Abstract
Summary
There are differences in the risk profile of patients prescribed alendronate, risedronate, or ibandronate. Observed reductions in fracture incidence over time suggest that the effectiveness of each bisphosphonate in clinical practice has been consistent with their efficacies demonstrated in randomized controlled trials.
Introduction
Observational studies of bisphosphonate effectiveness for fracture prevention are subject to bias from unknown characteristics of baseline fracture risk at the start of therapy. The fracture incidence during the short period after starting a bisphosphonate and before any expected clinical benefit likely reflects baseline fracture risk. Bisphosphonate effectiveness may then be estimated by measuring the change in fracture incidence over time on therapy.
Methods
Administrative billing data were used to follow three cohorts of women aged 65 and older (total n = 210,144) after starting therapy either on alendronate, risedronate, or ibandronate in the USA between market introduction and 2006. Within each cohort, the baseline incidence of clinical fractures at the hip, vertebral, and nonvertebral sites was defined by the initial 3-month period after starting therapy. Relative to these baselines, we then compared the fracture incidence during the subsequent 12 months on therapy.
Results
At the start of therapy, the ibandronate cohort was younger and had fewer prior fractures than either the risedronate or alendronate cohorts. Accordingly, the baseline incidence of hip fractures was higher in the risedronate cohort (0.90 per 100 person-years) and in the alendronate cohort (0.77) than in the ibandronate cohort (0.64). Relative to the baseline incidence, fracture incidence was significantly lower in the subsequent 12 months in both cohorts of alendronate (18% lower at hip, 28% at nonvertebral sites, and 57% at vertebral sites) and risedronate (27% lower at hip, 21% at nonvertebral sites, and 54% at vertebral sites). In the ibandronate cohort, the fracture incidence was lower (31%) only at vertebral sites.
Conclusions
Differences in the baseline fracture incidence among the cohorts may reflect differences in the risk profile of patients prescribed each bisphosphonate. The reductions observed in fracture incidence over time within each cohort suggest that the effectiveness of each bisphosphonate in clinical practice has been consistent with their efficacies demonstrated in randomized controlled trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demonstrated efficacy of a therapy in a randomized clinical trial may not predict its actual effectiveness in clinical practice because of differences in characteristics of patients and level of medical care [1]. As a therapy for osteoporosis, the oral bisphosphonates have been widely utilized in recent years. These bisphosphonates include once-a-week alendronate (marketed in the USA since 2000), once-a-week risedronate (since 2002), and once-a-month ibandronate (since 2005). Since health data on large numbers of bisphosphonate patients in clinical practice have now been collected (through administrative billing data, medical records, and registries), many recent observational studies have examined the effectiveness of oral bisphosphonates for reducing clinical fractures. The designs of these observational studies have included comparisons between patient populations with or without a fracture [2, 3], with or without bisphosphonate use [4, 5], compliant or not compliant with bisphosphonate use [6–19], or between patient populations on different bisphosphonate molecules [20–23]. A key limitation in interpreting any of these comparisons is uncertainty if known or unknown differences in baseline fracture risk between patient populations could account for some or all of the reported results.
An approach to directly measure the baseline risk of an outcome within patient populations that has been used in effectiveness studies of other therapies may be applicable to the study of bisphosphonates. In a comparison of patients receiving a bare or drug-eluting stent, the mortality 2 days after procedure was used to assess risk of the mortality outcome independent of possible drug effect [24]. In a comparison of patients receiving influenza vaccine or not, the mortality after vaccination but before flu season was used to assess risk of mortality outcome independent of possible vaccination effect [25]. Likewise, following initiation of bisphosphonate therapy, the realization of fracture reduction is likely not immediate. Bone mineral density, a surrogate marker of therapeutic effect, begins to change after start of therapy though does not reach its maximum level of change until at least 1 year on therapy [26]. As changes in bone density and quality take time, correspondingly, fracture reductions have not been noted earlier than 6 months after start of therapy within post hoc, pooled analysis of clinical trials [27, 28].
