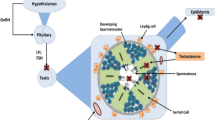
Summary
Ovary plays an important role in the female reproductive system. The maintenance and regulation of ovarian function are affected by various physical and chemical factors. With the development of industrialization, environmental pollutants have caused great harm to public health. Phthalates, as a class of endocrine-disrupting chemicals (EDCs), are synthesized and used in large quantities as plasticizers due to their chemical properties. They are easily released into environment because of their noncovalent interactions with substances, causing human exposure and possibly impairing ovary. In recent years, more and more attention has been paid to the role of epigenetics in the occurrence and development of diseases. And it is urgent to study the role of methylation, gene imprinting, miRNA, and other epigenetic mechanisms in reproductive toxicology.
Similar content being viewed by others
References
Chowdhary P, Raj A, Bharagava RN. Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environmental threats: A review. Chemosphere, 2018,194:229–246
Heudorf U, Mersch-Sundermann V, Angerer E. Phthalates: Toxicology and exposure. Int J Hyg Envir Heal, 2007,210(5):623–634
Wang YX, Zeng Q, Sun Y, et al. Semen phthalate metabolites, semen quality parameters and serum reproductive hormones: A cross-sectional study in China. Environ Pollut, 2016,211:173–182
Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect, 2004,112(3):331–338
Hannon PR, Flaws JA. The effects of phthala. Front Endocrinol, 2015,6:19
Lv HX, Mo CH, Zhao HM, et al. Soil contamination and sources of phthalates and its health risk in China: A review. Environ Res, 2018,164:417–429
Gallinger ZR, Nguyen GC. Presence of phthalates in gastrointestinal medications: Is there a hidden danger? World J Gastroenterol, 2013,19(41):7042–7047
Schettler T. Human exposure to phthalates via consumer products. Int J Androl, 2006,29(1):134–139
Koniecki D, Wang R, Moody RP, et al. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ Res, 2011,111(3): 329–336
Al-Saleh I, Al-Rajudi T, Al-Qudaihi G, et al. Evaluating the potential genotoxicity of phthalates esters (PAEs) in perfumes using in vitro assays. Environ Sci Pollut Res, 2017,24(30):23903–23914
Xu Y, Hubal EAC, Little JC. Predicting Residential Exposure to Phthalate Plasticizer Emitted from Vinyl Flooring: Sensitivity, Uncertainty, and Implications for Biomonitoring. Environ Health Perspect, 2010,118(2): 253–258
Przybylinska PA, Wyszkowski M. Environmental contamination with phthalates and its impact on living organisms. Ecol Chem Eng S, 2016,23(2):347–356
Li B, Wu S, Liang JM, et al. Distribution Characteristics and Risk Assessment of Phthalic Acid Esters in Agricultural Products Around the Pearl River Delta, South China. Huanjing Kexue (Chinese), 2016,37(1): 317–324
Abdolahnejad A, Gheisari L, Karimi M, et al. Monitoring and health risk assessment of phthalate esters in household’s drinking water of Isfahan, Iran. Int J Environ Sci Te, 2019,16(11):7409–7416
Anh HQ, Tomioka K, Tue NM, et al. A preliminary investigation of 942 organic micro-pollutants in the atmosphere in waste processing and urban areas, northern Vietnam: Levels, potential sources, and risk assessment. Ecotox Environ Safe, 2019,167:354–364
Blanchard O, Glorennec P, Mercier F, et al. Semivolatile Organic Compounds in Indoor Air and Settled Dust in 30 French Dwellings. Environ Sci Technol, 2014,48(7): 3959–3969
Polinski KJ, Dabelea D, Hamman RF, et al. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res, 2018,162:308–317
Kato K, Silva MJ, Reidy JA, et al. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect, 2004,112(3):327–330
Du Y, Guo N, Wang Y, et al. Follicular fluid concentrations of phthalate metabolites are associated with altered intrafollicular reproductive hormones in women undergoing in vitro fertilization. Fertil Steril, 2019,111(5):953–961
