Abstract
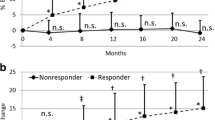
Our objective was to determine the effect of prior bisphosphonate exposure on the treatment response to teriparatide. All patients started on teriparatide in our hospital are entered into a database. All patients who had at least 12 months’ treatment were identified. Patients were divided into two groups depending on whether or not they had prior bisphosphonate exposure, and the response to teriparatide was compared using procollagen of type 1 N-terminal propeptide (P1NP) and bone mineral density (BMD). Fifty-two patients had been treated for at least 12 months, 38 with prior bisphosphonate exposure and 14 without. The mean duration of bisphosphonate treatment was 67 months, discontinued a mean of 1 month previously. P1NP increased significantly at 3 and 6 months in both groups. However, those without previous bisphosphonate treatment had a higher baseline P1NP (49 vs. 30 μg/L, P < 0.01), and this remained higher at 3 months (109 vs. 71 μg/L, P = 0.10) and 6 months (183 vs. 126 μg/L, P = 0.06), although the difference was not significant. In the prior bisphosphonate and bisphosphonate naive groups, respectively, the change in spinal BMD was 9.0% and 7.8% (P = 0.54) at 12 months and 9.8% and 6.1% (P = 0.30) at 18 months. The respective change in hip BMD was 1.0% and −0.3% (P = 0.36) at 12 months and 2.8% and 1.3% (P = 0.44) at 18 months. There was a trend toward a smaller but still significant increase in P1NP in response to teriparatide in bisphosphonate-treated patients. Although this suggests a blunting of the anabolic effects, in our clinic population this did not result in a reduction in BMD gain.



Similar content being viewed by others
References
Neer RM, Arnaud CD, Zanchetta JR, et al. (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Arlot M, Meunier PJ, Boivin G, et al. (2005) Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res 20:1244–1253
National Institute for Clinical Excellence (2005) Bisphosphonates (alendronate, etidronate, risedronate), selective oestrogen receptor modulators (raloxifene) and parathyroid hormone (teriparatide) for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. Published appraisals; Technology Appraisal Guidance 87. http://guidance.nice.org.uk/TA87
Bone HG, Hosking D, Devogelaer JP, et al. (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Hannon R, Blumsohn A, Naylor K, et al. (1998) Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variation. J Bone Miner Res 13:1124–1133
Looker AC, Bauer DC, Chestnut CH, et al. (2000) Clincial use of biochemical markers of bone remodelling: current status and future directions. Osteoporos Int 11:467–480
Graham R, Russell G (2007) Determinants of structure-function relationships among bisphosphonates. Bone 40:S21–S25
Black DM, Schwartz AV, Ensrud KE, et al. (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-Term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Neele SJ, Evertz R, De Valk-De Roo G, Roos JC, Netelenbos JC (2002) Effect of 1 year of discontinuation of raloxifene or estrogen therapy on bone mineral density after 5 years of treatment in healthy postmenopausal women. Bone 30:599–603
Black DM, Greenspan SL, Ensrud KE, et al. (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226
Boonen S, Marin F, Lyritis G, et al. (2006) Type of prior antiresorptive therapy, but not its duration or washout period, determines the bone mineral density response to 12 months of teriparatide. Calcif Tissue Int 78; Supp1 1:s28, OC012
Al-Shahi R, Vousden C, Warlow C (2005) Bias from requiring explicit consent from all participants in observational research: prospective, population based study. BMJ 33:942–945
Junghans C, Feder G, Hemingway H, Timmis A, Jones M (2005) Recruiting patients to medical research: double blind randomised trial of “opt-in” versus “opt-out” strategies. BMJ 331:940–942
Sheperd JA, Fan B, Lu Y, Lewiecki EM, Miller P, Genant HK (2006) Comparison of BMD precision for Prodigy and Delphi spine and femur scans. Osteoporos Int 17:1303–1308
Acknowledgements
We thank Eli Lilly and company for allowing us to use their BMD and P1NP data obtained by our center as part of a muliticenter trial.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Middleton, E.T., Steel, S.A. & Doherty, S.M. The Effect of Prior Bisphosphonate Exposure on the Treatment Response to Teriparatide in Clinical Practice. Calcif Tissue Int 81, 335–340 (2007). https://doi.org/10.1007/s00223-007-9066-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-007-9066-5