Hence, for an observational study of bisphosphonates, the fracture incidence during the short period after starting a therapy and before any expected clinical benefit likely reflects baseline fracture risk. We propose that bisphosphonate effectiveness may then be estimated by measuring the change in fracture incidence over time on therapy. For this study, administrative billing data were used to follow three cohorts of women aged 65 and older after starting therapy either on alendronate, risedronate, or ibandronate. Within each cohort, the baseline incidence of clinical fractures at the hip, vertebral, and nonvertebral sites was defined by the initial 3-month period after starting therapy. Relative to these baselines, we then compared the fracture incidence during the subsequent 12 months on therapy.
Materials and methods
Data source
Computerized records of administrative billing provide a convenient data source for studying drug use and outcomes in large populations. Records include patient-level data of: (1) inpatient and outpatient services specified by diagnoses codes of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM); (2) retail and mail-order pharmacy dispensations specified by national drug codes; and (3) demographic information including sex, age, and eligibility dates of health plan coverage. The data for this study, inclusive of January 2000–December 2007, originated from two mutually exclusive sources: Ingenix Lab/Rx (Eden Prairie, MN, USA) and Medstat MarketScan (Ann Arbor, MI, USA). During that period, the average number of eligible enrollees was 14 million in Medstat, representing multiple health plans, and 10 million in Ingenix, representing a single health plan. Geographically, one half of this population was located in Michigan, California, Florida, Ohio, Georgia, or Texas and one half in the other 44 states.
Study population
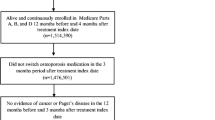
The study population consisted of three cohorts—one prescribed alendronate, one prescribed risedronate, and one prescribed ibandronate. Subjects entered a cohort on the date of their initial filled prescription for alendronate 70 mg/week, risedronate 35 mg/week, or ibandronate 150 mg/month during the time period of market introduction through December 2006. Market introduction was November 2000 for alendronate cohort, May 2002 for risedronate cohort, and April 2005 for ibandronate cohort. The initial bisphosphonate prescription was defined by a subject having at least 6 months of prior coverage in the data source without any other bisphosphonate use (e.g., another bisphosphonate type or dose). After 6 months without any bisphosphonate use, a subject was allowed to enter a new cohort (i.e., a subject could be in more than one cohort—1% of alendronate cohort, 4% of risedronate cohort, and 20% of ibandronate cohort was preceded by inclusion in another cohort).
In addition, subjects were required to: (1) be women aged 65 and over to provide a study population similar in age to that of the randomized controlled trials and for which clinical fractures are likely to be related to osteoporosis [29]; (2) have at least 3 months of coverage in data source after cohort entry to provide for minimum follow-up; and (3) have no diagnosis of malignant neoplasm (ICD-9-CM codes 140–208) or Paget’s disease (731.0) within 6 months prior- and 3 months post-cohort entry to maximize the probability that subjects were being treated for either post-menopausal osteoporosis or glucocorticoid-induced osteoporosis.
Risk factors for fracture
Available risk factors in the data source included age, history of prior fracture, glucocorticoid use, and diagnosis of rheumatoid arthritis. Age was calculated at the year of cohort entry. History of prior fracture was defined by any clinical fracture diagnosis at the hip, wrist, humerus, clavicle, pelvis, leg, or vertebrae in the 6 months prior to cohort entry. Glucocorticoid use was defined by receiving 450 mg prednisone-equivalent pills within ±90 days of cohort entry—an approximation of the American College of Rheumatology guideline of 5 mg prednisone for at least 90 days [30]. A diagnosis of rheumatoid arthritis was based on any inpatient or outpatient diagnosis (ICD-9-CM code 714.0) within 6 months prior- and 3 months post-cohort entry. Risk factors not available in the data source included bone mineral density, body mass index, smoking, alcohol consumption, and family history of fracture.
Fracture outcomes
After subjects entered a cohort, each was followed to identify three outcomes: a new hip fracture, a new nonvertebral fracture, or a new clinical vertebral fracture. During the follow-up, subjects were allowed to have each outcome once. Hip fractures were defined by an inpatient diagnosis at the hip (ICD-9-CM code 820, 733.14). Nonvertebral fractures were inclusive of inpatient diagnosis at the hip, and inpatient or outpatient diagnosis at the wrist (813, 733.12), humerus (812, 733.11), clavicle (810), pelvis (808), and leg (821, 823, 733.15, 733.16). Clinical vertebral fractures were defined by either inpatient or outpatient diagnosis at vertebral sites (805.2, 805.4, 805.8, 733.13). New fractures were defined as a fracture at each body site for which there was no fracture at that same site in the 6 months before cohort entry. To increase the probability of only including osteoporotic-related fractures, we excluded likely traumatic fractures by eliminating diagnoses of an open fracture or of a documented cause of injury other than an accidental fall (ecode of E880–E888). These exclusions removed less than 10% of fracture outcomes.