Main KM, Mortensen GK, Kaleva MM, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect, 2006,114(2):270–276
Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect, 2004, 112(3):331–338
Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol, 2013,43(3):200–219
Messerlian C, Souter I, Gaskins AJ, et al. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum Reprod, 2016,31(1):75–83
Machtinger R, Gaskins AJ, Racowsky C, et al. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ Int, 2018,111:23–31
Deng TR, Du YY, Wang YX, et al. The associations of urinary phthalate metabolites with the intermediate and pregnancy outcomes of women receiving IVF/ICSI treatments: A prospective single-center study. Ecotox Environ Safe, 2020,188:109884
Hannon PR, Peretz J, Flaws JA. Daily Exposure to Di(2-ethylhexyl) Phthalate Alters Estrous Cyclicity and Accelerates Primordial Follicle Recruitment Potentially Via Dysregulation of the Phosphatidylinositol 3-Kinase Signaling Pathway in Adult Mice. Biol Reprod, 2014, 90(6):136
Rattan S, Brehm E, Gao LY, et al. Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol Reprod, 2018,98(1):130–145
Pepling ME. Follicular assembly: mechanisms of action. Reproduction, 2012,143(2):139–149
Zhang T, Shen W, De Felici M, et al. Di(2-ethylhexyl) phthalate: Adverse effects on folliculogenesis that cannot be neglected. Environ Mol Mutagen, 2016,57(8):579–588
Del Mazo J, Brieno-Enriquez MA, Garcia-Lopez J, et al. Endocrine disruptors, gene deregulation and male germ cell tumors. Int J Dev Biol, 2013,57(2–4):225–239
Iona S, Klinger FG, Sisti R, et al. A comparative study of cytotoxic effects of N-ethyl-N-nitrosourea, adriamycin, and mono-(2-ethylhexyl)phthalate on mouse primordial germ cells. Cell Biol Toxicol, 2002,18(2):131–145
Mu XY, Liao XG, Chen XM, et al. DEHP exposure impairs mouse oocyte cyst breakdown and primordial follicle assembly through estrogen receptor-dependent and independent mechanisms. J Hazard Mater, 2015,298:232–240
Zhang T, Li L, Qin X-S, et al. Di-(2-ethylhexyl) Phthalate and Bisphenol A Exposure Impairs Mouse Primordial Follicle Assembly In Vitro. Environ Mol Mutagen, 2014,55(4):343–353
Zhang Y, Mu XY, Gao RF, et al. Foetal-neonatal exposure of Di (2-ethylhexyl) phthalate disrupts ovarian development in mice by inducing autophagy. J Hazard Mater, 2018,358:101–112
Liu JC, Li L, Yan HC, et al. Identification of oxidative stress-related Xdh gene as a di(2-ethylhexyl)phthalate (DEHP) target and the use of melatonin to alleviate the DEHP-induced impairments in newborn mouse ovaries. J Pineal Res, 2019,67(1):16
Zhang JN, Zhang RQ, Liu JC, et al. Di (2-ethylhexyl) Phthalate Exposure Impairs the microRNAs Expression Profile During Primordial Follicle Assembly. Front Endocrinol (Lausanne), 2019,10:877
Cuenca L, Shin N, Lascarez-Lagunas LI, et al. Environmentally-relevant exposure to diethylhexyl phthalate (DEHP) alters regulation of double-strand break formation and crossover designation leading to germline dysfunction in Caenorhabditis elegans. PLoS Genet, 2020,16(1):30
Zhang XF, Zhang T, Han Z, et al. Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod Fert Develop, 2015,27(8):1213–1221
Chen Y, Lyu R, Rong B, et al. Refined spatial temporal epigenomic profiling reveals intrinsic connection between PRDM9-mediated H3K4me3 and the fate of double-stranded breaks. Cell Res, 2020,30(3):256–268
Li X, Schimenti JC. Mouse pachytene checkpoint 2 (Trip13) is required for completing meiotic recombination but not Synapsis. PLoS Genet, 2007,3(8):1365–1376
Liu JC, Lai FN, Li L, et al. Di (2-ethylhexyl) phthalate exposure impairs meiotic progression and DNA damage repair in fetal mouse oocytes in vitro. Cell Death Dis, 2017,8(8):e2966
