Abstract
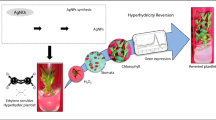
Hyperhydricity syndrome (HHS) occurs when plants are stressed resulting in sharp changes in the ethylene concentration in the in vitro culture. One of the characteristics of HHS is the reduction of cell wall lignification. The aim of this study was to examine the effects of silver nanoparticles (AgNPs) on HHS in micropropagated Thymus daenensis shoots. AgNPs (0, 1, 2.5, or 5 mg L−1) were mixed with Murashige and Skoog (MS) culture medium and the antioxidant response and variations in lignin content were measured on micropropagated shoots derived from seedlings cultured with AgNPs. In addition, the different forms of microshoot polyamines (PAs) were investigated through High Performance Thin Layer Chromatography (HPTLC). The results indicated that seedling pretreatment with AgNPs at concentrations of 1 and 2.5 mg L−1 led to a decrease in the activity of the microshoot antioxidant system resulting in an increase of H2O2. Furthermore, the increased lignin content in response to the 2.5 mg L−1AgNPs treatment was accompanied by an increase in the concentration of the bonded form of putrescine (Put) and spermine (Spm). There was a direct correlation between the lignin content and the concentration of bonded PAs. We hypothesize that the higher level of bonded PAs reduced the concentration of the free forms, leading to increased lignification. This may have led to the decreased HHS observed in shoot cultures.




Similar content being viewed by others
References
Bagni N, Tassoni A (2001) Biosynthesis oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 20:301–317
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 11:764–755
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Debergh P, Aitken-Christie J, Cohen D, Grout B, Von Arnold S, Zimmerman R, Ziv M (1992) Reconsideration of the term ‘vitrification’ as used in micropropagation. Plant Cell Tiss Organ Cult 30:135–140
Drolet G, Dumbroff E, Legge R, Thompson J (1986) Radical scavenging properties of polyamines. Phytochem 25:367–371
Durmuş N, Kadioğlu A (2005) Reduction of paraquat toxicity in maize leaves by benzyladenine. Acta Biol Hung 56:97–107
Franck T, Kevers C, Gaspar T (1995) Protective enzymatic systems against activated oxygen species compared in normal and vitrified shoots of Prunus avium L. raised in vitro. Plant Growth Regul 16:253–256
Franck T, Strasser RJ, Dommes J, Gaspar T (2004) Hyperhydricity of micropropagated shoots: a typically stress-induced change of physiological state. Plant Cell Tiss Organ Cult 77:181–191
Gaspar T (1991) Vitrification in micropropagation. In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry, vol 17, High-Tech and Micropropagation I. Springer, Berlin, pp 117–126
Graham MY, Graham TL (1991) Rapid accumulation of anionic peroxidases and phenolic polymers in soybean cotyledon tissues following treatment with Phytophthora megasperma f. sp. glycinea wall glucan. Plant Physiol 97:1445–1455
Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 124:21–30
Handy RD, Owen R, Valsami-Jones E (2008) The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicol 17:315–325
Hassannejad S, Bernard F, Mirzajani F, Gholami M (2012) SA improvement of hyperhydricity reversion in Thymus daenensis shoots culture may be associated with polyamines changes. Plant Physiol Biochem 51:40–46
Ivanova M, Novák O, Strnad M, Van Staden Z (2006) Endogenous cytokinins in shoots of Aloe polyphylla cultured in vitro in relation to hyperhydricity, exogenous cytokinins and gelling agents. Plant Growth Regul 50:219–230
Ivanova M, Van Staden J (2011) Influence of gelling agents and cytokinins on the control of hyperhydricity in Aloe polyphylla. Plant Cell Tiss Organ Cult 104:13–20
Kevers C, Franck T, Strasser RT, Dommes J, Gasper T (2004) Hyperhydricity of micropropagated shoot: a typically stress-induced change of physiological state. Plant Cell Tiss Organ Cult 77:181–191
Kevers C, Gaspar T (1986) Vitrification of carnation in vitro: changes in water content, extracellular space, air volume, and ion levels. Plant Cell Tiss Organ Cult 24:647–653
Kim HR, Kim MJ, Lee SY, Oh SM, Chung KH (2011) Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cells. Mutat Res, Genet Toxicol Environ Mutagen 726:129–135. doi:10.1016/j.mrgentox.2011.08.008