Follow-up
All subjects contributed 3 months of follow-up after cohort entry, during which the baseline fracture incidence was calculated. The denominator was the sum of observation time for all subjects within a cohort during the 3 months. For example, within the alendronate cohort, the 116,996 subjects contributed 91 days of follow-up each for 10.6 million days/364 days per year or 29,249 person-years of observation. The numerator was number of subjects with a new fracture during the 3 months.
After 3 months of follow-up, subsequent observation was available for subjects through December 2007 unless their individual coverage ceased in the data source (Fig. 1a). The fracture incidence was calculated for the subsequent 1 year on therapy. We limited our observation to the subsequent 1 year of therapy because of concerns that a subject's fracture risk may change over a period of multiple years independent of any therapeutic effect. Two examples of changing fracture risk over time include: the risk of hip fracture increasing with each year of age [31] and the risk of fractures increasing substantially within the year after a fracture but then decreasing thereafter [32]. All subjects who had received a sufficient quantity of pills (of the same bisphosphonate type initiated at cohort entry) to provide for a medical possession ratio ≥80% at the end of 3 months were followed into the subsequent 3-month period (Fig. 1b). The level utilized for the medical possession ratio has been frequently suggested to provide a high level of therapy effectiveness for bisphosphonates [6–19]. Subjects were followed until the end of this 3-month period or the end of their coverage in data source. The same process was applied at the end of 6, 9, and 12 months after cohort entry. For the calculation of incidence, the denominator was the sum of observation during follow-up preceded by a medical possession ratio of at least 80%. For example, within the alendronate cohort:
84,534 subjects had an average of 89 days of follow-up between 3 and 6 months of therapy, 61,594 subjects had an average of 89 days of follow-up between 6 and 9 months of therapy, 54,681 subjects had an average of 89 days of follow-up between 9 and 12 months of therapy, and 45,802 subjects had an average of 89 days of follow-up between 12 and 15 months of therapy—for a sum of 60,108 person-years of observation. The numerator included number of subjects with a new fracture, preceded by medical possession ratio of 80%, akin to previous study [7].
Statistical analysis
A simple ratio was used to compare the incidence of fractures between the period of 3 months after starting therapy and the subsequent 1-year period on therapy. Poisson regression was used to compute the 95% confidence intervals around the ratio. An independent review and replication of statistical analyses was completed by Esteban Walker, Ph.D., of the Department of Quantitative Health Sciences at the Cleveland Clinic.
Results
Cohort characteristics
The study population included women who entered into a cohort on the date of their initial filled prescription for alendronate 70 mg (n = 116,996) or risedronate 35 mg (n = 78,860) or ibandronate 150 mg (n = 14,288) (Fig. 1a). The data source provided a record of health care utilization for at least 1 year after initial bisphosphonate prescription for more than 80% of each cohort (Fig. 1b). Of those subjects in the data source for at least 1 year, between 43% and 46% of the cohorts were highly compliant to therapy (i.e., maintained a medical possession ratio to initiated therapy of at least 80%).
At cohort entry, the ibandronate cohort was the youngest and had the smallest percentage with a recent fracture history among the three cohorts (Table 1). Since a subject was allowed to enter a cohort after 6 months without any bisphosphonate use, some subjects had some previous use of bisphosphonates. Prior use of bisphosphonates in the 4 years prior to cohort entry ranged from 7% of alendronate cohort to 40% of ibandronate cohort.
Baseline incidence of hip fractures
During the 3 months after starting therapy in all three cohorts, the incidence of hip fractures was higher among those of greater age, prior fracture history, and glucocorticoid use, and lower among those with use of hormone replacement therapy (Table 2). During these 3 months, patients receiving risedronate had an incidence of hip fractures that was 141% of the incidence among those receiving ibandronate and 117% of the incidence among those receiving alendronate. After statistically adjusting (by direct standardization to risedronate cohort) for age, fracture history, and prior bisphosphonate use, patients receiving risedronate had an incidence of hip fractures that was 132% of the incidence among those receiving ibandronate and 114% of the incidence among those receiving alendronate.