Sun ZY, Zhang P, Wang JJ, et al. Melatonin alleviates meiotic defects in fetal mouse oocytes induced by Di (2-ethylhexyl) phthalate in vitro. Aging-US, 2018, 10(12):4175–4187
Tu ZH, Mu XY, Chen XM, et al. Dibutyl phthalate exposure disrupts the progression of meiotic prophase I by interfering with homologous recombination in fetal mouse oocytes. Environ Pollut, 2019,252:388–398
Liu XS, Craig ZR. Environmentally relevant exposure to dibutyl phthalate disrupts DNA damage repair gene expression in the mouse ovary. Biol Reprod, 2019,101(4):854–867
Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol, 2001,172(3):217–224
Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function — implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrin, 2005,3:41
Park C, Lee J, Kong B, et al. The effects of bisphenol A, benzyl butyl phthalate, and di(2-ethylhexyl) phthalate on estrogen receptor alpha in estrogen receptor-positive cells under hypoxia. Environ Pollut, 2019,248:774–781
Xie Y, Li S, Zhou L, et al. Rapamycin preserves the primordial follicle pool during cisplatin treatment in vitro and in vivo. Mol Reprod Dev, 2020,87(4):442–453
Zhang XF, Zhang LJ, Li L, et al. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ Mol Mutagen, 2013,54(5):354–361
Hannon PR, Brannick KE, Wang W, et al. Mono(2-Ethylhexyl) Phthalate Accelerates Early Folliculogenesis and Inhibits Steroidogenesis in Cultured Mouse Whole Ovaries and Antral Follicles. Biol Reprod, 2015,92(5):11
Hannon PR, Niermann S, Flaws JA. Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice. Toxicol Sci, 2016,150(1):97–108
Beranger R, Hoffmann P, Christin-Maitre S, et al. Occupational exposures to chemicals as a possible etiology in premature ovarian failure: A critical analysis of the literature. Reprod Toxicol, 2012,33(3):269–279.
Pocar P, Fiandanese N, Berrini A, et al. Maternal exposure to di(2-ethylhexyl)phthalate (DEHP) promotes the transgenerational inheritance of adult-onset reproductive dysfunctions through the female germline in mice. Toxicol Appl Pharmacol, 2017,322:113–121
Rattan S, Beers HK, Kannan A, et al. Prenatal and ancestral exposure to di(2-ethylhexyl) phthalate alters gene expression and DNA methylation in mouse ovaries. Toxicol Appl Pharmacol, 2019,379:114629
Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod, 2010, 25(12):2944–2954
Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrin Met, 2010,21(2):96–103
Wan X, Zhu Y, Ma X, et al. Effect of DEHP and its metabolite MEHP on in vitro rat follicular development. Wei Sheng Yan Jiu (Chinese), 2010,39(3):268–70, 74
Warner GR, Li Z, Houde ML, et al. Ovarian Metabolism of an Environmentally Relevant Phthalate Mixture. Toxicol Sci, 2019,169(1):246–259
Gupta RK, Singh JM, Leslie TC, et al. Di-(2-ethylhexyl) phthalate and mono- (2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol, 2010,242(2):224–230
Wang W, Craig ZR, Basavarajappa MS, et al. Mono-(2-Ethylhexyl) Phthalate Induces Oxidative Stress and Inhibits Growth of Mouse Ovarian Antral Follicles. Biol Reprod, 2012,87(6):10
Craig ZR, Hannon PR, Wang W, et al. Di-n-Butyl Phthalate Disrupts the Expression of Genes Involved in Cell Cycle and Apoptotic Pathways in Mouse Ovarian Antral Follicles. Biol Reprod, 2013,88(1):10
Hannon PR, Brannick KE, Wang W, et al. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol, 2015,284(1):42–53
Rasmussen LM, Sen N, Vera JC, et al. Effects of in vitro exposure to dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on ovarian antral follicle growth and viability. Biol Reprod, 2017,96(5):13
Zhou CQ, Flaws JA. Effects of an Environmentally Relevant Phthalate Mixture on Cultured Mouse Antral Follicles. Toxicol Sci, 2017,156(1):217–229