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. Plant Growth Metabol Process Biochem 47:651–658
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381
Lee Y-H, Kim H-S, Kim M-S, Kim Y-S, Joung H, Jeon J-H (2009) Reduction of shoot hyperhydricity in micropropagated potato plants via antisense inhibition of a chCu/ZnSOD gene. J Korean Soc Appl Biol Chem 52:397–400
Liu H-P, Dong B-H, Zhang Y-Y, Liu Z-P, Liu Y-L (2004) Relationship between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings. Plant Sci 166:1261–1267
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagents. J Biol Chem 193:265–275
Lu P, Cao J, He S, Liu J, Li H, Cheng G, Ding Y, Joyce DC (2010) Nano-silver pulse treatments improve water relations of cut rose cv. Movie Star flowers. Postharvest Biol Technol 57:196–202
Mauricio H-M, Jesus N-R, Catalina R (1999) Changes in polyamine content are related to low temperature resistance in potato plants. Acta Biol Colombiana 4:27–47
Mayor ML, Nestares G, Zorzoli R, Picardi LA (2003) Reduction of hyperhydricity in sunflower tissue culture. Plant Cell Tiss Organ Cult 72:99–103
Mirzajani F, Ghassempour A, Aliahmadi A, Esmaeili MA (2011) Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res Microbiol 162:542–549
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signaling. Curr Opinion Plant Biol 5:388–395
Niemietz CM, Tyerman SD (2002) New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. Science 531:443–447
Olmos E, Piqueras A, Martinez-Solano JR, Hellin E (1997) The subcellular localization of peroxidase and the implication of oxidative stress in hyperhydrated leaves of regenerated carnation plants. Plant Sci 130:97–105
Pandey S, Ranade SA, Nagar PK, Kumar N (2000) Role of polyamines and ethylene as modulators of plant senescence. J Biosci 25:291–299
Park SW, Jeon JH, Kim HS, Park YM, Aswath C, Joung H (2004) Effect of sealed and vented gaseous microenvironment on hyperhydricity of potato shoots in vitro. Sci Hortic 99:199–205
Piqueras A, Cortina M, Serna MD, Casas JL (2002) Polyamines and hyperhydricity in micropropagated carnation plants. Plant Sci 162:671–678
Rafiuddin ZZ (2012) Crystal growth of different morphologies (nanospheres, nanoribbons and nanoplates) of silver nanoparticles. Colloidal and Surf A: Physiochem Eng Aspects 393:1–5
Roussos PA, Pontikis CA (2007) Changes of free, soluble conjugated and bound polyamine titers of jojoba explants under sodium chloride salinity in vitro. J Plant Physiol 164:895–903
Ruffini Castiglione M, Cremonini R (2009) Nanoparticles in higher plants. Caryologia 62:161–165
Ruley AT, Sharma NC, Sahi SV (2004) Antioxidant defense in a lead accumulating plant, Sesbania drummondii. Plant Physiol Biochem 42:899–906
Serafini-Fracassini D, Di Sandro A, Del Duca S (2010) Spermine delays leaf senescence in Lactuca sativa and prevents the decay of chloroplast photosystems. Plant Physiol Biochem 48:602–611
Speranza A, Crinelli R, Scoccianti V, Taddei AR, Iacobucci M, Bhattacharya P, Ke PC (2013) In vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ Pollut 179:258–267
Turhan H (2004) The effect of silver nitrate (ethylene inhibitor) on in vitro shoot development in potato (Solanum tuberosum L.). Biotechnol 3:72–74
Veal E, Day A (2011) Hydrogen peroxide as a signaling molecule. Antioxid Redox Signal 15:147–151
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 152:59–66
Xiu Z-M, Zhang Q-B, Hema L, Puppala HL, Colvin VL, Alvarez PJJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275
Zar JH (1996) Biostatistical analysis. Prentice-Hall, Upper Saddle River, 662pp
Ziv M (1991) Vitrification: morphological and physiological disorders of in vitro plants. In: Debergh PC, Zimmerman RH (eds) Micropropagation: technology and application. Kluwer, Dordrecht, pp 45–69
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Bernard, F., Navab Moghadam, N. & Mirzajani, F. The effect of colloidal silver nanoparticles on the level of lignification and hyperhydricity syndrome in Thymus daenensis vitro shoots: a possible involvement of bonded polyamines. In Vitro Cell.Dev.Biol.-Plant 51, 546–553 (2015). https://doi.org/10.1007/s11627-015-9700-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9700-2