Change in fracture incidence over time
After the initial 3-month period, the incidence of fractures was observed in the subsequent 1 year while on therapy. Relative to the baseline incidence of the initial 3-month period, the incidence of clinical fractures at the hip, vertebral, and nonvertebral sites was significantly lower in the subsequent 12 months in both cohorts of alendronate and risedronate (Table 3). The incidence of vertebral fractures in the subsequent 1 year was lower in the ibandronate cohort.
We examined whether different levels of fracture risk at the start of therapy modified the longitudinal change in fracture incidence—by using stratification to limit the analyses to subgroups of similar baseline characteristics of fracture risk. The strata were based upon prior clinical fracture (yes or no), prior bisphosphonate use (yes or no), age (one quantile above or below population median of 75 years), and date of study entry (period before or after all three therapies were on the market). For every subgroup, its 95% confidence interval included the point estimate of overall analyses (Fig. 2).
Discussion
In this observational study of cohorts containing patients starting alendronate, risedronate, or ibandronate, there were apparent differences among the cohorts in age and prior fracture history, in prior use of bisphosphonates, and in the fracture incidence during the short period after starting therapy and before any expected clinical benefit. These differences in the risk profile of patients prescribed each bisphosphonate are significant for the consideration of bias in interpretation of results of any observational study of bisphosphonates.
In this study and prior studies [7, 20, 22, 23], the inclusion criterion for new use of bisphosphonate was defined by a subject having at least 6 months of no other bisphosphonate use. In order to further evaluate this criterion, we were able to examine up to four prior years for a subset of the population. As evident by the large number of ibandronate patients in this study with prior bisphosphonate use, the six month criterion was not always adequate to truly define new users of bisphosphonates. Indeed, the median gap between stopping and restarting bisphosphonate therapy has been reported to be 16 months [33]. Since bisphosphonates have residual treatment effects, for example, alendronate has effects for up to 5 years after stopping treatment [34], it may not be readily possible to disentangle the fracture outcome with the current bisphosphonate exposure from past bisphosphonate exposure [35].
The study approach to account for differences in patient profiles is often techniques of regression modeling, propensity score modeling, or instrument variable analysis [36]. However, statistical techniques cannot exclude the possibility of bias arising from the nonrandom allocation of subjects [37]. In the current study, given the differences between cohorts in patient profiles including prior use of bisphosphonates, we proposed a study design that estimated bisphosphonate effectives by measuring the change in fracture incidence over time within a cohort. In such an approach, the cohort population receiving the same therapy is its own control, thus limiting the potential bias associated with a comparison of outcomes between cohorts.
In this study, two observations suggested that change in fracture incidence over time within a cohort may indeed be utilized to measure bisphosphonate effectiveness. The first observation supporting the study design was that the baseline fracture incidence during the initial 3 months after starting therapy accurately reflected the underlying risk of cohort. During this baseline period, the incidence of hip fractures corresponded with well-accepted risk factors for fracture, including age, prior fracture history, and glucocorticoid use [38]. These relationships between risk factors and fracture incidence were consistently observed across all three cohorts (Table 2). The second observation supporting the study design was the consistency in results between this observational study and the prospectively planned analyses of respective phase III randomized controlled trials [39–45]. As summarized in a government-funded systematic review of bisphosphonates [46], alendronate, risedronate, and ibandronate have all been shown to reduce vertebral fractures, while only alendronate and risedronate have been shown to reduce nonvertebral fractures, including hip fractures. Of note, the results of subgroup [45] or post hoc [47] analyses of randomized controlled trial data have suggested a reduction of nonvertebral fractures among subjects using ibandronate. To date, no data from randomized controlled trials appear available in the literature concerning hip fractures and ibandronate.
