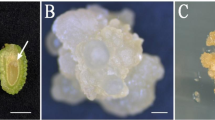
Abstract
A highly reproducible plant regeneration protocol through somatic embryogenesis and shoot organogenesis has been developed for Cenchrus ciliaris. Three explants (seeds, shoot apices, and immature inflorescences) of four genotypes (IG-3108, IG-718, IG-74, and DBC15-8/32/10) were used for callus induction and plant regeneration. The highest rate of callus formation was found using Murashige and Skoog (MS) medium supplemented with 0.5 mg L−1 benzylaminopurine (BA) and 3.0 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D). The largest number of somatic embryos was generated with the addition of 400 mg L−1 L-proline, 400 mg L−1 L-glutamine, and 300 mg L−1 casein hydrolysate. Somatic embryos were successfully germinated on MS medium with 3.0 mg L−1 BA and 0.25 mg L−1 2,4-D. In vitro plant regeneration was accomplished through somatic embryogenesis using all three explants. Ultra-structural features of somatic embryos confirmed proper formation and ontogeny.




Similar content being viewed by others
References
Armstrong CL, Green CE (1985) Establishment and maintenance of friable, embryogenic maize callus and the involvement of L.-proline. Planta 164:207–214
Ayerza R (1981) El’ Buffel grass: utilidad y manejo de una promisoria gramínea. Hemisferio Sur, Buenos Aires
Bai Y, Qu R (2001) Factors influencing tissue culture responses of mature seeds and immature embryos in turf-type tall fescue. Plant Breed 120:239–242
Batra S, Kumar S (2002) In-vitro high frequency plant regeneration in buffel grass (Cenchrus ciliaris L.). J Plant Biol 29:191–194
Batra S, Kumar S (2003) Agrobacterium-mediated transient GUS gene expression in buffel grass (Cenchrus ciliaris L.). J Appl Genet 44:449–458
Bhat V, Dalton SJ, Kumar S, Bhat BV, Gupta MG, Morris P (2001) Particle- inflow-gun-mediated genetic transformation of buffel grass (Cenchrus ciliaris L.): optimizing biological and physical parameters. J Appl Genet 42:405–412
Botti C, Vasil IK (1984) Ontogeny of somatic embryos of Pennisetum americanum. II. In cultured immature inflorescences. Can J Bot 62:1629–1635
Bray RA (1978) Evidence for facultative apomixis in Cenchrus ciliaris. Euphytica 27:801–804
Burson BL, Hussy MA, Actkinson JM, Shafer GS (2002) Effect of pollination time on the frequency of 2n+n fertilization in apomictic buffel grass. Crop Sci 42:1075–1080
Cai T, Butler L (1990) Plant regeneration from embryogenic callus initiated from immature inflorescences of several high-tannin Sorghum. Plant Cell Tiss Org Cult 20:101–110
Carvalho CHS, Bahorova N, Bordallo PN, Abreu LL, Valicente FH, Bressan W, Paiva E (1997) Type II callus production and plant regeneration in tropical maize genotypes. Plant Cell Rep 17:73–76
Colomba EL, Grunberg K, Griffa S, Ribotta A, Mroginski L, Biderbost E (2006) The effect of genotype and culture medium on somatic embryogenesis and plant regeneration from mature embryos of fourteen apomictic cultivars of buffel grass (Cenchrus ciliaris L.). Grass Forage Sci 6:2–8
Conger BV, Hanning GE, Gray DJ, McDaniel JK (1983) Direct embryogenesis from mesophyll cells of orchard grass. Science 221:850–851
Cummings DP, Green CE, Stuthman DD (1976) Callus induction and plant regeneration in oats. Crop Sci 16:465–470
Dal Vesco LL, Guerra MP (2001) The effectiveness of nitrogen sources in Feijoa somatic embryogenesis. Plant Cell Tiss Org Cult 64:19–25