Chen H, Feng WW, Chen K, et al. Transcriptomic analysis reveals potential mechanisms of toxicity in a combined exposure to dibutyl phthalate and diisobutyl phthalate in zebrafish (Danio rerio) ovary. Aquat Toxicol, 2019,216:105290
Meling DD, Warner GR, Szumski JR, et al. The effects of a phthalate metabolite mixture on antral follicle growth and sex steroid synthesis in mice. Toxicol Appl Pharmacol, 2020,388:114875
Sen N, Liu XS, Craig ZR. Short term exposure to din-butyl phthalate (DBP) disrupts ovarian function in young CD-1 mice. Reprod Toxicol, 2015,53:15–22
Li L, Liu JC, Lai FN, et al. Di (2-ethylhexyl) Phthalate Exposure Impairs Growth of Antral Follicle in Mice. Plos One, 2016,11(2):18
Wang YA, Yang Q, Liu W, et al. DEHP exposure in utero disturbs sex determination and is potentially linked with precocious puberty in female mice. Toxicol Appl Pharmacol, 2016,307:123–129
Liu J, Wang W, Zhu J, et al. Di(2-ethylhexyl) phthalate (DEHP) influences follicular development in mice between the weaning period and maturity by interfering with ovarian development factors and microRNAs. Environ Toxicol, 2018,33(5):535–544
Grossman D, Kalo D, Gendelman M, et al. Effect of di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate on in vitro developmental competence of bovine oocytes. Cell Biol Toxicol, 2012,28(6):383–396
Absalan F, Saremy S, Mansouri E, et al. Effects of Mono-(2-Ethylhexyl) Phthalate and Di-(2-Ethylhexyl) Phthalate Administrations on Oocyte Meiotic Maturation, Apoptosis and Gene Quantification in Mouse Model. Cell J, 2017,18(4):503–513
Li FP, Zhou JL, Guo AW, et al. Di(n-butyl) phthalate exposure impairs meiotic competence and development of mouse oocyte. Environ Pollut, 2019,246:597–607
Zhang Y, Wang T, Lan M, et al. Melatonin protects oocytes from MEHP exposure-induced meiosis defects in porcine. Biol Reprod, 2018,98(3):286–298
Ambruosi B, Uranio MF, Sardanelli AM, et al. In Vitro Acute Exposure to DEHP Affects Oocyte Meiotic Maturation, Energy and Oxidative Stress Parameters in a Large Animal Model. PLoS One, 2011,11(2):e0148350
Ray B, D’ Souza AS, Kumar V, et al. Ovarian development in Wistar rat treated prenatally with single dose diisobutyl phthalate. Bratisl Med J, 2012,113(10): 577–582
Yin JC, Liu R, Jian ZH, et al. Di (2-ethylhexyl) phthalate-induced reproductive toxicity involved in dna damage-dependent oocyte apoptosis and oxidative stress in Caenorhabditis elegans. Ecotox Environ Safe, 2018,163:298–306
Edson MA, Nagaraja AK, Matzuk MM. The Mammalian Ovary from Genesis to Revelation. Endocr Rev, 2009,30(6):624–712
Lai FN, Liu JC, Li L, et al. Di (2-ethylhexyl) phthalate impairs steroidogenesis in ovarian follicular cells of prepuberal mice. Arch Toxicol, 2017,91(3):1279–1292
Davis BJ, Maronpot RR, Heindel JJ. Di-(2-Ethylhexyl) Phthalate Suppresses Estradiol And Ovulation In Cycling Rats. Toxicol Appl Pharmacol, 1994,128(2):216–223
Davis BJ, Weaver R, Gaines LJ, et al. Mono-(2-Ethylhexyl) Phthalate Suppresses Estradiol Production Independent Of Fsh-Camp Stimulation In Rat Granulosa-Cells. Toxicol Appl Pharmacol, 1994,128(2):224–228
Meltzer D, Martinez-Arguelles DB, Campioli E, et al. In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod Toxicol, 2015,51:47–56
Li N, Liu T, Zhou LT, et al. Di-(2-ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol, 2012,34(3):869–875
Li N, Liu KQ, Yuan HT, et al. The effect of mono-(2-ethylhexyl) phthalate on apoptosis of rat ovarian granulosa cells in vitro. Environ Toxicol Pharmacol, 2015,39(2):643–650
Kawano M, Qin XY, Yoshida M, et al. Peroxisome proliferator-activated receptor a mediates di-(2-ethylhexyl) phthalate transgenerational repression of ovarian Esr1 expression in female mice. Toxicol Lett, 2014,228(3):235–240
Wang XJ, Xiong GP, Luo XM, et al. Dibutyl Phthalate Inhibits the Effects of Follicle-Stimulating Hormone on Rat Granulosa Cells Through Down-Regulation of Follicle-Stimulating Hormone Receptor. Biol Reprod, 2016,94(6):13
