture risk: results from a managed care administrative claims database. J Manag Care Pharm 10:142–152
National Institute for Health and Care Excellence (2014) Osteoporosis overview. Available at: http://pathways.nice.org.uk/pathways/osteoporosis#content=view-node%3Anodes-secondary-prevention-of-osteoporotic-fragility-fractures-in-postmenopausal-women. Accessed 20 July 2015.
Biskobing DM (2002) COPD and osteoporosis. Chest 121:609–620
Royal College of Physicians (2011) Falling standards, broken promises: report of the national audit of falls and bone health in older people 2010. Available at: https://www.rcplondon.ac.uk/sites/default/files/national_report.pdf. Accessed 20 July 2015
Balasubramanian A, Brookhart MA, Goli V, Critchlow CW (2013) Discontinuation and reinitiation patterns of osteoporosis treatment among commercially insured postmenopausal women. Int J Gen Med 6:839–848
Rossini M, Bianchi G, Di Munno O, Giannini S, Minisola S, Sinigaglia L, Adami S, Treatment of Osteoporosis in clinical Practice (TOP) Study Group (2006) Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int 914
Blouin J, Dragomir A, Ste-Marie L, Fernandes JC, Perreault S (2007) Discontinuation of antiresorptive therapies: a comparison between 1998–2001 and 2002–2004 among osteoporotic women. J Clin Endocrinol Metab 92:887–894
Lo JC, Pressman AR, Omar MA, Ettinger B (2006) Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int 17:922–928
Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Brookhart MA (2005) Compliance with osteoporosis medications. Arch Intern Med 165:2414–2419
Dennison EM, Compston JE, Flahive J et al (2013) Effect of comorbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 50:1288–1293
Ensrud KE (2013) Fracture risk in CKD. Clin J Am Soc Nephrol 8:1282–1283
Nazareth I, King M, Haines A, Rangel L, Myers S (1993) Accuracy of diagnosis on general practice computer system. BMJ 307:32–34
Hippisley-Cox J, Bayly J, Potter J, Fenty J, Parker C. Evaluation of standards of care for osteoporosis and falls in primary care. 2007. Available at: http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/general-practice/evaluation-of-standards-of-care-for-osteoporosis-and-falls-in-primary-care. Accessed 30 April 2015
Boonen S, Kay R, Cooper C, Haentjens P, Vanderschueren D, Callewaert F, Milisen K (2009) Osteoporosis management: a perspective based on bisphosphonate data from randomised clinical trials and observational databases. Int J Clin Pract 63:1792–1804
Soriano JB, Maier WC, Visick G, Pride NB (2001) Validation of general practitioner-diagnosed COPD in the UK general practice research database. Eur J Epidemiol 17:1075–1080
Li L, Roddam A, Ferguson S, Feudjo-Tepie M, Taylor A, Jick S (2014) Switch patterns of osteoporosis medication and its impact on persistence among postmenopausal women in the U.K. General Practice Research Database. Menopause 21:1106–1113
Acknowledgments
Writing assistance was provided by Oxford PharmaGenesis Ltd, funded by Amgen.
Conflicts of interest
The study was sponsored by Amgen Ltd and GlaxoSmithKline plc.
M. Feudjo-Tepie, S. Ferguson, A. Taylor, and C. Critchlow are all Amgen employees. A. Roddam is a GlaxoSmithKline plc employee, but was previously an Amgen employee. J. Bayly is an independent consultant, affiliated with the University of Derby. He has received consultation fees from Amgen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feudjo-Tepie, M., Ferguson, S., Roddam, A. et al. Comorbidities, bone-sparing agent prescription history and their determinants among postmenopausal women in UK primary care settings: a retrospective database study. Arch Osteoporos 10, 41 (2015). https://doi.org/10.1007/s11657-015-0233-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-015-0233-4