Desai NS, Suprasanna P, Bapat VA (2004) Simple and reproducible protocol for direct somatic embryogenesis from cultured immature inflorescence segments of sugarcane. Curr Sci 87:764–768
Dhillon NK, Gosal SS (2013) Effect of growth adjuvants of somatic embryogenesis in maize (Zeamays L.). J Cell Tiss Res 13:3557–3563
Dunstan DI, Short KC, Thomas E (1978) The anatomy of secondary morphogenesis in cultured scutellum tissues of Sorghum bicolor. Protoplasma 97:251–260
Elkonin LA, Pakhomova NV (2000) Influence of nitrogen and phosphorus on induction embryogenic callus of Sorghum. Plant Cell Tiss Org Cult 61:115–123
Fisher WD, Bashaw EC, Holt EC (1954) Evidence for apomixis in Pennisetum ciliare and Cenchrus setigerus. Agron J 46:401–404
Gamborg OL, Miller RA, Ohima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gleddie S, Keller W, Setterfield G (1983) Somatic embryogenesis and plant regeneration from leaf explants and cell suspensions of Solanum melongena (eggplant). Can J Bot 61:656–666
Griffa SDD, Ribotta A, Castelli SL, Munoz N, Colomba EL, Luna C, Grunberg K, Biderbost E (2006) Molecular genetic discrimination of Buffel grass genotypes and F1 hybrids for breeding purposes using amplified fragment length polymorphism analyses. Grass Forage Sci 61:454–458
Gupta S, Khanna VK, Singh R, Garg GK (2002) Effect of media and explant on callus formation and plant regeneration in sorghum. J Plant Biol 29:39–44
Hanzel JJ, Miller JP, Brinkmann MA, Fendos E (1985) Genotype and media effects on callus formation and regeneration in barley. Crop Sci 25:27–31
Ho W, Vasil IK (1983) Somatic embryogenesis in sugarcane (Saccharum officinarum L.) I. The morphology and physiology of callus formation and the ontogeny of somatic embryos. Protoplasma 118:169–180
Hoagland M, Arnon DI (1950) The water culture method to grow plants without soil. Calif Agric Expt Station Cir 347. Berkeley California
Ikeuchi M, Favero DS, Sakamoto Y, Iwase A, Coleman D, Rymen B, Sugimoto K (2019) Molecular mechanisms of plant regeneration. Annu Rev Plant Biol 70:377–406
Johnsen DA (1940) Plant micro-techniques. McGraw-Hill, NewYork
Kackar A, Shekhawat NS (1991) Plant regeneration from cultured immature inflorescences of Cenchrus setigerus and C. ciliaris. Indian J Exp Biol 29:62–64
Kumar J, Shukla SM, Bhat V, Gupta S, Gupta MG (2005) In vitro plant regeneration and genetic transformation of Dichantium annulatum. DNA Cell Biol 24:670–679
Kumar S, Sahu N, Singh A (2015) High-frequency in vitro plant regeneration via callus induction in a rare sexual plant of Cenchrus ciliaris L. In Vitro Cell Dev Biol - Plant 51:28–34
Lowe K, Rota ML, Hoerster G, Hastings C, Wang N, Chamberlin M, Wu E, Jones T, Gordon-Kamm W (2018) Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell Dev Biol – Plant 54:240–252
McDaniel JK, Conger BV, Graham ET (1982) A histological study of tissue proliferation, embryogenesis and organogenesis from tissue cultures of Dactylis glomerata L. Protoplasma 110:121–128
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murty UR, Bharathi M, Visadara M, Annapurna A (1992) Embryogenic callus formation and plant regeneration in Cenchrus ciliaris (L). Cereal Res Commun 20:7–12
Ozawa K, Komamine A (1989) Establishment of a system of high frequency embryogenesis from long-term cell suspension cultures of rice (Oryza sativa L.). Theor Appl Genet 77:205–211