Li N, Liu T, Guo K, et al. Effect of mono-(2-ethylhexyl) phthalate (MEHP) on proliferation of and steroid hormone synthesis in rat ovarian granulosa cells in vitro. J Cell Physiol, 2018,233(4):3629–3637
Tripathi A, Pandey V, Sahu AN, et al. Di-(2-ethylhexyl) phthalate (DEHP) inhibits steroidogenesis and induces mitochondria-ROS mediated apoptosis in rat ovarian granulosa cells. Toxicol Res, 2019,8(3):381–394
Guo M, Lai L, Zong T, et al. Exposure to di(2-ethylhexyl) phthalate inhibits luteal function via dysregulation of CD31 and prostaglandin F2alpha in pregnant mice. Reprod Biol Endocrin, 2015,13:1–8
Herreros MA, Gonzalez-Bulnes A, Inigo-Nunez S, et al. Toxicokinetics of di(2-ethylhexyl) phthalate (DEHP) and its effects on luteal function in sheep. Reprod Biol, 2013,13(1):66–74
Kurzynska A, Bogacki M, Chojnowska K, et al. Peroxisome Proliferator Activated Receptor Ligands Affect Progesterone And 17 Beta-Estradiol Secretion By Porcine Corpus Luteum During Early Pregnancy. J Physiol Pharmacol, 2014,65(5):709–717
Parillo F, Maranesi M, Brecchia G, et al. In Vivo Chronic and In Vitro Acute Effects of Di(2-Ethylhexyl) Phthalate on Pseudopregnant Rabbit Corpora Lutea: Possible Involvement of Peroxisome Proliferator-Activated Receptor Gamma. Biol Reprod, 2014,90(2):14
Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reprod Toxicol, 2007,23(3):297–307
Xie X, Gao Y, Zhang Y, et al. Genome-wide analysis of DNA methylation changes in the rat ovary after prenatal exposure to di-(2-ethylhexyl)-phthalate. Zhonghua Yu Fang Yi Xue Za Zhi (Chinese), 2012,46(9):840–844
Wiklund ED, Kjems J, Clark SJ. Epigenetic architecture and miRNA: reciprocal regulators. Epigenomics, 2010,2(6):823–840
Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science, 2001,293(5532): 1089–1093
Choudhuri S, Cui Y, Klaassen CD. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol Appl Pharmacol, 2010,245(3):378–393
Singh S, Li SSL. Epigenetic Effects of Environmental Chemicals Bisphenol A and Phthalates. Int J Mol Sci, 2012,13(8):10143–10153
Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature, 2007, 447(7143):425–432
Tindula G, Murphy SK, Grenier C, et al. DNA methylation of imprinted genes in Mexican-American newborn children with prenatal phthalate exposure. Epigenomics, 2018,10(7):1011–1026
Ferguson-Smith AC, Surani MA. Imprinting and the epigenetic asymmetry between parental genomes. Science, 2001,293(5532):1086–1089
Reik W, Walter J. Genomic imprinting: Parental influence on the genome. Nat Rev Genet, 2001,2(1):21–32
Clayton-Smith J. Genomic imprinting as a cause of disease — Is increasingly recognised, especially after assisted reproduction. Br Med J, 2003,327(7424):1121–1122
Li L, Zhang T, Qin XS, et al. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol Biol Rep, 2014,41(3):1227–1235
Chao HH, Zhang XF, Chen B, et al. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem Cell Biol, 2012,137(2):249–259
Hudder A, Novak RF. miRNAs: Effectors of environmental influences on gene expression and disease. Toxicol Sci, 2008,103(2):228–240
Yanez-Mo M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles, 2015,4: 27066
Martinez RM, Hauser R, Liang L, et al. Urinary concentrations of phenols and phthalate metabolites reflect extracellular vesicle microRNA expression in follicular fluid. Environ Int, 2019,123:20–28
Brehm E, Flaws JA. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology, 2019,160(6):1421–1435
Dodge LE, Williams PL, Williams MA, et al. Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment. Reprod Toxicol, 2015,58:184–193
Hauser R, Gaskins AJ, Souter I, et al. Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing in Vitro Fertilization: Results from the EARTH Study. Environ Health Perspect, 2016,124(6):831–839