There are several limitations in interpretation of change in fracture incidence as a measure of bisphosphonate effectiveness. One limitation arises from the differences in risk profile of patients between cohorts. It is conceivable that the lack of an observable effectiveness on nonvertebral fractures for ibandronate could relate to the lower risk profile of those patients. In a study of another bisphosphonate, clodronate, the magnitude of fracture reduction was greatest among those at highest probability of fracture [48]. Another limitation in interpretation of results comes from the relatively small sample size of ibandronate cohort relative to the other cohorts. Hence, the 95% confidence interval (0.71–1.88) around the estimate of longitudinal change in hip fracture incidence was the widest in the ibandronate cohort. A third limitation in interpretation of results is the data source does not indicate the reason starting therapy (e.g., post-menopausal osteoporosis or glucocorticoid-induced osteoporosis), hence these results may not generalize to defined populations. An additional limitation in interpretation may arise from misclassification of outcomes. In a prior study, the proportion of fracture claims confirmed by chart review to be a fracture was highest for the hip relative to other fracture sites [49]. Since the effect of misclassification of outcomes is likely no different between cohorts (i.e., misclassification does not depend on cohort), the study results for the measure of nonvertebral sites and for vertebral sites are likely more attenuated by misclassification than results at the hip.
In conclusion, for this large observational study of more than 200,000 bisphosphonate patients, the apparent differences in the baseline incidence of hip fractures among the alendronate, risedronate, and ibandronate cohorts likely reflect differences in the risk profile of patients prescribed each bisphosphonate. Statistical adjustments could not account for these differences and therefore the design of epidemiological studies should be given careful consideration to account for these differences. Relative to the baseline fracture incidence, the longitudinal analyses indicated that alendronate and risedronate decreased nonvertebral and hip fractures over time, whereas ibandronate did not. All three bisphosphonates decreased vertebral fractures. The reductions observed in fracture incidence over time within each cohort suggest that the effectiveness of each bisphosphonate in clinical practice has been consistent with their efficacies demonstrated in randomized controlled trials.
References
Avorn J (2007) In defense of pharmacoepidemiology—embracing the yin and yang of drug research. N Engl J Med 357:2219–2221
Perreault S, Dragomir A, Blais L et al (2008) Population-based study of the effectiveness of bone-specific drugs in reducing the risk of osteoporotic fracture. Pharmacoepidemiol Drug Saf 17:248–259
Langsetmo LA, Morin S, Richards JB et al (2009) Effectiveness of antiresorptives for the prevention of nonvertebral low-trauma fractures in a population-based cohort of women. Osteoporos Int 20:283–290
Morin S, Rahme E, Behlouli H et al (2007) Effectiveness of antiresorptive agents in the prevention of recurrent hip fractures. Osteoporos Int 18:1625–1632
Feldstein AC, Weycker D, Nichols GA et al (2009) Effectiveness of bisphosphonate therapy in a community setting. Bone 44:153–159
Blouin J, Dragomir A, Moride Y et al (2008) Impact of noncompliance with alendronate and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly women. Br J Clin Pharmacol 66:117–127
Curtis JR, Westfall AO, Cheng H et al (2008) Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res 23:1435–1441
Penning-van Beest FJ, Erkens JA, Olson M, Herings RM (2008) Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int 19:511–517
Gallagher AM, Rietbrock S, Olson M, van Staa TP (2008) Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res 23:1569–1575
Meijer WM, Beest FJ, Olson M, Herings RM (2008) Relationship between duration of compliant bisphosphonate use and the risk of osteoporotic fractures. Curr Med Res Opin 24:3217–3222
Rabenda V, Mertens R, Fabri V et al (2008) Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int 19:811–818
Sunyecz JA, Mucha L, Baser O et al (2008) Impact of compliance and persistence with bisphosphonate therapy on health care costs and utilization. Osteoporos Int 19:1421–1429
Curtis JR, Westfall AO, Cheng H et al (2008) Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int 19:1613–1620
McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J (2004) Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 48:271–287
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38:922–928
van den Boogaard CH, Breekveldt-Postma NS, Borggreve SE et al (2006) Persistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: a database analysis study. Curr Med Res Opin 22:1757–1764