Rajyalakshmi K, Grover A, Maheshwari N, Tyagi AK, Maheshwari SC (1991) High frequency regeneration of plantlets from the leaf bases via somatic embryogenesis and comparison of polypeptide profiles from morphogenic and non-morphogenic calli in wheat (Triticum aestivum). Plant Physiol 82:617–623
Ramakrishnan M, Ceasar SA, Duraipandiyan V, Daniel MA, Ignacimuthu S (2013) Efficacious somatic embryogenesis and fertile plant recovery from shoot apex explants of onion (Allium cepa L.). In Vitro Cell Dev Biol - Plant 49:285–293
Rogers SMD, Dahmer ML, Stair DW (1993) Characterization of buffel grass (Cenchrus ciliaris L.) cell suspension cultures. In Vitro Cell Dev Biol - Plant 29:51–54
Ross AH, Manners JM, Birch RG (1995) Embryogenic callus production, plant regeneration and transient gene expression following particle bombardment in the pasture grass, C. ciliaris (Graminae). Aust J Bot 43:193–199
Sanderson MA, Voigt P, Jones RM (1999) Yield and quality of warm season grasses in central Texas. Rangel Ecol Manag 52:45–50
Sankhla A, Sankhla N (1989) Tissue culture studies on desert plants: Cenchrus ciliaris cv. 75. Curr Sci 58:872–874
Satish L, Ceasar SA, Shilpha J, Rency AS, Rathinapriya P, Ramesh M (2015) Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn). In Vitro Cell Dev Biol - Plant 51:192–200
Sharma S, Sandhu MK, Kaur P, Kaur A, Gosal SS (2012) Factors affecting somatic embryogenesis in maize (Zea mays L.). J Cell Tiss Res 12:3103–3108
Sherwood RT, Young BA, Bashaw EC (1980) Facultative apomixis in buffel grass. Crop Sci 20:375–379
Shohael AM, Akanda MAL, Parvez S, Mahfuja S, Alam MF, Islam R, Joarder N (2003) Somatic embryogenesis and plant regeneration from immature embryo derived callus of inbred maize (Zea mays L.). Biotechnology 2:154–161
Sinha H, Gill M, Gosal S (2000) Regulation of somatic embryogenesis and plant regeneration in sugarcane (Saccharum officinarum L.). Indian J Agric Sci 70:181–183
Vasil V, Vasil IK (1982) The ontogeny of somatic embryos of Pennisetum americanum (L.) K. Schum. I. In cultured immature embryos. Bot Gaz 143:454–465
Vasil V, Vasil IK (1985) Initiation and maintenance of cell suspension cultures of Gramineae. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol 1. Laboratory procedures and their applications. Academic Press, New York, pp 152–157
Vikrant, Rashid A (2003) Somatic embryogenesis or shoot formation following high 2,4-D pulse-treatment of mature embryos of Paspalum scrobiculatum. Biol Plant 46:297–300
Whyte RO, Moir TRG, Cooper JP (1959) Grasses in agriculture. FAO agricultural study no. 42. Rome, FAO
Yadav CB, Jha P, Mahalakshmi C, Anjaiah V, Bhat V (2009) Somatic embryogenesis and regeneration of Cenchrus ciliaris genotypes from immature inflorescence explants. Biol Plant 53:603–609
Yadava R, Chawla HS (2002) Role of genotypes, growth regulators and amino acids on callus induction and plant regeneration from different developmental stages of inflorescence in wheat. Indian J Genet 62:55–60
Funding
This research was supported by the R & D grant, University of Delhi. This paper is presented by Shashi in partial fulfillment of requisites for a thesis of Doctor of Philosophy in Botany at the University of Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Charles Armstrong
Rights and permissions
About this article
Cite this article
Shashi, Bhat, V. Enhanced somatic embryogenesis and plantlet regeneration in Cenchrus ciliaris L.. In Vitro Cell.Dev.Biol.-Plant 57, 499–509 (2021). https://doi.org/10.1007/s11627-020-10148-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10148-y