Wu HT, Ashcraft L, Whitcomb BW, et al. Parental contributions to early embryo development: influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment. Hum Reprod, 2017,32(1):65–75
Cao MF, Pan WY, Shen XY, et al. Urinary levels of phthalate metabolites in women associated with risk of premature ovarian failure and reproductive hormones. Chemosphere, 2020,242:125206
Hatcher KM, Smith RL, Chiang C, et al. Association of phthalate exposure and endogenous hormones with self-reported sleep disruptions: results from the Midlife Women’s Health Study. Menopause, 2020,27(11):1251–1264
Sathyanarayana S, Barrett E, Butts S, et al. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction, 2014,147(4):401–409
Meeker JD, Ferguson KK. Urinary Phthalate Metabolites Are Associated With Decreased Serum Testosterone in Men, Women, and Children From NHANES 2011–2012. J Clin Endocrinol Metab, 2014,99(11):4346–4352
Lin S, Ku HY, Su PH, et al. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere, 2011,82(7):947–955
Huang PC, Kuo PL, Chou YY, et al. Association between prenatal exposure to phthalates and the health of newborns. Environ Int, 2009,35(1):14–20
Su PH, Chen JY, Lin CY, et al. Sex Steroid Hormone Levels and Reproductive Development of Eight-Year-Old Children following In Utero and Environmental Exposure to Phthalates. PLoS One, 2014,9(9):e102788
Wen HJ, Chen CC, Wu MT, et al. Phthalate exposure and reproductive hormones and sex-hormone binding globulin before puberty — Phthalate contaminated-foodstuff episode in Taiwan. Plos One, 2017,12(4): e0175536
Wen HJ, Sie L, Su PH, et al. Prenatal and childhood exposure to phthalate diesters and sex steroid hormones in 2-, 5-, 8-, and 11-year-old children: A pilot study of the Taiwan Maternal and Infant Cohort Study. J Epidemiol, 2017,27(11):516–523
Du YY, Guo N, Wang YX, et al. Urinary phthalate metabolites in relation to serum anti-Mullerian hormone and inhibin B levels among women from a fertility center: a retrospective analysis. Reprod Health, 2018,15(1):33
Li N, Li Y, Meng H, et al. Associations between Urinary Phthalate Metabolites and Serum Anti-Muller Hormone Levels in US Men Based on National Health and Nutrition Examination Survey 2003–2004. Int J Environ Res Public Health, 2017,14(12):1513
Nishimura Y, Moriya K, Kobayashi S, et al. Association of exposure to prenatal phthalate esters and bisphenol A and polymorphisms in the ESR1 gene with the second to fourth digit ratio in school-aged children: Data from the Hokkaido study. Steroids, 2020,159:108637
Martinez-Nava GA, Burguete-Garcia AI, Lopez-Carrillo L, et al. PPAR gamma and PPARGC1B polymorphisms modify the association between phthalate metabolites and breast cancer risk. Biomarkers, 2013,18(6):493–501
Joensen UN, Jorgensen N, Meldgaard M, et al. Associations of Filaggrin Gene Loss-of-Function Variants with Urinary Phthalate Metabolites and Testicular Function in Young Danish Men. Environ Health Perspect, 2014,122(4):345–350
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest Statement
The authors declare that there is no conflict of interest with any financial organization or corporation or individual that can inappropriately influence this work.
The study was supported by the National Natural Science Foundation of China (No. 81571508 and No. 81771654), the National Natural Science Youth Foundation of China (No. 81701520) and the Self-dependent Innovation Research Funding of Huazhong University of Science and Technology (No. 2017KFYXJJ121).
Rights and permissions
About this article
Cite this article
Jiang, Hh., Du, Yy. & Li, Yf. Ovarian Toxicity and Epigenetic Mechanisms of Phthalates and Their Metabolites. CURR MED SCI 41, 236–249 (2021). https://doi.org/10.1007/s11596-021-2342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-021-2342-1