Caro JJ, Ishak KJ, Huybrechts KF et al (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008
Weycker D, Macarios D, Edelsberg J, Oster G (2007) Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int 18:271–277
Siris ES, Harris ST, Rosen CJ et al (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022
Silverman SL, Watts NB, Delmas PD et al (2007) Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int 18:25–34
Cadarette SM, Katz JN, Brookhart MA et al (2008) Relative effectiveness of osteoporosis drugs for preventing nonvertebral fracture. Ann Intern Med 148:637–646
Curtis JR, Westfall AO, Cheng H et al (2009) RisedronatE and ALendronate Intervention over Three Years (REALITY): minimal differences in fracture risk reduction. Osteoporos Int 20(6):973–978
Harris ST, Reginster JY, Harley C et al (2009) Risk of fracture in women treated with monthly oral ibandronate or weekly bisphosphonates: the eValuation of IBandronate Efficacy (VIBE) database fracture study. Bone 44(5):758–765
Mauri L, Silbaugh TS, Garg P et al (2008) Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med 359:1330–1342
Jackson LA, Jackson ML, Nelson JC et al (2006) Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 35:337–344
Bonnick S, Saag KG, Kiel DP et al (2006) Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91:2631–2637
Harrington JT, Ste-Marie LG, Brandi ML et al (2004) Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int 74:129–135
Black DM, Thompson DE, Bauer DC et al (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 85:4118–4124
Melton LJ 3rd, Thamer M, Ray NF et al (1997) Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res 12:16–23
American College Of Rheumatology Ad Hoc Committee On Glucocorticoid-Induced Osteoporosis (2001) Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheum 44:1496–1503
Riggs BL, Melton LJ 3rd, Robb RA et al (2006) Population-based analysis of the relationship of whole bone strength indices and fall-related loads to age- and sex-specific patterns of hip and wrist fractures. J Bone Miner Res 21:315–323
Johnell O, Kanis JA, Odén A et al (2004) Fracture risk following an osteoporotic fracture. Osteoporos Int 15:175–179
Brookhart MA, Avorn J, Katz JN et al (2007) Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med 120:251–256
Black DM, Schwartz AV, Ensrud KE et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158:915–920
D'Agostino RB Jr, D'Agostino RB Sr (2007) Estimating treatment effects using observational data. JAMA 297:314–316
Shapiro S (2000) Bias in the evaluation of low-magnitude associations: an empirical perspective. Am J Epidemiol 151:939–945
Kanis JA, Johnell O, Oden A et al (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
Cummings SR, Black DM, Thompson DE et al (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. JAMA 280:2077–2082
Liberman UA, Weiss SR, Bröll J et al (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Reginster J, Minne HW, Sorensen OH et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 11:83–91
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:333–340
Chesnut CH III, Skag A, Christiansen C et al (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
MacLean C, Newberry S, Maglione M et al (2008) Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 148:197–213
Harris ST, Blumentals WA, Miller PD (2008) Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Curr Med Res Opin 24:237–245
McCloskey EV, Johansson H, Oden A et al (2009) Ten-year fracture probability identifies women who will benefit from clodronate therapy—additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int 20:811–817
Ray WA, Griffin MR, Fought RL, Adams ML (1992) Identification of fractures from computerized Medicare files. J Clin Epidemiol 45:703–714
Acknowledgement
Funding by The Alliance for Better Bone Health (Procter & Gamble Pharmaceuticals and sanofi-aventis).
Conflicts of interest
Dr. Abelson reports receiving consulting fees from sanofi-aventis, Procter & Gamble, Novartis; serving on speaker’s bureaus for Amgen, Procter & Gamble, Roche, Novartis, and sanofi-aventis. Dr. Gold reports receiving consulting or advisory committee fees from Amgen, Eli Lilly, GlaxoSmithKline, Merck, Procter & Gamble, Roche, sanofi-aventis; serving on speaker's bureaus for Amgen, Eli Lilly, GlaxoSmithKline, Procter & Gamble, Roche, and sanofi-aventis. Dr. Thomas reports receiving consulting or advisory committee fees from Amgen, Daïchi-Sankyo, Ipsen, Lilly, MSD, Novartis, Procter & Gamble, Roche/GlaxoSmithKline, sanofi-aventis, and Servier; grant support from Lilly, MSD, Nicomed, Novartis, Procter & Gamble, sanofi-aventis, and Servier. Dr. Lange is an employee of Procter & Gamble.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Abelson, A., Ringe, J.D., Gold, D.T. et al. Longitudinal change in clinical fracture incidence after initiation of bisphosphonates. Osteoporos Int 21, 1021–1029 (2010). https://doi.org/10.1007/s00198-009-1046-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-1046-3